Abstract
Context:
Nowadays, it has been proved that there is a relation between dietary habits and incidence of different types of cancers. Consumption of processed foods exposes human to a wide range of toxicants such as heterocyclic aromatic amines, polycyclic aromatic hydrocarbons, acrylamide and nitrosamines that have mutagenic and carcinogenic effects on body and especially induce colon cancer. Under such circumstances, search for antimutagenic agents and helpful strategies have gained interest.Evidence Acquisition:
We performed a computerized search of Scopus, Pubmed and google scholar databases with keywords: cancer, food toxicants, lactic acid bacteria, and probiotics.Results:
Natural dietary compounds like lactic acid bacteria and probiotics can be beneficial in decline of detrimental effects associated with toxicants formed during food processing. It has been stated that binding ability of lactic acid bacteria and probiotics via their cell wall have prominent roles in detoxifying these toxicants. Also, this capability is influenced by various factors.Conclusions:
It can be concluded that probiotics can play a vital role in prevention of colon cancer that is induced by food toxicants and their incorporation into food can be helpful in this respect.Keywords
Anticarcinogenic Cancer Food Toxicants Lactic Acid Bacteria Probiotics
1. Context
Today, the production of healthy and safe food is a key element in food industry. Unlike the food industry's efforts to produce safe food, it is likely that food can be either contaminated during the process or prepared from contaminated raw materials (1). Therefore, food products consumers can be environmentally exposed to both intentional and unintentional additives and pollutants which can have adverse effects on health over time. Environmental contaminants such as heavy metals, pesticides and mycotoxins can be released to water and food production chain and lead to formation of unwanted harmful chemical compounds during processing, resulting in various adverse health effects and chronic toxicity, especially cancer (1-3). Cancer is a very serious and complicated disease created by out of control and irregular growth of cell (4), whose prevalence is remarkably increasing. Except for genetic defects which contribute to 5 to 10% of cancer incidences, the rest (90% to 95%) can be limited by changing lifestyle, increasing physical activity, avoiding smoking and utilizing nutritionally balance diet together with the foods free from contaminants (4, 5). Lung, colon/rectum, breast, and prostate cancers are the most widespread among 100 human cancers (4). It is reported that bowel cancer is the second and third most prevalent type of cancer in Europe and worldwide, respectively (6, 7). Among the different aspects of lifestyle, it is generally approved that diet and nutritional factors have a major role in incident of cancer, particularly gastrointestinal tract-related ones such as colorectal cancer (CRC) (8, 9) and it is observed that 30% - 40% of cancer cases can be possibly prevented by improvement of diet and getting proper nutritional factors (10). Researches have shown that diets rich with fruits and vegetables seem to have a protective effect on CRC development (11) while the risk of CRC is enhanced by increasing consumption of red meat and animal fat (9, 12). Heterocyclic aromatic amines (HCA), polycyclic aromatic hydrocarbons (PAH), nitrites and nitrates are related to meat consumption. On the other hand, mycotoxins (aflatoxins) and acrylamide (13) might have important roles in the etiology of CRC (14-16). Although various approaches are utilized as common treatment options for CRC such as surgery, chemotherapy and radiotherapy (13, 17), lactic acid bacteria and probiotics gained a lot of attention as antimutagens and preventive agents in colon carcinogenesis (17-23).
2. Evidence Acquisition
In this article, binding ability of different LABs and probiotics strains to some food toxicants and the underlying mechanisms as well as parameters affecting this ability are reviewed. For the literature review, we have used standard search strategies involving the querying of available online databases (Scopus, Pubmed and google scholar) by using terms including “Anticarcinogenic”, “Cancer”, “Heterocyclic aromatic amines”, “Polycyclic aromatic hydrocarbons”, “Acrylamide”, “Nitrosamine”, “Lactic acid bacteria”, and “Probiotics”. No specific key words have required as inclusion criteria. The reference lists of each article have been reviewed in details to find additional articles. Articles found were categorized according to binding of lactic acid bacteria and probiotics to four types of food toxicants and were reviewed independently in full text.
3. Results
3.1. Lactic Acid Bacteria Probiotics, and Colon Cancer Prevention
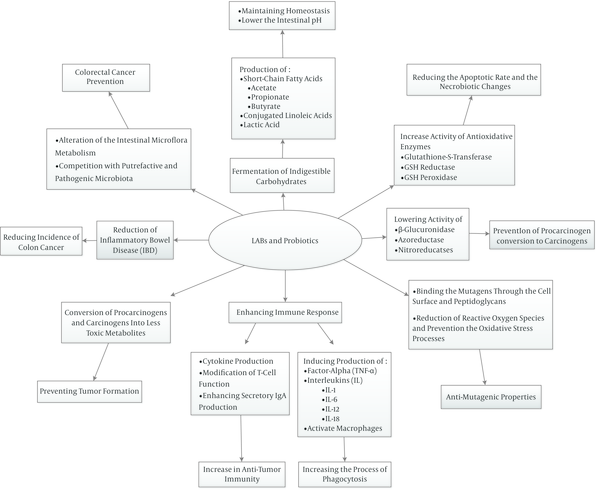
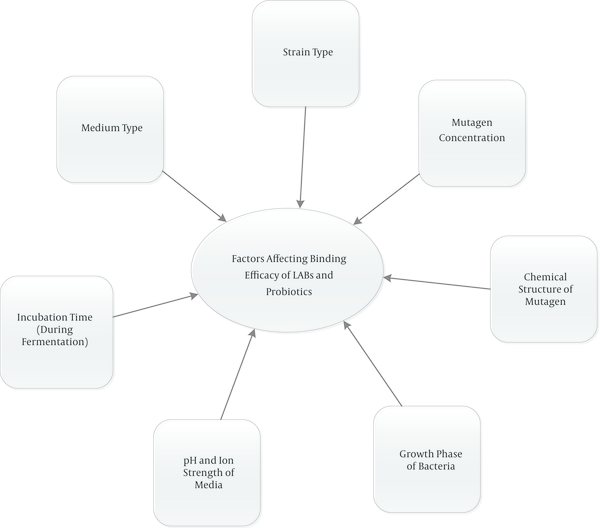
‘Lactic acid bacteria (LAB)’ are varied groups of bacteria that produce mainly lactic acid as a consequence of carbohydrate fermentation. LABs are gram-positive, non-motile and cocci or rods which have been widely utilized to produce various foods especially fermented ones (1, 24). Lactobaciillus, Lactococcus, leuconostoc, piediococcus, and Streptococcus are the major genera of LABs (25). Most of them are presented in the oral cavity, the intestinal tract, and vagina normal flora having different beneficial effects and preservative properties (26, 27). Most of their useful properties are attributed to their ability to adhere to the intestinal mucosa (28). In addition, it is mentioned that LABs have protective effects against different toxic compounds in foods such as mycotoxin, acrylamide, polycyclic aromatic hydrocarbons (PAHs), heterocyclic amines (HCAs) and amino acid pyrolysates (16). ‘Probiotics’ that mainly belong to the type ‘lactobacillus’ or ‘Bifidobacteria’ are non-toxic and non-pathogenic, show in vivo functional properties and are considered probiotics (1, 29, 30). The term ‘probiotic’ is originated from Greek meaning ‘for life’ and these microorganisms bring health benefits to humans/animals principally by balancing intestinal flora. Apart from Lactobacillus and Bifidobacterium spp., Pediococcus acidilatici, Enterococcus spp. and saccharomyces boulardii are also used as probiotics (1, 29, 31). Various health properties such as anti-mutagenic and anti-carcinogenic effects, modulation of the immune system, suppression of allergies, decreasing cholesterol levels, anti-infection properties, relief of lactose intolerance symptoms, and improving the nutritional value have been ascribed to LABs and probiotics (25, 32-35). The anti-carcinogenic effect, especially preventing colorectal cancer, is one of the most important health consequences of LABs and probiotics which are taken into consideration by most researchers (20, 22, 23, 25, 36-38). Although the exact mechanisms of colon cancer prevention have not been identified entirely, there are several possible mechanisms that could explain their anti-cancer properties. LABs and probiotics may inhibit colon cancer by enhancing the host’s immune response, changing the metabolism of intestinal microflora (19), reducing intestinal inflammation such as inflammatory bowel disease (IBD) (39-41), binding/adsorption of carcinogens by cell surface and peptidoglycans (42-46), altering the xenobiotic metabolizing enzyme, preventing the oxidative stress processes, and reducing reactive oxygen species (4, 47, 48). Additionally, it is indicated that production of various free fatty acids, organic acids, and other metabolites as a consequence of non-digestible carbohydrate fermentation in the gut and reducing pH are the other pathways hindering incidence of colon cancer (49). It is expressed that probiotic bacteria participate in detoxification and biotransformation of xenobiotics and converting them to less toxic metabolites as well as slowing down conversion of nontoxic procarcinogens to highly toxic metabolites and hinder tumor formation (50). Figure 1 shows main mechanisms of LABs and probiotics action in prevention of cancer. Also the main factors that have effects on the binding ability of lactic acid bacteria (LAB) and probiotics are shown in Figure 2.
Main Mechanisms of Lactic Acid Bacteria (LABs) and Probiotics Action in Prevention of Cancer
Main Factors Affect Binding Ability of Lactic Acid Bacteria (LAB) and Probiotics
3.2. Binding Ability of LABs and Probiotics to Heterocyclic Aromatic Amines (HAA) and Polycyclic Aromatic Hydrocarbons (PAH)
Heterocyclic aromatic amines are a class of chemical compounds that possess at least one heterocyclic ring and are classified into two major groups. One group, known as 'pyrolytic HAAs', are formed as a result of pyrolysis of some amino acids like tryptophan, glutamic acid, phenylalanine, and ornithine at elevated temperatures (> 250°C). The second group of HAAs are the aminoimidazoarenes (AIAs). These compounds are formed in muscle foods (meat and fish) that are cooked to medium and well-done states at high temperatures (150 - 250°C). The Maillard reaction is assumed to have a significant role in the formation of AIAs (51-53). Epidemiological studies revealed the relation between intakes of HAAs and cancers of various organs like colon, rectum, breast, pancreas, lung, prostate, stomach and esophagus (54). The international agency for research on cancer (IARC) regards some of the HAAs as possible human carcinogens (MeIQ, MeIQx and PhIP, class 2B) and one as a probable human carcinogen (IQ, class 2A) (55). In order to mitigate the risk of cancers driven by HAAs, using lower temperatures and avoiding prolonged cooking and broiling of meat, and direct exposure to a naked flame can be profitable. On the other hand, the other methods involve inhibition and abrogation of these compounds activities in biological systems (53, 56).
Polycyclic aromatic hydrocarbons (PAH) are a group of organic compounds that contain two or more fused aromatic rings consisting carbon and hydrogen atoms. PAHs are formed as a consequence of incomplete combustion of fossil fuels, and since they are air pollutants, the soil and ground water can be contaminated and therefore, they can enter into the food chain (57). More than 100 different PAHs have been recognized, but the US environmental protection agency (EPA) has listed 16 PAHs as the main contaminants of food sources (58). The most important HAAs and PAHs are listed in Table 1. Different strategies of decontamination processes to reduce PAH levels in fish oils, including solvent extraction (ethanol) and adsorbent extraction (e.g., activated carbon, mussel shell, and wood ashes), and in smoked meat products, such as different smoking conditions, type of casing, and different types of wood chips, have been explored (59, 60). Apart from the proposed strategies and methods to diminish the level of formed HAAs and PAHs, some investigation have been performed considering the potential inhibitory activity of probiotics versus mutagenic compounds in foods induced by processing.
The Most Important PAHs and HAA Compounds Formed in Food
| Polycyclic Aromatic Hydrocarbons | Heterocyclic Aromatic Amine |
|---|---|
| Naphthalene | 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) |
| Acenaphthylene, | 2-Amino-3,4-dimethyl-3H-imidazo [4,5-f] quinoline (MeIQ) |
| Acenaphthene | 2-Amino-1-methyl-6-phenylimidazo[4,5-b] pyridine (PhIP) |
| Fluorene | 2-amino-6-methyldipyrido [1,2-a:3',2'-d] imidazole (Glu-P-1) |
| Anthracene | 3-Amino-1,4-dimethyl-5H-pyrido [4,3-b] indole (Trp-P-1) |
| Phenanthrene | 3-amino-1-methyl-5H-pyrido [4,3-b] indole (Trp-P-2) |
| Fluoranthene | 2-amino-3,8-dimethylimidazo [4,5-f] quinoxaline (MeIQx) |
| Pyrene | |
| Chrysene | |
| Benz[a]anthracene | |
| Benzo[b]fluoranthene | |
| B[a]P | |
| Benzo[k]fluoranthene, | |
| Indeno[1,2,3-cd]pyrene | |
| Benzo[g,h ,i]perylene | |
| Dibenz[a,h]anthracene |
The binding capacity of either whole cells, cell wall skeleton (CWS), or any component of CWS of Lactobacillus acidophilus IFO 13951 and Bifidobacterium bifidum IFO 14252 to six HCAs (Trp-P1, Glu-P-l, Phe-P-l, MeIQ, IQ, and MeIQx) were examined. The binding efficacy was variable between the mentioned strains according to the mutagen compound. The binding of Trp-P-l and Trp-P-2 were the highest, but the binding of Glu-P-l, Phe-P-l, and IQ were lower by the two bacteria. Treating whole cells and CWS by lysozyme and α-amylase, decreased the binding of Trp-P-l and Trp-P-2 by about 30%. The authors pointed out that the main component responsible for binding activity of these bacteria was peptidoglycan of CWS (45). In another study, binding ability of mutagens Trp-P1, PhIP, IQ, and MeIQx to eight human intestinal or LAB strains (L. acidophilus NCFB 174, Lactobacillus fermentum KLD and Bifidobacterium longum BB 5368, Clostridium perfringens ATCC 1314, Bacteroides fragilis NCTC 9343, Escherichia coli ATCC 25922, Lactococcus lactis ssp. lactis NCFB 604 and Lactococcuslactis ssp. cremoris NCFB 607) were reported. The results exhibited that all the tested strains were able to bind Trp-P1 and by using 20 µg or more of lyophilized cell, 90% - 96% binding of the mutagen was recorded by LAB strains. In contrast, E. coli, B. fragilis and Cl. perfringens were less effective at these high levels (61). In a work carried out by Sreekumar and Hosono, 28 strains of Lactobacillus gasseri and 2 strains of B. Longum were verified regarding their binding properties to amino acid pyrolysates (Trp-P-1, Trp-P-2, MeIQ, IQ and Glu-P-1) and antimutagenic properties with Trp-P1. Among the inspected strains, the greatest percentage of antimutagenicity and binding were rendered by four strains of L. acidophilus (SBT0274, SBT1703, SBT10239, and SBT10241) and 1 strain of B. longum (SBT 2928) which were selected for subsequent studies on the effect of cell concentration, pH, incubation time and mutagen concentration. It was illustrated that in all of these selected strains, cell concentration of 2 mg caused a binding degree of 88% - 95% to 0.2 mg Trp-P1 during 30 minutes incubation at pH 7. It was also implied that purified cell wall of the strains were more impressive compared to crude extracts, peptidoglycan, or cell extracts in mutagen binding. Treating cell walls with meta- periodate or trichloroacetic acid that oxidize OH groups in the cis position to aldehydes and carbon acid groups, and degrade carbohydrate or remove polymer from the structure, reduced binding capability whereas enzymic treatment with trypsin or proteinase K had no effect. Thus, it could be concluded that the bacterial binding receptors lie in the bacterial cell wall polysaccharides, and the intact glucose molecules have a significant role in binding (42). In contrast to this study, it was displayed that cells and peptidoglycan of Lactobacillus plantarum mutant strain had no binding ability while capsular cells of the mutant (cell and EPS attached to the cell surface) showed binding ability (62).
The growing and survivability of four Lactobacillus strains (L. casei LOCK 0900, L. casei LOCK 0908, L. paracasei LOCK 0919 and L. plantarum LOCK 0945) were examined in the presence of three heterocyclic aromatic amines (IQ, MeIQx, or PhIP). In order to examine the growing ability of bacteria, they were incubated with HAA compounds at concentrations of 5 and 25 µg/mL for 24 hours in MRS broth and survival of lactobacilli was monitored by incubation in phosphate buffer for maximum 120 hours. It was demonstrated that the growth of the strains was not influenced by the presence of IQ, MeIQx, or PhIP at two levels, except in the case of L. casei 0900 where the number of the living cell decreased slightly at the level of 25 µg/mL PhIP. HCA compounds at concentrations of 5 µg/mL had no impact on survival of bacteria in the phosphate buffer, but at 25 µg/mL, various results were obtained depending on the type of strain. Three of four strains were not influenced by PhIP and IQ until the period of 120 hours. The most resistant strain was L. plantarum 0945, while L. casei 0908 and L. paracasei 0919 were the most sensitive to MeIQx and L. casei 0900 to IQ. Totally, it was implicated that probiotic bacteria are able to bind HCAs in human body and are removed in feces (63). In a study carried out by Stidl et al. 12 strains from eight different LAB species (B. longum, L. acidophilus, L. bulgaricus, L. casei, L. helveticus, L. kefir, L. plantarum and S. thermophiles) either contained in fermented foods or in the human gastrointestinal tract were explored regarding their binding capacities to five HCA including AαC, PhIP, IQ, MeIQx and DiMeIQx. Among the tested eight species of Lactobacillus, L. helveticus and S. thermophilus were seven to eight times more effective than L. Kefir and L. plantarum strains in detoxification. The results also revealed that the detoxification of mutagens was as follows: AαC > DiMeIQx > MeIQx > IQ > PhIP (22). Faridnia et al. (64) assessed the binding ability of Bifidobacterium pseudocatenulatum G4, B. longum BB536, and E. coli ATCC 25922 to heterocyclic aromatic amines, including Trp-p-2, IQ, MeIQx, 7,8DiMeIQx and PhIP at pH 5.6 and 6.8.
The effect of bacterial cell concentration on binding ability was also monitored. Results indicated that B. pseudocatenulatum G4 was the most effective strain in binding to HCA compounds followed by B. longum, and E. coli. It was concluded that gram-positive bacterium, due to its cell wall structure, was more efficient compared with gram-negative strains. Binding to mutagens was pH dependent and the maximum binding ability were observed at pH 6.8 in all bacteria, but the two bifidobacteria showed higher activity than E. coli. The interaction between HCA compounds and various concentrations of B. pseudocatenulatum G4 (106, 108 and 1010) was evaluated which showed that the highest reduction in HCA amount occurred at a level of 1010 cfu/ mL (64).
The impact of pH on binding capacity has been illustrated in previous studies (38, 45, 65, 66). In rats, absorption of HAAs in stomach occurred at pH above 4 (61). Binding of HAAs to L. acidophilus and B. longum cell walls was 80% at pH 5 (67). Contrary to these results, Tsuda et al. reported a maximum binding of Trp-P-1 to L. plantarum mutant strain at pH 8 (62).
Detoxifying effect of L. casei DN 114001 in MRS broth and modified MRS broth in the presence of 5 - 25 µg/mL of IQ, MelQx or PhIP were studied. It was implied that none of the HCA compounds affected the growth and survival of L. casei DN 114001 during 24 hours and 168 hours incubation in MRS broth and modified MRS broth, respectively. After 24 hours cultivation in MRS broth, the amount of IQ and PhIP were reduced significantly (98% - 99%) and in the case of MelQx, the degree of reduction was 27%. In modified MRS broth, decreasing HCA concentration was lower as a result of lower cell density and was dependent on the growth phase of bacteria. IQ decreased by 49% - 54% in the stationary phase of growth (after 24 hours of cultivation), while the MelQx amount was reduced by 11.2% in the logarithmic (till 24 hours), stationary and early death phase of growth which imply that dead cells have the additional ability to absorb carcinogens (66). The antigenotoxic capability of the probiotic L. rhamnosus IMC501 was investigated in mice treated with PhIP. 10 days before administration of PhIP, mice were fed with the suspension of lactobacilli and abundance of lactobacilli in feces, effects on fecal enzymatic activity and DNA damage in the colon and liver cells were determined. It was observed that after 5 days of probiotic administration, the number of lactobacilli increased in the feces and activity of β-glucuronidase and β-N-acetyl-glucosaminidase (high activity in patients with colorectal cancer) decreased 63% and 26%, respectively. It was also reported that the extent of DNA damage in colon cells significantly decreased, whereas no genotoxic effect was recognized in liver cells (23). On approval of this study, genotoxicity of fecal water (FW) and the activity of two enzymes (β-glucuronidase and β-glucosidase) in human feces in three age groups (children, adults and elderly) after incubation with 50 µg/mL IQ and three probiotic strains including (L. casei LOCK 0900, L. casei LOCK 0908, and L. paracasei LOCK0919) demonstrated reduction in β-glucuronidase activity in feces and the reduced amount was 64% in the case of L. casei 0900, 76% for L. paracasei 0908 in children and in the elderly group, it was alleviated by 58% for L. casei 0900 to 82% for L. paracasei 0919. The same pattern was observed in the decline of β-glucosidase activity. In the elderly group, the decrease range was from 83% (L. casei 0900) to 90% (L. paracasei 0919), and in the children group from 37% (L. casei 0900) to 55% (L. casei 0908) and in the adult group, there was no significant change in the activity of the enzymes. The greatest inhibition extent (64.5%) of fecal water genotoxicity by lactobacilli was achieved in the adult group (63). It is assumed that probiotics can adhere to colonocytes and restrict absorption of mutagens to the intestine (45) or decrease their bioavailability (68).
In another study by the aforementioned author, the impact of the same probiotic strains was evaluated on the fecal enzyme and genotoxic activity in human fecal water (children, adults and the elderly) in the presence of PhIP. It was reported that the mean β- glucuronidase activity was 64% lower among children feces after incubation with L. casei 0900, 75% lower for L. casei 0908 and 65% lower for L. paracasei 0919 and in elderly group, the most remarkable decline was observed as 82% for L. casei 0900, 83% for L. casei 0908 and 90% for L. paracasei 0919. In terms of β-glucosidase activity, probiotic strains induced a slight change in children and adults. In elderly, the enzyme activity was 87% lower for L. casei 0900, 79% for L. casei 0908 and 92% for L. paracasei 0919. L. casei 0908 and L. paracasei 0919 were the most effective in decreasing genotoxicity in children and adults. In the elderly group, L. casei 0900 was the most efficient strains (63). In another study by Klewicka et al. the protective effect of feeding beetroot juice fermented with Lactobacillus brevis 0944 and L. paracasei 0920 against aberrant crypt foci (ACF) formation in rat colon in the presence of PhIP. PhIP was used as a carcinogen at a dose of 10 µg/day. Lactofermented beetroot juice decreased the number of ACF from 59 to 26, malondialdehyde in the liver and cytotoxic and genotoxic effects of fecal water in PhIP- treated rats (69).
In a study by Tavan et al. a mixture of three HCA- IQ, MeIQ and PhIP were given to male rats for a 7 week period with a cumulative dose of 250 mg of the HCA per kg body weight. The impact of four different diets including supplemented with 20% water, 30% non-fermented milk, 30% Bifidobacterium animalis DN-173010 fermented milk, 30% S. thermophilus DN-001 fermented milk on aberrant crypt foci (ACF) induction initiated by HCA compounds were determined. It was stated that consumption of milk, especially fermented milk significantly abolished the number of ACFs in rats. The inhibition degree was 66% in milk-supplemented diet, 96% with B. animalis fermented milk and 93% in S. thermophilus fermented milk. Additionally, HCA metabolism, fecal mutagenicity and colon DNA lesions were declined and there was no difference between two strains in terms of protective effect (70). In a related work by Duangjitcharoen et al. degrading of two HCA compound PhIP and IQ (50 µg/mL-1) by three strains of L. plantarum were determined. At the beginning of the test (0 minute), sudden binding of all strains to mutagen compounds was observed. The highest binding percentage to PhIP (46.32% after 24 hours) and IQ (85.34% after 144 hours) occurred in the strain L. plantarum CM4. It was deduced that this new strain could be utilized for human consumption as a protective agent against undesirable compounds in food and reactive metabolites in the gastrointestinal tract (71). Degradation of 16 different PAH compounds (0.25 μg of each compound /ml media) by three lactic acid bacteria (B. bifidium, S. thermophilus and L. bulgaricus) was studied in MRS medium during different incubation periods (2, 4, 6, 8, 10, 12, 24, 48 and 72 hours) at 37°C. Furthermore, yogurt by a mixture of buffalo and cow’s milk inoculated with yogurt starter (a mixture of S. thermophilus and L. bulgaricus) was prepared. 0.02 µg/mL of each PAH was added and degradation of PAH compounds was also determined during 3 hours. It was claimed that PAH decline was related to bacterial species and incubation period. During the incubation periods (2 - 72 hours), the reduction (%) relative to the initial concentration of PAHs (4 μg/mL) ranged from (46.6 to 92.9), (51.8 to 94.9) and (77.7 to 92.4), by B. bifidium, S. thermophilus and L. bulgaricus, respectively. It is noteworthy that the maximum reduction of PAHs by B. bifidium and S thermophilus was observed after incubation for 10 and 12 hours, and was found to be 92.6 and 96.0 %, respectively. However, the highest reduction by L. bulgaricus was recorded after 48 hours and was found to be 92.4%. At the end of incubation time (72 hours), the PAHs reducing capability was as follows: L. bulgaricus (91.5%), S. thermophilus (87.7%) and B. bifidium (46.6%). The results of PAHs biodegradation in yogurt showed an inconsiderable reduction within incubation periods (1, 2 and 3 hours) and the reduction percentage was 3.46 in the final product. It was assumed that the existence of PAHs depend on a number of factors such as the type of microorganism, the interaction between microorganisms, the microbial concentration, the composition of the medium, and the microbial growth conditions of temperature and pH (36).
The binding ability of lactic acid bacteria isolated from rice and wheat miso to eight different HCA was investigated. The experiment illustrated that all of the bacterial isolates could bind to Trp-P-1, Trp-P-2, MeAaC, and PhIP efficiently. Considering the mutagen type, it was expressed that except one isolate, others bounded to Trp-P-1 and Trp-P-2 more than 85%. In the case of MeAaC, the extent of binding was more than 90% whilst Glu-P-1, IQ, and MeIQ were bound relatively low. Hence, bacterial and mutagen types were the factors affecting the binding proportion (37). It was assumed that Van der Waals (hydrophobic) interactions were important factors in the binding of mutagens. It was stated that more hydrophobic compounds like AαC and DiMeIQx are bound more efficiently than IQ and PhIP. Furthermore, the tryptophan pyrolysates are more hydrophobic than the quinolines, quinoxalines and PhIP and are removed better than other HAA compounds (22). For subsequent studies, two isolates were selected which were distinguished as Pediococcus. acidilactici and named as P. acidilactici 1 and P. acidilactici 2. In the next step, cell wall fractions, heat-treated cells, and cytoplasmic contents were evaluated for their binding ability to HCA compounds. It was shown that except cytoplasmic content, pure cell wall and peptidoglycan fraction in both strains possessed more binding capability in comparison to bacterial cells. Heat treatment of lyophilized cells of both strains did not modify binding capacity and therefore, binding of the mutagens by cells is not the mechanism involved. Also, enzymic treatment with various enzymes had no impact on binding except a decrease in enzyme activity.
The authors inferred that binding activity of the cell walls of bacteria and cells as a whole were not influenced by the damage; therefore, extracellular substances or structures had no function in this procedure. When HCA compounds were acetylated, none of the two strains were able to bind the mutagens which were attributed to substitution of the amino group by the acetyl group and indicating the role of the amino group in the binding property. The proposed mechanism of binding activity was the reaction of peptidoglycan with amino group of mutagen compounds (37).
In a study by Zsivkovits et al. the effect of four Lactobacillus strains consisting of L. bulgaricus 291, S. thermophilus F4, S. thermophilus V3 and B. longum BB536 on DNA damage induced by HCAs, which are generally found in fried beef (beef mix) and chicken mix, in the liver and colon of female rats were examined. Lactic acid bacteria were either administered simultaneously or at different time intervals before giving HCA. It was indicated that all strains prevented damage caused by beef mix after giving of 1 × 1010 cells/animal while in the case of chicken mix, the effect was not significant. It was also found that the impact was considerable at 1 × 107 cells/animal and even when was given 12 hours before beef mix. Hence, consumption of probiotic dairy products several hours before cooked and fried meats would be beneficial considering the reduction of DNA damage (72). Terahara et al. surveyed absorption of Trp-P-1 and MeIQx by L. delbrueckii ssp. bulgaricus 2038 and S. thermophilus 1131 in distilled water, buffer solutions and intestine. They pointed out that the amount of bound Trp-P-1 and MeIQx in strain 2038 was 94.1% and 60.8% respectively, as well as 83.2% and 32.2% in strain 1131. In addition to these findings, it was specified that the absorption of the mutagens was pH dependent. The highest binding of strain 1131 to Trp-P-1 happened at the range of 4-8 but strain 2038 bounded to Trp-P-1 and MeIQx at pH 7. The results of HCA absorption in the small intestine of rats by loop test showed that strain 1131 was effective in the binding reduction of Trp-P-1than strain 2038. This was pertained to the similarity of the pH of absorption of Trp-P-1 by strain 1131 is similar and the small intestine (6, 7, 38). It was proved that incorporation of lyophilized cultures of B. longum BB536 (0.5%) in male and female rats diet during 56 weeks restrained colon, liver and mammary carcinogenesis induced by IQ (73). In a study potential binding ability of the goat probiotics (L. reuteri DDL 19, L. alimentarius DDL 48, Enterococcus faecium DDE 39 and B. bifidum DDBA) at cell concentrations of 1 × 106, 1 × 108 and 1 × 1011 cfu/mL against B[a] P and sodium azide was reported. A higher antimutagenecity (74%) was recognized in the mixture of goat probiotics than any individual strains at the same cell concentration. Also, the B[a] P- probiotic complex was stable after washing with DMSO (74).
3.3. Binding Ability of LABs and Probiotics to Acrylamide
In April 2002, the Swedish Food Administration found a remarkable amount of acrylamide in various heat treated carbohydrate-rich foods such as potato chips and crisps, coffee and bread (75) and thereafter it was classified as a probable human carcinogen by the International agency for research on cancer (76). Acrylamide is an electrophile molecule and thus can react to nucleophilic groups such as amines, carboxylates, and those that are commonly found in biological molecules such as DNA. Exposure to acrylamide causes DNA damage and at high doses, neurotoxic and reproductive effects have been observed while exposure to low, but prolonged doses, results in peripheral neuropathy with the presence or absence of central nervous system complications (76, 77). Because of the undesirable impacts of acrylamide on human health, many strategies have been investigated in order to alleviate the amount of acrylamide in foods. These approaches include reduction of precursors in raw materials (78-80), changing the process parameters such as temperature, pH and addition of amino acid and salts (81-84) and post processing approaches like chromatography, evaporation, polymerization (85-87). Recently, application of specific strains of lactic acid bacteria has been explored owing to their ability to reduce the acrylamide content in foods. Serrano-Nino et al. evaluated the potential ability of 14 lactic acid strains (L. casei Shirota (SHI), L. reuteri northern regional research laboratory (NRRL) 14171 (LR), L. johnsonii (ATCC) 3200 (JH), L. acidophilus ATCC 4796 (AC), L. fermentum ATCC 11976 (FER), L. rhamnosus ATCC 13075 (RHA), L. helveticus ATCC 27558 (HL), L. casei ATCC 334 (L334), L. casei L9 (L9), L. casei L30 (L30), L. casei 12A (12A), L. casei 21/1 (L21/1), L. casei 7R1 (7R1), L. casei (DPC) 3968) to remove acrylamide (5 and 10 µg/mL) in vitro after 0, 4 and 12 hours incubation at 37°C and different pH (3, 5 and 8). Stability of bacterial- acrylamide complex was also determined. It was implicated that the acrylamide binding abilities were pH, concentration, and strain dependent. Binding to acrylamide varied with respect to incubation time. At 0 hour, the amount of bound acrylamide was from 11.89 to 29.13%, depending on the strains and the maximum binding was observed in strains AC and SHI. It was proposed that binding was a rapid process and occurred passively on the bacterial surface (88). This is in accordance with the findings by Hernandez-Mendoza et al that observed aflatoxin B1 bound 15 to 58% at 0 hour (89). After 4 hours, L334 had the best binding ability (29.13%) and after 12 hours, the maximum binding was demonstrated by SHI and LR (24.95 and 24.01%, respectively). Generally, Strains JH, FER and L334 displayed the weakest ability to bind AA, whereas LR strain was the best one at any of the incubation period time. By increasing the time from 0 to 4 and 12 hours, the amount of AA bound by strains JH, SHI, L 30, 12A, DCP and 7R1 enhanced notably. For other strains including HL, RHA, L9, L334 and L21/1, the amount of acrylamide bound to bacteria after 12 hours was less compared to 0 and 4 h. By enhancement of acrylamide concentration from 5 to 10 µg/mL, the binding ability of strains was decreased considerably and in this case, LR showed the highest binding ability among the examined strains. The binding ability of strains was investigated at different pH levels (3, 5 and 8). The maximum binding at pH 8 was observed in the strain L334 while at pH 3, a substantial reduction in binding ability occurred (88). Similarly, Zhang et al. (45) reported a more binding of pyrolyzed mutagen at pH 6 - 7 as well as Hernandez-Mendoza et al. that implied, L. reuteri NRRL 14171 and L. casei Shirota bound more effectively at pH 7 than pH 8 (89). It was announced that the probable mechanism of pH influence on binding ability was due to competition between toxic compounds and protons to attach to the negatively charged binding sites (90). The Bacterial-toxin complex was not degraded after three washes with PBS solution which demonstrated that acrylamide binds to the strains irreversibly.
Finally, it was concluded that all the strains had the ability to bind acrylamide at different incubation periods and can be a new detoxification tool for improving the amount of acrylamide. In another attempt by Serrano-Niño et al, the interaction of acrylamide and aflatoxin B1 with teichoic acids (TA) in the cell wall of the aforementioned lactic acid bacteria was studied. TA was extracted from the cell wall and in order to analyze its components, it was subjected to acid hydrolysis. TA was composed of ribitol, glycerol, glucose, D-alanine and phosphate. The results of binding assay (at 0, 4 and 12 hours incubation at 37°C) revealed that binding of acrylamide was relevant to strain type and incubation time. The maximum binding at 0, 4 and 12 h was detected in the strain L.21/1 (> 15%), L334 (28%) and LR, SHI, 12A and DCP (about 50-65%), respectively. As it was illustrated before, the bacterial-toxin complex was stable and no detectable amount of acrylamide was liberated after three washes. The mechanism of physical binding of toxins to lactic acid bacteria was explicated. It was proposed that there was a relation between components of TA and percentage of bound acrylamide. The presence of the lower amount of glucose, D-alanine or teichoic acid caused more binding of acrylamide to the cell wall of bacteria. H-bonds may develop between carbonyl oxygen and the amino group between adjacent acrylamide and D-Alanine directly attached to position D-4 (L-2) of ribitol (88). Furthermore, the amine group of D-alanine might react with acrylamide units by means of a Michael addition reaction (91). Also, hydrogen bonds may occur between carbonyl (C = O) oxygens of both AFB1 and acrylamide, and the hydroxyl groups of either glucose residues or glycerol phosphate substituent attached to the poly (ribitol phosphate) chain.
3.4. Binding Ability of LABs and Probiotics to Nitrosamine
Concerns about the occurrence of N-nitroso compounds (NOCs) in foods is growing, since these compounds can induce tumor growth in human and IARC has classified a number of nitrosamines as probably (Group 2A) or possibly (Group 2B) carcinogenic to humans (92). They are formed by the interaction of secondary or tertiary amines with proper nitrosating species and their existence in food is a consequence of various processes during production, storage, cooking and in some circumstances through migration from packaging materials (93). The ways to mitigate nitrosamine formation in food include reducing nitrite level in curing salt, application of nitrite substituent, using ascorbic acid as an inhibitor agent, utilization of lower temperatures and indirect heating (94). There are also few studies concerning the inhibitory effects of probiotics which can bind to these compounds.
Antimutagenic activity of some lactic acid bacteria from fermented milk was assessed against N-nitroso-diethylamine (NDEA), N-nitroso-dimethylamine (NDMA), N-nitroso-piperidine (NPIP) and N-nitroso-pyrrolidine (NPYR). Among the tested bacteria, the highest inhibitory activity was observed in genus Leuconostoc. The strains consisting Streptococcus lactis ssp. diacetylactis R-63, Streptococcus cremoris R-48, and Leuconostoc paramesenteroides R-62 and R-8 depicted the most inhibitory activity versus the mutagenicity of NDEA. Therefore, these four strains were selected to evaluate their impact on mutagenecities of NDMA, NPYR, and NPIP. Mutagenecity of NDMA was partly inhibited by the lactic acid bacteria tested and in the case of NPYR and NPIP, these four strains were not so effective. The inhibitory effect of filtrates of cell suspension of lactic acid bacteria on NDEA mutagenicity was also investigated and a strong antimutagenic activity was observed (95). In a study by Grill et al, the influence of NDMA, NPIP and NPYR on growth of six bifidobacteria strain (B. breve ATCC 15698, B. infantis ATCC 25962, B. longum ATCC 15707, B. longum ATCC 15708, B. longum BB536 and B. animalis ATCC 25527) during 24 hours in TYP medium was studied. It was noted that in the concentration range of 2 - 200 μg/mL, the nitrosamines had no effect on the growth of bifidobacteria and only B. longum BB536 was able to metabolize nitrosamines. At the level of 2 μg/mL, 20% degradation for NPYR, 16% for NDMA and 10% for NPIP were detected. At 20 μg/mL, 0.5% - 1% decrease and in the case of 200 μg/mL no antimutagenic activity were observed. The inhibitory effect of bifidobacteria was attributed to an intracellular enzymic activity (96). Nowak et al studied binding and degrading ability of five probiotic Lactobacillus strains (L. rhamnosus LOCK 0900, L. rhamnosus LOCK 0908, L. casei LOCK0919, L. casei DN114001 and L. brevis 0945) versus N-nitrosodimethylamine (NDMA) under different culture conditions (24 hours in MRS, 168 hours in modified MRS N, and 168 hours in phosphate buffer). They also investigated the growth and survival of the strains during 24 hours in the presence of NDMA. It was stated that the highest growth was obtained for strain L. casei DN 114001 (1 × 1010 CFU/ mL) and the lowest for Lb. rhamnosus 0908 (1 × 109 CFU/mL). The morphology of the bacteria was not affected by NDMA even at high concentration of 100 μg/mL and NDMA at different levels was not toxic for lactobacilli. NDMA (2 - 100 μg/mL) did not influence the survival of the probiotic strains during 168-h incubation in phosphate buffer. In the case of decreasing the amount of NDMA, all strains had the capability of decreasing NDMA concentration in MRS from 2 µg/mL to 0.40 - 0.92 µg/mL after 24 hours cultivation while at the concentration of 20 µg/mL, the NDMA was reduced to 6 µg/mL by only two strains including L. rhamnosus 0908 and L. casei DN 114001 and at the initial level of 100 µg/mL no change was observed. The ability of bacteria to reduce NDMA amount in MRS N after 168 hours was weak and related to the growth phase and strain of bacteria. During logarithmic phase, decline of NDMA level was about 0.3 - 0.8 µg/mL that amplified in stationary phase to the initial level of 10 µg/mL and again in death phase, 0.6 - 0.9 µg/mL reduction was found in the case of L. rhamnosus 0900, L. casei DN 114001 and L. brevis 0945 whereas for L. rhamnosus 0908 and L. casei 0919, NDMA level remained constant. In phosphate buffer, L. rhamnosus 0900 lowered the NDMA level from 2 µg/mL to 1.45 µg/mL. At the concentration of 20 µg/mL, three strains were able to decrease NDMA, but at the level of 100 µg/mL none of the tested lactobacilli were capable of reducing the NDMA. The lower decrease in NDMA level in MRS N than MRS was attributed to lower numbers of bacteria and higher pH level. It was also announced that the most efficient strain in lowering the concentration and genotoxicity of NDMA was Lb. brevis 0945.
As a final note, the decrease of NDMA was dependent on the medium, incubation time, phase of growth, strain type, pH, and NDMA concentration (63). Degrading of two nitroso compounds, including diphenylnitrosamine (DPN) and 1-nitrosopyrrolidine (NPR) by three strains of L.plantarum was determined. Among the tested bacteria, L. plantarum CM4 which is a new probiotic of non-human origin strain demonstrated the utmost degrading ability of DPN (1 - 100 µg/mL) in a dose-response manner and the highest degradation activity was seen at the concentration of 100 µg/mL that yielded 11.10 µ/mol nitrite per mL during 20 hours of incubation time. The breakdown of NPR by all strains was slower than DPN and was not dose-response relationship activity (71).
4. Conclusions
This article reviewed the potential application of different lactic acid bacteria and probiotics in detoxification of various toxicants that are formed during food processing. Reports demonstrated that lactic acid bacteria and particularly probiotics can decline mutagenicity and genotoxicity of these toxicants remarkably by physical binding or enzymic degrading mechanisms. The efficacy of probiotic protective activity depends on several factors such as strain type, medium type, incubation time, pH, growth phase, chemical structure of mutagen, mutagen concentration, and probably existence of different binding sites on the cell wall of bacteria. Thereby, considering the findings in various studies, it can be concluded that probiotics can play a vital role in prevention of colon cancer that is induced by food toxicants. However, most of these studies have been carried out in vitro and further in vivo and clinical trials are still required to support the obtained results and specify the real effects of probiotic in human lumen and elucidate the underlying mechanisms.
Acknowledgements
References
-
1.
Zoghi A, Khosravi-Darani K, Sohrabvandi S. Surface binding of toxins and heavy metals by probiotics. Mini Rev Med Chem. 2014;14(1):84-98. [PubMed ID: 24329992].
-
2.
Arab M, Sohrabvandi S, Mortazavian AM, Mohammadi R, Tavirani M. Reduction of aflatoxin in fermented milks during production and storage. Toxin Rev. 2012;31(3-4):44-53.
-
3.
Topcu A, Bulat T. Removal of cadmium and lead from aqueous solution by Enterococcus faecium strains. J Food Sci. 2010;75(1):T13-7. [PubMed ID: 20492209]. https://doi.org/10.1111/j.1750-3841.2009.01429.x.
-
4.
Cho SS, Finocchiaro T. Handbook of prebiotics and probiotics ingredients: health benefits and food applications. CRC Press; 2009.
-
5.
Davoodi H, Esmaeili S, Mortazavian AM. Effects of milk and milk products consumption on cancer: a review. Comprehens Rev Food Sci Food Safety. 2013;12(3):249-64.
-
6.
Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann Oncol. 2005;16(3):481-8. [PubMed ID: 15718248]. https://doi.org/10.1093/annonc/mdi098.
-
7.
Walton GE, Gibson GR. Prebiotics and bowel cancer. Curr Topics Nutraceut Res. 2007;5(1):19.
-
8.
Gonzalez CA, Riboli E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur J Cancer. 2010;46(14):2555-62. [PubMed ID: 20843485]. https://doi.org/10.1016/j.ejca.2010.07.025.
-
9.
Chong ES. A potential role of probiotics in colorectal cancer prevention: review of possible mechanisms of action. World J Microbiol Biotechnol. 2014;30(2):351-74. [PubMed ID: 24068536]. https://doi.org/10.1007/s11274-013-1499-6.
-
10.
Marmot M, Atinmo T, Byers T, Chen J, Hirohata T, Jackson A, et al. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington: American Institute for Cancer Research; 2007.
-
11.
Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78(3 Suppl):559S-69S. [PubMed ID: 12936950].
-
12.
Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657-64. [PubMed ID: 16991129]. https://doi.org/10.1002/ijc.22170.
-
13.
Raman M, Ambalam P, Kondepudi KK, Pithva S, Kothari C, Patel AT, et al. Potential of probiotics, prebiotics and synbiotics for management of colorectal cancer. Gut Microbes. 2013;4(3):181-92. [PubMed ID: 23511582]. https://doi.org/10.4161/gmic.23919.
-
14.
Lee I, Tran M, Evans-Nguyen T, Stickle D, Kim S, Han J, et al. Detoxification of chlorella supplement on heterocyclic amines in Korean young adults. Environ Toxicol Pharmacol. 2015;39(1):441-6. [PubMed ID: 25590673]. https://doi.org/10.1016/j.etap.2014.11.015.
-
15.
Miller PE, Lazarus P, Lesko SM, Cross AJ, Sinha R, Laio J, et al. Meat-related compounds and colorectal cancer risk by anatomical subsite. Nutr Cancer. 2013;65(2):202-26. [PubMed ID: 23441608]. https://doi.org/10.1080/01635581.2013.756534.
-
16.
Knasmuller S, Steinkellner H, Hirschl AM, Rabot S, Nobis EC, Kassie F. Impact of bacteria in dairy products and of the intestinal microflora on the genotoxic and carcinogenic effects of heterocyclic aromatic amines. Mutat Res. 2001;480-481:129-38. [PubMed ID: 11506806].
-
17.
Geier MS, Butler RN, Howarth GS. Probiotics, prebiotics and synbiotics: a role in chemoprevention for colorectal cancer? Cancer Biol Ther. 2006;5(10):1265-9. [PubMed ID: 16969130].
-
18.
Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci. 2008;9(5):854-63. [PubMed ID: 19325789]. https://doi.org/10.3390/ijms9050854.
-
19.
Uccello M, Malaguarnera G, Basile F, D'Agata V, Malaguarnera M, Bertino G, et al. Potential role of probiotics on colorectal cancer prevention. BMC Surg. 2012;12 Suppl 1. S35. [PubMed ID: 23173670]. https://doi.org/10.1186/1471-2482-12-S1-S35.
-
20.
Desrouilleres K, Millette M, Vu KD, Touja R, Lacroix M. Cancer preventive effects of a specific probiotic fermented milk containing Lactobacillus acidophilus CL1285, L. casei LBC80R and L. rhamnosus CLR2 on male F344 rats treated with 1, 2-dimethylhydrazine. J Function Foods. 2015;17:816-27.
-
21.
Yu AQ, Li L. The Potential Role of Probiotics in Cancer Prevention and Treatment. Nutr Cancer. 2016;68(4):535-44. [PubMed ID: 27144297]. https://doi.org/10.1080/01635581.2016.1158300.
-
22.
Stidl R, Sontag G, Koller V, Knasmuller S. Binding of heterocyclic aromatic amines by lactic acid bacteria: results of a comprehensive screening trial. Mol Nutr Food Res. 2008;52(3):322-9. [PubMed ID: 18320573]. https://doi.org/10.1002/mnfr.200700034.
-
23.
Dominici L, Villarini M, Trotta F, Federici E, Cenci G, Moretti M. Protective effects of probiotic Lactobacillus rhamnosus IMC501 in mice treated with PhIP. J Microbiol Biotechnol. 2014;24(3):371-8. [PubMed ID: 24346468].
-
24.
Batish VK, Roy U, Lal R, Grover S. Antifungal attributes of lactic acid bacteria--a review. Crit Rev Biotechnol. 1997;17(3):209-25. [PubMed ID: 9306649]. https://doi.org/10.3109/07388559709146614.
-
25.
Masood MI, Qadir MI, Shirazi JH, Khan IU. Beneficial effects of lactic acid bacteria on human beings. Crit Rev Microbiol. 2011;37(1):91-8. [PubMed ID: 21162695]. https://doi.org/10.3109/1040841X.2010.536522.
-
26.
Bai JA. Beneficial Microbes in Fermented and Functional Foods. CRC Press; 2014.
-
27.
Carr FJ, Chill D, Maida N. The lactic acid bacteria: a literature survey. Crit Rev Microbiol. 2002;28(4):281-370. [PubMed ID: 12546196]. https://doi.org/10.1080/1040-840291046759.
-
28.
Shetty PH, Jespersen L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci Technol. 2006;17(2):48-55.
-
29.
Mohammadi R, Mortazavian AM. Review article: technological aspects of prebiotics in probiotic fermented milks. Food Rev Int. 2011;27(2):192-212.
-
30.
Sadaghdar Y, Mortazavian AM, Ehsani MR. Survival and activity of 5 probiotic lactobacilli strains in 2 types of flavored fermented milk. Food Sci Biotechnol. 2012;21(1):151-7.
-
31.
Hojati Z, Salehi Z, Motovali-Bashi M, Korbekandi H, Jami S. Molecular Analysis of the Clavulanic Acid Regulatory Gene Isolated from an Iranian Strain of Streptomyces Clavuligerus , PTCC 1709. Cell J. 2011;13(3):179-86. [PubMed ID: 23508694].
-
32.
Roberfroid MB. Prebiotics and probiotics: are they functional foods? Am J Clin Nutr. 2000;71(6 Suppl):1682S-7S. discussion 1688S-90S. [PubMed ID: 10837317].
-
33.
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, et al. Health benefits of probiotics: a review. ISRN Nutr. 2013;2013:481651. [PubMed ID: 24959545]. https://doi.org/10.5402/2013/481651.
-
34.
Butel MJ. Probiotics, gut microbiota and health. Med Mal Infect. 2014;44(1):1-8. [PubMed ID: 24290962]. https://doi.org/10.1016/j.medmal.2013.10.002.
-
35.
Kailasapathy K. Commercial sources of probiotic strains and their validated and potential health benefits-a review. Int J Fermented Foods. 2013;2(1):1.
-
36.
Abou-Arab AAK, Salim A, Maher RA, El-Hendawy HH, Awad AA. Degradation of polycyclic aromatic hydrocarbons as affected by some lactic acid bacteria. J Am Sci. 2010;6(10).
-
37.
Rajendran R, Ohta Y. Binding of heterocyclic amines by lactic acid bacteria from miso, a fermented Japanese food. Can J Microbiol. 1998;44(2):109-15. [PubMed ID: 9543712].
-
38.
Terahara M, Meguro S, Kaneko T. Effects of lactic acid bacteria on binding and absorption of mutagenic heterocyclic amines. Biosci Biotechnol Biochem. 1998;62(2):197-200. [PubMed ID: 9532774].
-
39.
Collins PD, Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev. 2006;(2). CD000279. [PubMed ID: 16625534]. https://doi.org/10.1002/14651858.CD000279.pub3.
-
40.
Rachmilewitz D, Katakura K, Karmeli F, Hayashi T, Reinus C, Rudensky B, et al. Toll-like receptor 9 signaling mediates the anti-inflammatory effects of probiotics in murine experimental colitis. Gastroenterology. 2004;126(2):520-8. [PubMed ID: 14762789].
-
41.
Bibiloni R, Fedorak RN, Tannock GW, Madsen KL, Gionchetti P, Campieri M, et al. VSL#3 probiotic-mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol. 2005;100(7):1539-46. [PubMed ID: 15984978]. https://doi.org/10.1111/j.1572-0241.2005.41794.x.
-
42.
Sreekumar O, Hosono A. Antimutagenicity and the influence of physical factors in binding Lactobacillus gasseri and Bifidobacterium longum cells to amino acid pyrolysates. J Dairy Sci. 1998;81(6):1508-16. [PubMed ID: 9684159]. https://doi.org/10.3168/jds.S0022-0302(98)75716-9.
-
43.
Sreekumar O, Hosono A. The heterocyclic amine binding receptors of Lactobacillus gasseri cells. Mutat Res. 1998;421(1):65-72. [PubMed ID: 9748508].
-
44.
Rhee CH, Park HD. Three glycoproteins with antimutagenic activity identified in Lactobacillus plantarum KLAB21. Appl Environ Microbiol. 2001;67(8):3445-9. [PubMed ID: 11472917]. https://doi.org/10.1128/AEM.67.8.3445-3449.2001.
-
45.
Zhang XB, Ohta Y. Binding of mutagens by fractions of the cell wall skeleton of lactic acid bacteria on mutagens. J Dairy Sci. 1991;74(5):1477-81. [PubMed ID: 1908865]. https://doi.org/10.3168/jds.S0022-0302(91)78306-9.
-
46.
Matar C, Nadathur SS, Bakalinsky AT, Goulet J. Antimutagenic effects of milk fermented by Lactobacillus helveticus L89 and a protease-deficient derivative. J Dairy Sci. 1997;80(9):1965-70. [PubMed ID: 9313136]. https://doi.org/10.3168/jds.S0022-0302(97)76139-3.
-
47.
Grajek W, Olejnik A, Sip A. Probiotics, prebiotics and antioxidants as functional foods. Acta Biochim Pol. 2005;52(3):665-71. [PubMed ID: 16086074].
-
48.
Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J Dairy Res. 2008;75(2):189-95. [PubMed ID: 18474136]. https://doi.org/10.1017/S0022029908003129.
-
49.
Wollowski I, Rechkemmer G, Pool-Zobel BL. Protective role of probiotics and prebiotics in colon cancer. Am J Clin Nutr. 2001;73(2 Suppl):451S-5S. [PubMed ID: 11157356].
-
50.
Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens -- focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591(1-2):74-92. [PubMed ID: 16083918]. https://doi.org/10.1016/j.mrfmmm.2005.04.020.
-
51.
Busquets R, Bordas M, Toribio F, Puignou L, Galceran MT. Occurrence of heterocyclic amines in several home-cooked meat dishes of the Spanish diet. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;802(1):79-86. [PubMed ID: 15035999]. https://doi.org/10.1016/j.jchromb.2003.09.033.
-
52.
Jägerstad M, Reuterswärd AL, Olsson R, Grivas S, Nyhammar T, Olsson K, et al. Creatin (in) e and Maillard reaction products as precursors of mutagenic compounds: Effects of various amino acids. Food Chem. 1983;12(4):255-64.
-
53.
Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95(4):290-9. [PubMed ID: 15072585].
-
54.
Food, nutrition and the prevention of cancer: a global perspective. American Institute for Cancer Research; 1997.
-
55.
Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins/this publication represents the views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, which met in Lyon. International Agency for Research on Cancer; 1992.
-
56.
Cheng KW, Chen F, Wang M. Heterocyclic amines: chemistry and health. Mol Nutr Food Res. 2006;50(12):1150-70. [PubMed ID: 17131456]. https://doi.org/10.1002/mnfr.200600086.
-
57.
Mottier P, Parisod V, Turesky RJ. Quantitative determination of polycyclic aromatic hydrocarbons in barbecued meat sausages by gas chromatography coupled to mass spectrometry. J Agric Food Chem. 2000;48(4):1160-6. [PubMed ID: 10775366].
-
58.
Wenzl T, Simon R, Anklam E, Kleiner J. Analytical methods for polycyclic aromatic hydrocarbons (PAHs) in food and the environment needed for new food legislation in the European Union. TrAC Trends Analytical Chem. 2006;25(7):716-25.
-
59.
Jira W, Pohlmann M, Hitzel A, Schwägele F. Smoked meat products-innovative strategies for reduction of polycyclic aromatic hydrocarbons by optimisation of the smoking process. Proceedings of International 57th Meat Industry Conference. Belgrade. 2013.
-
60.
Yebra-Pimentel I, Fernandez-Gonzalez R, Martinez-Carballo E, Simal-Gandara J. Optimization of purification processes to remove polycyclic aromatic hydrocarbons (PAHs) in polluted raw fish oils. Sci Total Environ. 2014;470-471:917-24. [PubMed ID: 24231673]. https://doi.org/10.1016/j.scitotenv.2013.10.061.
-
61.
Orrhage K, Sillerstrom E, Gustafsson JA, Nord CE, Rafter J. Binding of mutagenic heterocyclic amines by intestinal and lactic acid bacteria. Mutat Res. 1994;311(2):239-48. [PubMed ID: 7526189].
-
62.
Tsuda H, Hara K, Miyamoto T. Binding of mutagens to exopolysaccharide produced by Lactobacillus plantarum mutant strain 301102S. J Dairy Sci. 2008;91(8):2960-6. [PubMed ID: 18650272]. https://doi.org/10.3168/jds.2007-0538.
-
63.
Nowak A, Katarzyna S, Elzbieta K. Effect of probiotic lactobacilli on faecal enzyme and genotoxic activity in human faecal water in the presence of the carcinogen PhIP in vitro. Int J Dairy Technol. 2012;65(2):300-7.
-
64.
Faridnia F, Hussin AS, Saari N, Mustafa S, Yee LY, Manap MY. In vitro binding of mutagenic heterocyclic aromatic amines by Bifidobacterium pseudocatenulatum G4. Benef Microbes. 2010;1(2):149-54. [PubMed ID: 21831754]. https://doi.org/10.3920/BM2009.0035.
-
65.
Bolognani F, Rumney CJ, Rowland IR. Influence of carcinogen binding by lactic acid-producing bacteria on tissue distribution and in vivo mutagenicity of dietary carcinogens. Food Chem Toxicol. 1997;35(6):535-45. [PubMed ID: 9225011].
-
66.
Nowak A, Libudzisz Z. Ability of probiotic Lactobacillus casei DN 114001 to bind or/and metabolise heterocyclic aromatic amines in vitro. Eur J Nutr. 2009;48(7):419-27. [PubMed ID: 19448966]. https://doi.org/10.1007/s00394-009-0030-1.
-
67.
Nowak A, Arabski M, Libudzisz Z. Ability of intestinal lactic acid bacteria to bind and/or metabolise indole. Food Technol Biotechnol. 2008;46(3):299-304.
-
68.
Lidbeck A, Overvik E, Rafter J, Nord CE, Gustafsson JA. Effect of Lactobacillus acidophilus supplements on mutagen excretion in faeces and urine in humans. Microb Ecol Health Dis. 1992;5(1):59-67.
-
69.
Klewicka E, Nowak A, Zdunczyk Z, Juskiewicz J, Cukrowska B. Protective effect of lactofermented red beetroot juice against aberrant crypt foci formation, genotoxicity of fecal water and oxidative stress induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b] pyridine in rats model. Environ Toxicol Pharmacol. 2012;34(3):895-904. [PubMed ID: 22995401]. https://doi.org/10.1016/j.etap.2012.08.009.
-
70.
Tavan E, Cayuela C, Antoine JM, Trugnan G, Chaugier C, Cassand P. Effects of dairy products on heterocyclic aromatic amine-induced rat colon carcinogenesis. Carcinogenesis. 2002;23(3):477-83. [PubMed ID: 11895863].
-
71.
Duangjitcharoen Y, Kantachote D, Prasitpuripreecha C, Peerajan S, Chaiyasut C. Selection and characterization of probiotic lactic acid bacteria with heterocyclic amine binding and nitrosamine degradation properties. J Appl Pharm Sci. 2014;4(7):14.
-
72.
Zsivkovits M, Fekadu K, Sontag G, Nabinger U, Huber WW, Kundi M, et al. Prevention of heterocyclic amine-induced DNA damage in colon and liver of rats by different lactobacillus strains. Carcinogenesis. 2003;24(12):1913-8. [PubMed ID: 12970070]. https://doi.org/10.1093/carcin/bgg167.
-
73.
Reddy BS, Rivenson A. Inhibitory effect of Bifidobacterium longum on colon, mammary, and liver carcinogenesis induced by 2-amino-3-methylimidazo[4,5-f]quinoline, a food mutagen. Cancer Res. 1993;53(17):3914-8. [PubMed ID: 8358717].
-
74.
Apas AL, Gonzalez SN, Arena ME. Potential of goat probiotic to bind mutagens. Anaerobe. 2014;28:8-12. [PubMed ID: 24785349]. https://doi.org/10.1016/j.anaerobe.2014.04.004.
-
75.
Swedish National Food Administration. Information about acrylamide in food Uppsala. Sweden; 2002. Available from: http://192.71.90.8/engakrylanalysresultat.htm.
-
76.
Some industrial chemicals. IARC monographs on the evaluation of carcinogenic risks to humans. 1994.
-
77.
Garcia A, Alfaro M. Acrilamida en alimentos para consumo humano. Rev Sanid Milit Mex. 2007;61(6):384-8.
-
78.
Mestdagh F, Maertens J, Cucu T, Delporte K, Van Peteghem C, De Meulenaer B. Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chem. 2008;107(1):26-31.
-
79.
Pedreschi F, Kaack K, Granby K. Reduction of acrylamide formation in potato slices during frying. LWT-Food Sci Technol. 2004;37(6):679-85.
-
80.
Pedreschi F, Kaack K, Granby K. The effect of asparaginase on acrylamide formation in French fries. Food Chem. 2008;109(2):386-92. [PubMed ID: 26003362]. https://doi.org/10.1016/j.foodchem.2007.12.057.
-
81.
Ciesarova Z, Kiss E, Kolek E. Study of factors affecting acrylamide levels in model systems. Czech J Food Sci. 2006;24(3):133.
-
82.
Lindsay RC, Jang S. Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. Adv Exp Med Biol. 2005;561:393-404. [PubMed ID: 16438314]. https://doi.org/10.1007/0-387-24980-X_30.
-
83.
Lingnert H, Grivas S, Jägerstad M, Skog K, Törnqvist M, Åman P. Acrylamide in food: mechanism of formation and influencing factors during heating of foods. Scandinavian J Food Nutr. 2002;46(4):159-72.
-
84.
Rydberg P, Eriksson S, Tareke E, Karlsson P, Ehrenberg L, Tornqvist M. Investigations of factors that influence the acrylamide content of heated foodstuffs. J Agric Food Chem. 2003;51(24):7012-8. [PubMed ID: 14611163]. https://doi.org/10.1021/jf034649+.
-
85.
Banchero M, Pellegrino G, Manna L. Supercritical fluid extraction as a potential mitigation strategy for the reduction of acrylamide level in coffee. J Food Engin. 2013;115(3):292-7.
-
86.
Friedman M, Levin CE. Review of methods for the reduction of dietary content and toxicity of acrylamide. J Agric Food Chem. 2008;56(15):6113-40. [PubMed ID: 18624452]. https://doi.org/10.1021/jf0730486.
-
87.
Guenther H, Anklam E, Wenzl T, Stadler RH. Acrylamide in coffee: review of progress in analysis, formation and level reduction. Food Addit Contam. 2007;24 Suppl 1:60-70. [PubMed ID: 17687700]. https://doi.org/10.1080/02652030701243119.
-
88.
Serrano‐Nino JC, Cavazos‐Garduno A, Gonzalez‐Cordova AF, Vallejo‐Cordoba B, Hernández‐Mendoza A, Garcia HS. In vitro study of the potential protective role of Lactobacillus strains by acrylamide binding. J Food Safety. 2014;34(1):62-8.
-
89.
Hernandez-Mendoza A, Garcia HS, Steele JL. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem Toxicol. 2009;47(6):1064-8. [PubMed ID: 19425181].
-
90.
Huang C, Huang CP, Morehart AL. Proton competition in Cu (II) adsorption by fungal mycelia. Water Res. 1991;25(11):1365-75.
-
91.
Zamora R, Delgado RM, Hidalgo FJ. Model reactions of acrylamide with selected amino compounds. J Agric Food Chem. 2010;58(3):1708-13. [PubMed ID: 20078067]. https://doi.org/10.1021/jf903378x.
-
92.
ARC. Nitroso Compounds IARC. Monographs on the Evaluation of Risks to Humans. International Agency for Research on Cancer (IARC); 1978.
-
93.
Sen NP, Baddoo PA, Seaman SW. Nitrosamines in cured pork products packaged in elastic rubber nettings: An update. Food Chem. 1993;47(4):387-90.
-
94.
Habermeyer M, Eisenbrand G, Stadler RH, Lineback DR. N-nitrosamines, including N-nitrosoaminoacids and potential further nonvolatiles. Process-induced food toxicants: Occurrence, formation, mitigation, and health risks. John Wiley & Sons; 2009.
-
95.
Hosono A, Wardojo R, Otani H. Inhibitory effects of lactic acid bacteria from fermented milk on the mutagenicities of volatile nitrosamines. Agric Biol Chem. 1990;54(7):1639-43.
-
96.
Grill JP, Crociani J, Ballongue J. Effect of bifidobacteria on nitrites and nitrosamines. Lett Appl Microbiol. 1995;20(5):328-30. [PubMed ID: 7766234].
reply