Abstract
Background:
Use of complementary and alternative medicine (CAM) among cancer patients is not exactly defined.Objectives:
The aim of this study was to determine the use of CAM and associated factors among Iranian cancer patients, reasons behind this use, satisfaction and information about complementary and alternative therapy.Methods:
To assess CAM usage by adult cancer patients, a questionnaire was designed and distributed among the patients admitted in Amir Oncology hospital affiliated to the Shiraz University of Medical Sciences in Shiraz, South of Iran. The questionnaire consisted of 29 items including patients’ socio-demographic characteristics and questions about CAM use.Results:
A total number of 36 cancer patients participated in this study. The majority of respondents (94.4%) used or were using at least one CAM product or practice till the study date. Altogether, the most common type of CAM used among cancer patients was praying (86.1%). The most popular herbs in herbals subgroup were Mint and Garlic with 41.7%. Multivitamins and vitamin C were more favored than other vitamins and minerals (33.3%). No major side effects were reported regarding CAM use. The most reliable source of information about complementary medicine was reported to be physicians.Conclusions:
Iranian cancer patients frequently use CAM in addition to medical treatments. Therefore, it is important for oncologists to be aware of CAM use, its effects and adverse reactions to guide their patients.Keywords
1. Background
Complementary and alternative medicine (CAM) is defined as a group of medical practices, ingested therapies and other products that are not generally considered as an adjunct to conventional treatments. Many types of CAM, such as acupuncture, manipulative therapies, herbs and vitamins, homeopathic remedies and spiritual techniques have been recognized (1-4).
Cancer is one of the most common worldwide diseases that none of the conventional strategies (surgery, chemotherapy, and radiotherapy) can eliminate its cells completely. Furthermore, many serious side effects are often left using these routine therapies. Because conventional therapies cannot differentiate between cancer cells and healthy cells, they damage both types of cells. Therefore, many cancer patients seek a wide range of CAM. The most popular therapies seem to be dietary treatments, herbalism (5), homeopathy and hypnotherapy (6).
Recent reports from the United States and European countries showed that 91% and 35.9% of cancer patients were using at least one form of CAM, respectively (7, 8). A Japanese study reported 44.6% CAM use among cancer patients (9). According to Montazeri et al. study, 219 out of 625 Iranian cancer patients used CAM and indicated that apart from prayer and spiritual healing, the use of other common methods of CAM among Iranian cancer patients is unpopular (10).
Use of CAM has many reasons including symptom/side effect relief, strengthening the immune system of the body to fight the disease (11, 12), improving physical and/or emotional wellbeing and improving quality of life (11, 13). However, the use of alternative therapies by Iranian cancer patients and their satisfaction from using these remedies still needs to be elucidated.
Thus, the aims of this study were to determine the use of complementary and alternative therapy and associated factors among cancer patients, reasons behind this use, satisfaction and information about complementary and alternative therapy.
2. Methods
This study was a cross-sectional study among Iranian cancer patients who were hospitalized in Amir Oncology hospital which is a tertiary-care center affiliated to the Shiraz University of Medical Sciences in Shiraz, Southern Iran. The protocol of the study was approved by the ethics committee of the Shiraz University of Medical Sciences Grant number 92-01-32-6977. All patients with hematologic malignancies or solid tumors who were admitted to the Oncology ward were investigated during a 6-month period from Jan to Jun 2014. Patients who refused to participate in the study were excluded. During the study period, 350 patients were admitted in the oncology ward to get chemotherapy. Ninety-eight patients accepted to take part in the study. In order to assess the use of CAM by cancer patients, a standard questionnaire was distributed among the patients while a trained nurse explained it to them and took consent forms. The questionnaire consisted of 29 items including patients’ socio-demographic characteristics (Table 1) such as age, gender, general health status and annual household income, and patients’ clinical characteristics such as cancer type and cancer duration and questions about CAM use. Data were analyzed using SPSS 18 software.
| Values | Variables |
|---|---|
| Sex | |
| male/ female, No. | 17/ 19 |
| Age, y | |
| Mean ± SD (min - max) | 39.3 ± 13.2 (16 - 62) |
| General health status (excellent, very good and good vs. moderate and poor) | 23 (63.8) |
| Reasons for present visit | |
| regular follow up without treatment | 1 (2.8) |
| diagnostic assessments | 4 (11.1) |
| ongoing treatment | 26 (72.2) |
| others | 3 (8.3) |
| Annual household income, $/ month | |
| ≤ 300 | 30 (83.3) |
| > 300 | 4 (11.1) |
| Health insurance coverage | |
| Yes | 17 (47.2) |
| No | 8 (22.2) |
| Not sure | 9 (25.0) |
3. Results
A total number of 36 cancer patients completed the questionnaire and turn them back (response rate 36.7%). The characteristics of the participants who completed the forms are shown in Table 1. The majority of patients visited their physicians for their ongoing treatments. In total, 94.4% (n = 34) reported that they used or were using at least one CAM product or practice till the study date. The major reason of not using CAM was lack of knowledge about its effectiveness. The CAM used had three subtypes including minerals and vitamins, herbals and practices. Table 2 shows the rate of using these products, their common reasons of use and their perceived effectiveness. The reasons for using CAM among cancer patients were their physicians’ suggestions, strengthening the immune system and improving their overall health, relieving bone and body pain, decreasing gastrointestinal symptoms, getting relaxed and treating their concomitant anemia. The advice by treating physicians was the most common reason for using different types of CAM. The details of reasons are described in Table 2.
Products and Practices Used by Patients with Different Cancers, Their Common Reasons of Use and Perceived Effectivenessa
| Product | Ever Used/N | Most Common Reason of Use, Choice, Number/ Responders | Effective, Yes, N/ % |
|---|---|---|---|
| Minerals and Vitamins | |||
| Multivitamin | 12 | (2) 4/8 | 5/ 41.6 |
| (1) 4/8 | |||
| Folic acid | 7 | (4) 3/5 | 3/ 42.8 |
| (1) 2/5 | |||
| Vitamin C | 12 | (2) 3/7 | 6/ 85.7 |
| (1) 4/7 | |||
| Vitamin B | 5 | (2) 1/3 | 3/ 60.0 |
| (1) 2/3 | |||
| Calcium | 9 | (3) 2/6 | 4/ 44.4 |
| (1) 4/6 | |||
| Zinc | 5 | (3) 1/2 | 2/ 40.0 |
| (1) 1/2 | |||
| Herbals | |||
| Mint | 15 | (6) 6/7 | 5/ 33.3 |
| Chamomile | 10 | (5) 2/5 | 4/ 40.0 |
| (1) 2/5 | |||
| Leek | 2 | (4) 1/2 | 2/ 100.0 |
| (1) 1/2 | |||
| Garlic | 15 | (3) 2/5 | 2/ 13.3 |
| Miscellaneous | |||
| Jinseng | 1 | - | - |
| Homeopathy | 1 | (1) 1/1 | - |
| Probiotic | 3 | - | - |
| Fish oil | 6 | (3) 1/2 | 1/ 16.6 |
| (2) 1/2 | |||
| Others 1 | 8 | (1) 3/3 | - |
| (4) 1/2 | |||
| (2) 1/2 | |||
| Practice | |||
| Acupuncture | 2 | (3) 1/1 | - |
| Massage | 2 | - | - |
| Energy healing | 1 | - | - |
| Praying | 31 | (3) 7/16 | 15/48.3 |
| Others 2 | 1 | - | - |
Altogether, the most common type of CAM used among cancer patients was praying (86.1%). In detail, the most popular herbs in herbals subgroup were Mint and Garlic with 41.7%. Multivitamins and vitamin C were more favored in patients than other vitamins and minerals (33.3%). Figure 1 shows the different types of CAM used by cancer patients.
Types of CAM Used Among the Study Participants (%)
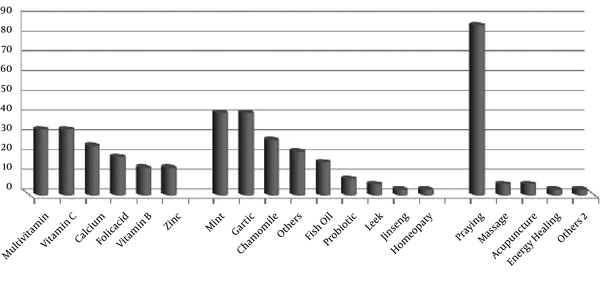
More than 22% of cancer patients used CAM along with their routine medical treatments mostly under medical supervision. No major side effects were reported regarding CAM use. A few minor adverse reactions were noticed in those using vitamin C, vitamin B, calcium and chamomile with no need to seek medical care.
Among different sources of information, the most trusted source which was scored on a 10-point scale (1 = no trust, 10 = full trust) was reported to be physicians (mean: 9.50, 95% CI: 7.91 - 11.09) (Table 3).
Distribution of Number, Mean, Standard Deviation and 95% Confidence Interval of Trust of Patients to Different Sources of Information About Complementary Medicine
| Source of Information | Na | Mean | Standard Deviation | 95% CI |
|---|---|---|---|---|
| Physician | 18 | 9.50 | 1.00 | 7.91 - 11.09 |
| Complementary medicine clinic | 15 | 4.50 | 4.35 | -2.44 - 11.44 |
| Drugstore | 14 | 5.50 | 3.41 | 0.06 - 10.94 |
| Grocery | 13 | 4.25 | 2.50 | 0.27 - 8.23 |
| Internet | 12 | 3.75 | 4.27 | -3.05 - 10.55 |
| Television | 12 | 6.25 | 2.06 | 2.97 - 9.53 |
| Book and magazine | 11 | 7.75 | 1.50 | 5.36 - 10.14 |
| Friends and families | 10 | 7.50 | 2.08 | 4.19 - 10.81 |
| Others | 10 | 8.25 | 2.87 | 3.68 - 12.82 |
4. Discussion
Our data indicate a high prevalence of CAM usage (94.4%) among cancer patients (10, 14-16). Considering plenty of previous studies, the prevalence of CAM use is usually more than 40%. The frequency of CAM use in this study was higher than that reported in the U.S. (53.7%), Australia and Malaysia (64%), Japan (44.6%), and Turkey (57%) (17-19). The socio-demographic characteristics of the participants used CAM in this study were somehow comparable to similar previous studies with female subjects more inclined to using CAM therapies (10, 14, 20-22). However, the annual household incomes of our participants were low (15, 16, 23, 24). It seems that health insurance programs in the country increasingly cover CAM services and providers, much like the case in other developed nations (25, 26).
Although our patients used CAM for a variety of reasons, they mostly agreed to use these therapies as their responsible physicians recommended to do so. It seems that our adult oncologists are familiar with this growing field of medicine and believe in their efficacy as an adjunct therapy.
It has been shown that prayer is the most common CAM therapy being used in the general population of the United State (27) and one of the most frequently reported by oncology patients (28-30). Wells et al. reported that 34.9% of cancer patients used prayer for resuming their health and it was the most commonly used CAM (31). Spiritual healing in Montazeri et al. study was the most popular CAM modality used by almost 80% of Iranian cancer patients (10). Similarly, in our study, prayer was reported as the most common CAM therapy employed by CAM users in cancer patients.
Lengacher et al. found that prayer, massage, herbal products, and meditation were the CAM therapies rated as being the most effective in women with breast cancer (28). In our study, praying, herbal products (Mint and Garlic), multivitamins and vitamin C were the most preferable CAM.
Our study faced some limitations. A potential bias is that our patients were selected from a referral and tertiary hospital. Moreover, our sample size was too small to generalize our results to all Iranian cancer patients. Despite these limitations, the frequency of CAM usage in our study was comparable to other regional and global studies.
In conclusion, we determined the most frequent types of CAM in Iranian patients with cancer as well as reasons behind this use, satisfaction and information about complementary and alternative therapy. Based on our results CAM is increasingly being used in cancer patients. Due to potential side effects and interactions these products may have with the mainstream treatments, it is crucial for oncologists to be aware of CAM use among their patients. Since the patients mostly trust their physicians, it is a good opportunity to guide and encourage them to use appropriate CAM while monitoring the expected adverse reactions.
Acknowledgements
References
-
1.
Akbari ME. Cancer screening, effective or harmful? Iran J Cancer Prev. 2016;In Press(In Press). https://doi.org/10.17795/ijcp-6633.
-
2.
Khodabandehloo H, Zahednasab H, Ashrafi Hafez A. Nanocarriers usage for drug delivery in cancer therapy. Iran J Cancer Prev. 2016;In Press(In Press). https://doi.org/10.17795/ijcp-3966.
-
3.
Davis MP, Darden PM. Use of complementary and alternative medicine by children in the United States. Arch Pediatr Adolesc Med. 2003;157(4):393-6. [PubMed ID: 12695237]. https://doi.org/10.1001/archpedi.157.4.393.
-
4.
Boon H, Stewart M, Kennard MA, Gray R, Sawka C, Brown JB, et al. Use of complementary/alternative medicine by breast cancer survivors in Ontario: prevalence and perceptions. J Clin Oncol. 2000;18(13):2515-21. [PubMed ID: 10893281]. https://doi.org/10.1200/JCO.2000.18.13.2515.
-
5.
Hashempur MH, Heydari M, Mosavat SH, Heydari ST, Shams M. Complementary and alternative medicine use in Iranian patients with diabetes mellitus. J Integr Med. 2015;13(5):319-25. [PubMed ID: 26343103]. https://doi.org/10.1016/S2095-4964(15)60196-0.
-
6.
Burstein HJ, Gelber S, Guadagnoli E, Weeks JC. Use of alternative medicine by women with early-stage breast cancer. N Engl J Med. 1999;340(22):1733-9. [PubMed ID: 10352166]. https://doi.org/10.1056/NEJM199906033402206.
-
7.
Molassiotis A, Fernadez-Ortega P, Pud D, Ozden G, Scott JA, Panteli V, et al. Use of complementary and alternative medicine in cancer patients: a European survey. Ann Oncol. 2005;16(4):655-63. [PubMed ID: 15699021]. https://doi.org/10.1093/annonc/mdi110.
-
8.
Yates JS, Mustian KM, Morrow GR, Gillies LJ, Padmanaban D, Atkins JN, et al. Prevalence of complementary and alternative medicine use in cancer patients during treatment. Support Care Cancer. 2005;13(10):806-11. [PubMed ID: 15711946]. https://doi.org/10.1007/s00520-004-0770-7.
-
9.
Hyodo I, Amano N, Eguchi K, Narabayashi M, Imanishi J, Hirai M, et al. Nationwide survey on complementary and alternative medicine in cancer patients in Japan. J Clin Oncol. 2005;23(12):2645-54. [PubMed ID: 15728227]. https://doi.org/10.1200/JCO.2005.04.126.
-
10.
Montazeri A, Sajadian A, Ebrahimi M, Haghighat S, Harirchi I. Factors predicting the use of complementary and alternative therapies among cancer patients in Iran. Eur J Cancer Care (Engl). 2007;16(2):144-9. [PubMed ID: 17371423]. https://doi.org/10.1111/j.1365-2354.2006.00722.x.
-
11.
Molassiotis A, Scott JA, Kearney N, Pud D, Magri M, Selvekerova S, et al. Complementary and alternative medicine use in breast cancer patients in Europe. Support Care Cancer. 2006;14(3):260-7. [PubMed ID: 16143871]. https://doi.org/10.1007/s00520-005-0883-7.
-
12.
Chen Z, Gu K, Zheng Y, Zheng W, Lu W, Shu XO. The use of complementary and alternative medicine among Chinese women with breast cancer. J Altern Complement Med. 2008;14(8):1049-55. [PubMed ID: 18928393]. https://doi.org/10.1089/acm.2008.0039.
-
13.
Lengacher CA, Bennett MP, Kip KE, Gonzalez L, Jacobsen P, Cox CE. Relief of symptoms, side effects, and psychological distress through use of complementary and alternative medicine in women with breast cancer. Oncol Nurs Forum. 2006;33(1):97-104. [PubMed ID: 16470237]. https://doi.org/10.1188/06.ONF.97-104.
-
14.
Harris P, Finlay IG, Cook A, Thomas KJ, Hood K. Complementary and alternative medicine use by patients with cancer in Wales: a cross sectional survey. Complement Ther Med. 2003;11(4):249-53. [PubMed ID: 15022659]. https://doi.org/10.1016/S0965-2299(03)00126-2.
-
15.
Swisher EM, Cohn DE, Goff BA, Parham J, Herzog TJ, Rader JS, et al. Use of complementary and alternative medicine among women with gynecologic cancers. Gynecol Oncol. 2002;84(3):363-7. [PubMed ID: 11855870]. https://doi.org/10.1006/gyno.2001.6515.
-
16.
Spadacio C, Barros NF. [Use of complementary and alternative medicine by cancer patients: systematic review]. Rev Saude Publica. 2008;42(1):158-64. [PubMed ID: 18200356].
-
17.
Tilden VP, Drach LL, Tolle SW. Complementary and alternative therapy use at end-of-life in community settings. J Altern Complement Med. 2004;10(5):811-7. [PubMed ID: 15650470]. https://doi.org/10.1089/acm.2004.10.811.
-
18.
Alferi SM, Antoni MH, Ironson G, Kilbourn KM, Carver CS. Factors predicting the use of complementary therapies in a multi-ethnic sample of early-stage breast cancer patients. J Am Med Womens Assoc (1972). 2001;56(3):120-3. 126. [PubMed ID: 11506149].
-
19.
Shaharudin SH, Sulaiman S, Emran NA, Shahril MR, Hussain SN. The use of complementary and alternative medicine among Malay breast cancer survivors. Altern Ther Health Med. 2011;17(1):50-6. [PubMed ID: 21614944].
-
20.
Chrystal K, Allan S, Forgeson G, Isaacs R. The use of complementary/alternative medicine by cancer patients in a New Zealand regional cancer treatment centre. N Z Med J. 2003;116(1168):U296. [PubMed ID: 12601420].
-
21.
Downer SM, Cody MM, McCluskey P, Wilson PD, Arnott SJ, Lister TA, et al. Pursuit and practice of complementary therapies by cancer patients receiving conventional treatment. BMJ. 1994;309(6947):86-9. [PubMed ID: 8038672]. https://doi.org/10.1136/bmj.309.6947.86.
-
22.
Rakovitch E, Pignol JP, Chartier C, Ezer M, Verma S, Dranitsaris G, et al. Complementary and alternative medicine use is associated with an increased perception of breast cancer risk and death. Breast Cancer Res Treat. 2005;90(2):139-48. [PubMed ID: 15803360]. https://doi.org/10.1007/s10549-004-3779-1.
-
23.
Lee MM, Lin SS, Wrensch MR, Adler SR, Eisenberg D. Alternative therapies used by women with breast cancer in four ethnic populations. J Natl Cancer Inst. 2000;92(1):42-7. [PubMed ID: 10620632].
-
24.
Paltiel O, Avitzour M, Peretz T, Cherny N, Kaduri L, Pfeffer RM, et al. Determinants of the use of complementary therapies by patients with cancer. J Clin Oncol. 2001;19(9):2439-48. [PubMed ID: 11331323]. https://doi.org/10.1200/JCO.2001.19.9.2439.
-
25.
Cassileth BR. Evaluating complementary and alternative therapies for cancer patients. CA Cancer J Clin. 1999;49(6):362-75. [PubMed ID: 11198952]. https://doi.org/10.3322/canjclin.49.6.362.
-
26.
Kilgore C. Perspectives. Expanding coverage signals growing demand, acceptance for alternative care. Med Health. 1998;52(18):suppl 1-4. [PubMed ID: 10179407].
-
27.
Barnes PM, Powell-Griner E, McFann K, Nahin RL. Complementary and alternative medicine use among adults: United States, 2002. Adv Data. 2004;(343):1-19. [PubMed ID: 15188733]. https://doi.org/10.1016/j.sigm.2004.07.003.
-
28.
Lengacher CA, Bennett MP, Kip KE, Keller R, LaVance MS, Smith LS, et al. Frequency of use of complementary and alternative medicine in women with breast cancer. Oncol Nurs Forum. 2002;29(10):1445-52. [PubMed ID: 12432415]. https://doi.org/10.1188/02.ONF.1445-1452.
-
29.
Von Gruenigen VE, White LJ, Kirven MS, Showalter AL, Hopkins MP, Jenison EL. A comparison of complementary and alternative medicine use by gynecology and gynecologic oncology patients. Int J Gynecol Cancer. 2001;11(3):205-9. [PubMed ID: 11437926]. https://doi.org/10.1046/j.1525-1438.2001.01011.x.
-
30.
Richardson MA, Sanders T, Palmer JL, Greisinger A, Singletary SE. Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology. J Clin Oncol. 2000;18(13):2505-14. [PubMed ID: 10893280]. https://doi.org/10.1200/JCO.2000.18.13.2505.
-
31.
Wells M, Sarna L, Cooley ME, Brown JK, Chernecky C, Williams RD, et al. Use of complementary and alternative medicine therapies to control symptoms in women living with lung cancer. Cancer Nurs. 2007;30(1):45-55. quiz 56-7. [PubMed ID: 17235219]. https://doi.org/10.1097/00002820-200701000-00008.