Abstract
Background:
A prospective study was conducted to investigate the response rate of patients newly diagnosed with acute myeloid leukemia (AML) to modified intermediate-dose cytarabine with daunorubicin.Methods:
A total of 45 patients received cytarabine at a modified intermediate-dose (115 mg/m2) given by continuous intravenous infusion for 12 hours twice daily over 7 days and daunorubicin 45 mg /m2 given on days 1, 2, and 3 of induction therapy. Patients with a complete response received reinduction with cytarabine at the same dose and infusion over 5 days with 2 doses of daunorubicin. After remission, patients who were socioculturoeconomically eligible for transplantation were evaluated for other prognostic factors, except for cytogenetic factors that were not available in the study center, to identify patients that were eligible for stem cell transplantation.Results:
Patients were 17 to 60 years of age. 6 patients had early death due to complications and treatment failure. 39 patients (87%) achieved complete remission. Only 16 patients were eligible for transplantation on evaluation and underwent allogeneic stem-cell transplantation. 18 patients were not eligible for this transplantation and underwent consolidation therapy with chemotherapy. 5 patients did not receive any treatment and died during the follow up. In the follow up period between April 2006 and January 2014 in 39 out of 45 patients (min 0.2 yr, max 7. 8 yr) 31 % of patients were alive.Conclusions:
Modified intermediate dose cytarabine was effective for the treatment of AML, achieving a high rate of complete remission, and might improve outcomes in patients.Keywords
1. Introduction
The incidence of acute myeloid leukemia (AML) is 3.6 per 100,000 persons per year, with a median age at diagnosis of 66 years (1, 2). An estimated 18,860 and 10,460 new cases and deaths, respectively, were attributed to AML in the United States in 2014 (3). Conventional treatment in AML includes induction chemotherapy followed by post remission therapy. Post remission therapy appears to be effective when given immediately after remission is achieved (4). Post remission therapy includes cytarabine-based regimens similar to the standard induction or high-dose chemotherapy with autologous bone marrow transplantation, and myelo -ablative therapy with allogeneic stem cell transplantation (Allo-SCT) (4-6). The main drug for AML treatment is cytarabine arabinoside (Ara-C), in which three protocol types are used: conventional or standard dose, intermediate dose, and high dose Ara-C (HDAC). The most commonly used induction regimens for AML are the so-called “7 + 3” regimens, which combine a seven-day continuous intravenous infusion of cytarabine (100 mg/ m2 per day) with a short infusion or bolus of an anthracycline [Daunorubicin (DNR) 45 mg/m2 per day] given on days one through three (7) although high-dose cytarabine arabinoside is now being used for induction therapy (8, 9) or consolidation therapy (10, 11). HDAC has not conventionally been used for remission induction, (12) but HDAC alone could be considered in patients that are not candidates for anthracyclines during induction (7). Use of modified intermediate-dose cytarabine arabinoside (about one-third of the total high doses) could result in maximal antitumor effects with less toxicity. The aim of the present study was to evaluate the effect and response rate of modified intermediate dose cytarabine arabinoside in acute leukemia. A second cycle of induction chemotherapy was routinely applied without delay after the first cycle of induction chemotherapy if bone marrow sampling after the fifteenth-day of treatment indicated complete remission (13, 14).
2. Methods
Between April 2006 and January 2014 at Talaghani and Imam Reza hospital, University of Medical Sciences Kermanshah, Iran, patients 15 to 60 years of age with de novo AML were registered in a cohort study. Eligibility criteria included: a diagnosis of de novo AML (except for cases of acute promyelocytic leukemia), ages between15 and 60 years. Patients with a prior diagnosis of myelodysplasia or refractory anemia or those who received prior chemotherapy were not eligible; in addition, patients with irreversible major organ failure, significant hepatic or renal dysfunction, and a left ventricular ejection fraction less than 45% - 50% were ineligible. The cohort study was approved by regional ethical committee at Kermanshah University of Medical Sciences (Ref.NO:IR.KUMS.REC.1394.300). Diagnosis was morphologically confirmed according to the French- American-British classification. Cytogenetic evaluation was not performed in the primary center due to costs. Patients were assigned to induction treatment with intermediate-dose that consisted Ara-C 115 mg/m2 given as a continuous infusion 12 hours twice daily on days 1 to 7, with the addition of bolus DNR at 45 mg/m2/d on days 1 to 3. Post induction bone marrow samples were obtained on day 15. If patients were in complete remission, the second course of induction was started without delay with intermediate-dose Ara-C 115 mg/m2 given as a continuous infusion 12 hours twice daily on days1 to 5 with the addition of bolus DNR at 45 mg/m2/d on days 1 and 2. After remission, patients with good socioeconomic and cultural conditions were selected for transplant evaluation. If these patients were eligible for allogenic stem cell transplantation because of stem cell transplantation criteria, they underwent SCT and if not eligible for SCT, due to lack of financial support, they underwent consolidation with etoposide, cytosar, and mitoxantron for 4 days monthly over twelve months. Many of these patients did not receive any further treatment. When consolidation treatment was completed, patients were monitored at 3-month intervals for a minimum of 5 years. Patient survival was measured from the day of the respective registration to death from any cause. During hospitalization of patients, febrile neutropenia was evaluated and treatment for fever and neutropenia was performed. All patients required packed cell and platelet transfusions. We used the following formula for the calculation of sample size based on survival rate from previous studies:
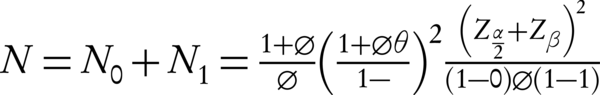
In this formula following items are defined and we considered alpha as 0.05 and beta as 0.2.
N0: required number of individuals in the control group.
N1: required number of individuals in the risk/intervention group.
n0: actual number of individuals in the control group.
n1: actual number of individuals in the risk/intervention group.
ϕ = n1/n2: the ratio of number of individuals in the risk/intervention group to number of control group.
ρ0: survival rate or efficacy of control group.
ρ1: survival rate or efficacy of risk/intervention group.
We used Kaplan-Meier estimator to estimate survival function for time to death. To compare survival curves and distributions between patients who received SCT with those who did not receive SCT, we used Log-rank test.
3. Results
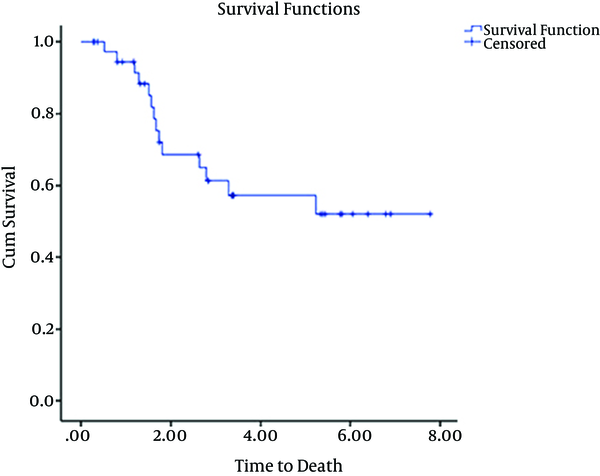
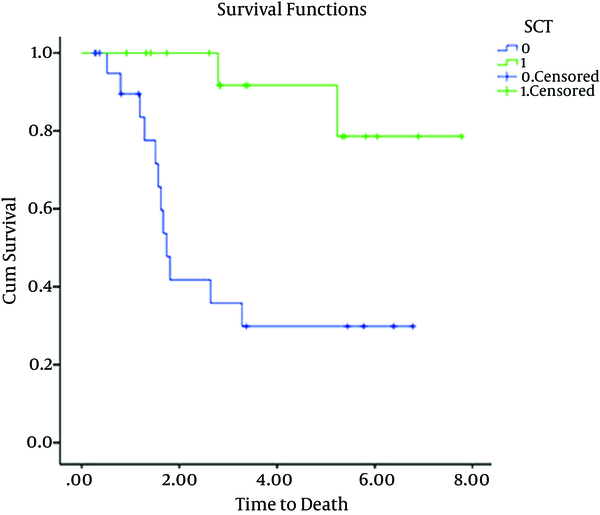
Of the 45 patients included in the study, 27 were male and 18 were female. Characteristics of these 45 patients are summarized in Tables 1 and 2. The median age was 32 years (range, 15 - 60 years). Early mortality occurred in 6 (13%) out of 45 patients. Five out 6 patients died due to complications of treatment or failure of treatment and one patient died from influenza infection during pancytopenia that developed after treatment was initiated. 39 (86%) of 45 patients achieved CR. These patients were then evaluated and underwent consolidation therapy. 16 out of 39 patients underwent SCT on the basis of good socio-economic status and high risk factors for relapse although cytogenetics was not evaluated. 18 patients were not eligible for evaluation of SCT because they were of low socioeconomic status. These patients then underwent consolidation with a chemotherapy regimen “etoposide100 mg/m2 day 1 - 3, mitoxantron 15 mg/m2 day1 and cytarabine 100 mg SQ day1 - 4”. Five patients were not selected to receive any type of consolidation treatment. At follow-up of all patients (45 patients) performed between April 2006 and January 2014, 12 of 45 (26%) patients were alive. The mean follow up time for 39 out of 45 patients was 2.9 yr (min 0.26, max 7.7 yr) and 23 of 39 patients died. This left 12 (31%) patients alive at the follow up. The results of 39 patients are as follows: Mean follow up of 5 patients that were not selected for consolidation therapy after double induction was 0.8 yr (min 0.2 yr max 1.6 yr). 100 percent of 5 patients without consolidation therapy after recurrence died. 8 of 16 patients with SCT died. 3 of 8 patients died due to GVHD and infection and 5 patients died due to relapse. The mean time taken to perform SCT was 0.6 yr (min 0.3 yr, max 1.5 yr). 12 of 18 patients with consolidation therapy composed of chemotherapy died after recurrence in follow up. Mean overall survival of 45 patients over 5 yr is shown in Figure 1. Mean overall survival time for patients undergoing SCT was 7yr. Mean and median overall survival time for patients who were not eligible for SCT was 3yr and 1.7 yr, respectively, as shown in Figure 2. (P values lower than 0.05 considered as significant).
| Characteristics | Values |
|---|---|
| Median age, y (range) | 32 (15 - 60) |
| < 50 | 34 |
| > 50 | 16 |
| Median WBC count, × 109/L (range) | 21.8 |
| > 30 × 109 | 31 (68.9) |
| 10 - 20 × 109 | 15 (28.9) |
| < 10 × 109 | 4 (2.2) |
| FAB type | |
| M0 | 8.8 |
| M1 | 17.6 |
| M2 | 30.8 |
| M4 | 22 |
| M5 | 15.4 |
| M6 | 2.2 |
| M7 | 2.2 |
| Early Mortality, patients | 6 |
| Male | 27 |
| Female | 18 |
Patient Characteristics
| Consolidation Allogenic SCT Group (Number Patients) | Consolidation Chemotherapy Group (Number Patients) | No Consolidation (Number Patients | |
|---|---|---|---|
| Number | 16 | 18 | 5 |
| Alive | 8 | 4 | 0 |
| Death | 8 | 14 | 5 |
Means Overall Survival for 45 Patients Underwent Induction Chemotherapy
Overall Survival Time Consolidation Treatment for Patient Underwent SCT (Green) and for Patient Group B Not Eligible for SC (Blue)
4. Discussion
The use of high-dose Ara-C therapy (15) first instituted 2 to 3 decades ago has been re-assessed.
High-dose cytarabine has been shown to be more effective than the low conventional dose in AML, (16) but is associated with increased toxicity and does not appear to improve outcomes when compared with the standard dose (17-21). Multicenter studies have compared intermediate-dose cytarabine with high dose cytarabine during induction therapy. The results of these comparisons suggest that the anti-leukemic effects of cytarabine might reach a maximum at intermediate doses and are well tolerated (16). In the present study, double induction chemotherapy was instituted. A higher full remission rate was achieved (87%) compared to some studies, such as Pagano et al. (22) (54%) and Lowenberg et al. (23) (58%) that used the conventional dose of cytarabine induction chemotherapy. These two studies also investigated other doses, but in the present study comparison was only made with patients that received conventional or standard dose. Moreover, in Lowenberg study, the initial mortality rate was 12%, compared to 12.5% in the present study. In a study by Wiernik et al. (24) (published in Blood in 1992) 59% of patients achieved full remission. Toxicity of cytarabine at modified intermediate dose has an insignificant difference with its toxicity at conventional or standard dose (used in studies such as Wiernik PH, 1992). In Wiernik PH study (24), nausea (mainly grades 1 and 2) occurred in 82% of patients and vomiting in 66%, compared to less severe nausea and vomiting in the present study, which may be attributed to the use of 4 anti-emetic regimen (dexamethasone, cimetidine, Kytril, and metoclopramide). As a result, increased dose of cytarabine as modified Intermediate Dose has no significant effect on nausea and vomiting. Mild mucositis occurred in 63%, esophagitis in 13%, and diarrhea in 78% of patients, and these complications were also mild in the present study. Mild bleeding occurred in 56% of patients and grade 3 and 4 alopecia in 37%. In Wiernik PH study, skin rash was not common, and it was mild when occurred. In the present study, skin rash occurred in 7 patients with greater intensity and quantity. In both Wiernik PH study and the present study, fever occurred in all patients. Given the higher dose used in the present study, the risk of grades 3 and 4 infection was not higher compared to studies that used conventional or standard dose. In a study by Bishop et al. (19) (1996), published in the Blood Journal, full remission was achieved in 74% of a group of 159 patients whose conventional or standard dose was consolidated with 7 days of etoposide. Interestingly, of 159 patients, in 112 patients, 93 (61%) received one course of induction, 54 (36%) two courses, and 5 (3%) three courses to go into remission, compared to our patients receiving only one course of induction and 87% of patients go to remission.
In James F Bishop study, duration of relapse-free survival was 12 months, and 80% of patients relapsed. In their study, 1 cases of bleeding, 1 case of respiratory failure, 1 of hypoxic brain damage, 1 renal failure and cardiovascular collapse occurred, and 11% of patients died in the course of induction. In the present study, in terms of grades 3 and 4 neurological toxicity, only 1 case of brain hemorrhage was observed, and fewer complications occurred, despite higher treatment dose. Also, toxicity of drugs used in therapy was assessed in patients: Grades 1 and 2, nausea and vomiting were found in 50% of patients. Grades 3 and 4 vomiting did not occur in any patients. Mucositis and esophagitis were observed in 55% of patients, which were resolved by mouthwash cocktail available in the ward. Grade 4 mucosistis occurred in none of the patients. Fever occurred in all patients, but grades 3 and 4 infection was found in 11 patients (24%), of whom 5 cases of septicemia (non-bone marrow recovery and prolonged pancytopenia), and 1 case of influenza ultimately died, but the rest had full bone marrow recovery. Diarrhea occurred in 30% of patients, and maculopopular rash in 15%, in most of whom, twice daily administration of dexamethasone (8 mg) for 48 hours (if they had no fever) led to relative recovery, full recovery of all patients during follow-up period and removing rash. One case of brain hemorrhage occurred, for whom backup procedures were performed including 10 units of platelet transfusion, and patient had bone marrow recovery with no nervous complication to be discharged from ICU. In the present study, no vascular-brain complication occurred in patients, similar to what happened with high dose of cytosar. The mean time for 16 transplant candidates to prepare for transplant in the present study was 7 months. This is a considerable time period without recurrence. In 5 patients without consolidation treatment, the mean time without recurrence was 8 months. Time to relapse in five patients without any consolidation treatment, the time taken to prepare for Allo-Sct was significant and these delays contributed to relapse. Summing up complications and percentage of patients that went into full remission, Modified intermediate dose regimen with acceptable toxicity can be recommended as an induction regimen for patients with acute myeloid leukemia. Although further studies, with larger sample size, are also recommended. Treatment did not result in significant toxicity in the present study. Although the number of patients in the study was low, the high response rate and considerable period without recurrence in patients suggests that the double induction treatment using continuous infusion 12 hours twice daily of modified intermediate-dose cytarabine could be recommended for AML.
Acknowledgements
References
-
1.
SEER Stat Fact Sheets: Acute Myeloid Leukemia. 2012. Available from: http://seer.cancer.gov/statfacts/html/amyl.html.
-
2.
Szer J. The prevalent predicament of relapsed acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2012;2012:43-8. [PubMed ID: 23233559]. https://doi.org/10.1182/asheducation-2012.1.43.
-
3.
Cancer Facts and Figures 2014. Atlanta: American Cancer Society; 2014.
-
4.
Archimbaud E, Thomas X, Leblond V, Michallet M, Fenaux P, Cordonnier C, et al. Timed sequential chemotherapy for previously treated patients with acute myeloid leukemia: long-term follow-up of the etoposide, mitoxantrone, and cytarabine-86 trial. J Clin Oncol. 1995;13(1):11-8. [PubMed ID: 7799010]. https://doi.org/10.1200/JCO.1995.13.1.11.
-
5.
Mayer RJ, Davis RB, Schiffer CA, Berg DT, Powell BL, Schulman P, et al. Intensive postremission chemotherapy in adults with acute myeloid leukemia. Cancer and Leukemia Group B. N Engl J Med. 1994;331(14):896-903. [PubMed ID: 8078551]. https://doi.org/10.1056/NEJM199410063311402.
-
6.
Champlin R, Gajewski J, Nimer S, Vollset S, Landaw E, Winston D, et al. Postremission chemotherapy for adults with acute myelogenous leukemia: improved survival with high-dose cytarabine and daunorubicin consolidation treatment. J Clin Oncol. 1990;8(7):1199-206. [PubMed ID: 1694236]. https://doi.org/10.1200/JCO.1990.8.7.1199.
-
7.
Thomas X, Raffoux E, Renneville A, Pautas C, de Botton S, de Revel T, et al. Outcome of treatment after first relapse in younger adults with acute myeloid leukemia initially treated by the ALFA-9802 trial. Leuk Res. 2012;36(9):1112-8. [PubMed ID: 22647869]. https://doi.org/10.1016/j.leukres.2012.04.020.
-
8.
Naina HV, Patnaik MM, Harris S. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361(26):2578. author reply 2578. [PubMed ID: 20032330]. https://doi.org/10.1056/NEJMc0910366.
-
9.
Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-74. [PubMed ID: 19880497]. https://doi.org/10.1182/blood-2009-07-235358.
-
10.
Buchner T, Berdel WE, Schoch C, Haferlach T, Serve HL, Kienast J, et al. Double induction containing either two courses or one course of high-dose cytarabine plus mitoxantrone and postremission therapy by either autologous stem-cell transplantation or by prolonged maintenance for acute myeloid leukemia. J Clin Oncol. 2006;24(16):2480-9. [PubMed ID: 16735702]. https://doi.org/10.1200/JCO.2005.04.5013.
-
11.
Bradstock KF, Matthews JP, Lowenthal RM, Baxter H, Catalano J, Brighton T, et al. A randomized trial of high-versus conventional-dose cytarabine in consolidation chemotherapy for adult de novo acute myeloid leukemia in first remission after induction therapy containing high-dose cytarabine. Blood. 2005;105(2):481-8. [PubMed ID: 15213095]. https://doi.org/10.1182/blood-2004-01-0326.
-
12.
Lowenberg B, Pabst T, Vellenga E, van Putten W, Schouten HC, Graux C, et al. Cytarabine dose for acute myeloid leukemia. N Engl J Med. 2011;364(11):1027-36. [PubMed ID: 21410371]. https://doi.org/10.1056/NEJMoa1010222.
-
13.
Castaigne S, Chevret S, Archimbaud E, Fenaux P, Bordessoule D, Tilly H, et al. Randomized comparison of double induction and timed-sequential induction to a "3 + 7" induction in adults with AML: long-term analysis of the Acute Leukemia French Association (ALFA) 9000 study. Blood. 2004;104(8):2467-74. [PubMed ID: 15142880]. https://doi.org/10.1182/blood-2003-10-3561.
-
14.
Breems DA, Boogaerts MA, Dekker AW, Van Putten WL, Sonneveld P, Huijgens PC, et al. Autologous bone marrow transplantation as consolidation therapy in the treatment of adult patients under 60 years with acute myeloid leukaemia in first complete remission: a prospective randomized Dutch-Belgian Haemato-Oncology Co-operative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) trial. Br J Haematol. 2005;128(1):59-65. [PubMed ID: 15606550]. https://doi.org/10.1111/j.1365-2141.2004.05282.x.
-
15.
Wolff SN, Herzig RH, Fay JW, Phillips GL, Lazarus HM, Flexner JM, et al. High-dose cytarabine and daunorubicin as consolidation therapy for acute myeloid leukemia in first remission: long-term follow-up and results. J Clin Oncol. 1989;7(9):1260-7. [PubMed ID: 2769327]. https://doi.org/10.1200/JCO.1989.7.9.1260.
-
16.
De Witte T, Suciu S, Selleslag D, Labar B, Roozendaal K, Zittoun R, et al. Salvage treatment for primary resistant acute myelogenous leukemia consisting of intermediate-dose cytosine arabinoside and interspaced continuous infusions of idarubicin: a phase-II study (no. 06901) of the EORTC Leukemia Cooperative Group. Ann Hematol. 1996;72(3):119-24. [PubMed ID: 8766252].
-
17.
Lowenberg B. Sense and nonsense of high-dose cytarabine for acute myeloid leukemia. Blood. 2013;121(1):26-8. [PubMed ID: 23287624]. https://doi.org/10.1182/blood-2012-07-444851.
-
18.
Fukushima T, Urasaki Y, Yamaguchi M, Ueda M, Morinaga K, Haba T, et al. A randomized comparison of modified intermediate-dose Ara-C versus high-dose ara-c in post-remission therapy for acute myeloid leukemia. Anticancer Res. 2012;32(2):643-7. [PubMed ID: 22287757].
-
19.
Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996;87(5):1710-7. [PubMed ID: 8634416].
-
20.
Weick JK, Kopecky KJ, Appelbaum FR, Head DR, Kingsbury LL, Balcerzak SP, et al. A randomized investigation of high-dose versus standard-dose cytosine arabinoside with daunorubicin in patients with previously untreated acute myeloid leukemia: a Southwest Oncology Group study. Blood. 1996;88(8):2841-51. [PubMed ID: 8874180].
-
21.
Lowenberg B, van Putten W, Theobald M, Gmur J, Verdonck L, Sonneveld P, et al. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003;349(8):743-52. [PubMed ID: 12930926]. https://doi.org/10.1056/NEJMoa025406.
-
22.
Pagano L, Pulsoni A, Vignetti M, Tosti ME, Falcucci P, Fazi P, et al. Secondary acute myeloid leukaemia: results of conventional treatments. Experience of GIMEMA trials. Ann Oncol. 2005;16(2):228-33. [PubMed ID: 15668275]. https://doi.org/10.1093/annonc/mdi051.
-
23.
Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341(14):1051-62. [PubMed ID: 10502596]. https://doi.org/10.1056/NEJM199909303411407.
-
24.
Wiernik PH, Banks PL, Case DJ, Arlin ZA, Periman PO, Todd MB, et al. Cytarabine plus idarubicin or daunorubicin as induction and consolidation therapy for previously untreated adult patients with acute myeloid leukemia. Blood. 1992;79(2):313-9. [PubMed ID: 1730080].