Abstract
Context:
To date, CSCs have been identified in a variety of hematopoietic and solid tumors. Applying CSC in clinical implication still depends on future studies to remove complexities including CSC heterogeneity and CSC similarity to normal stem cells. However, several potential clinical applications including therapeutic, diagnostic and prognostic implications have been introduced for cancer stem cells (CSC). In this review, we discuss previously considered and unconsidered potential clinical application of CSCs including how CSCs could be applied for pan-specific cancer screening and therapy.Evidence Acquisition:
We will first discuss the previously proposed CSC clinical implications using a brief review of the literature. Subsequently, we will discuss some theoretical potential CSC implications which have not been discussed before including pan-specific cancer screening and therapy, and present confirmatory references for each part of our hypothesis.Results:
We hypothetically demonstrated the presence of similar markers in the CSC subset of different tumors and introduced it as a way to simultaneously screen several cancers using one CSC marker.Conclusions:
Simultaneous screening of several cancers applying one CSC marker could be regarded as a novel high-value cost-conscious cancer screening approach which might evolve cancer screening concept. However, this application remains to be explored in the future instigations.Keywords
1. Context
Several potential clinical applications including therapeutic, diagnostic and prognostic implications have been previously introduced for cancer stem cells (CSC). In this review, we discuss previously considered and unconsidered potential clinical application of CSCs including how CSCs could be applied for pan-specific cancer screening and therapy.
2. Evidence Acquisition
Currently, two cancer initiation models have been explained for tumor formation. In stochastic model tumor cells are biologically equivalent but behave differently due to stochastic effects. In this model, each tumor cell has the same potential to contribute to tumor growth and their behavior is influenced by both intrinsic and extrinsic factors. Under a certain set of factors, some tumor cells acquire tumor-initiating properties. As a result, isolation of an enriched subpopulation with tumorogenic potential is not consistent with this model, while each cell is predicted to have potential tumor initiating properties. This model does not explain lots of tumor characteristics (1, 2).
In contrast, cancer stem cell (CSC) model or hierarchical model postulates that like normal tissues of the body, tumors contain a stem cell population at the apex of an organized system that possess capacity to both self-renew and differentiate, leading to more CSCs and tumor differentiated cells, respectively. In this model, tumor contains CSCs with acquired mutations that lead to deregulated growth at the clonal level and a proliferating progeny of CSCs that finally form differentiated tumor cells and tumor bulk. As a result, the existence of distinct subpopulations with biological and functional differences makes it possible to isolate cells with tumor-initiating properties. CSC theory explains different tumor characteristics including tumor initiation, development, metastasis and recurrence. It also explains the ineffectiveness of conventional cancer therapies (1, 2) (Figure 1).
Comparison of New Tumor Formation According to Two Different Tumor Models: Stochastic Vs. CSC Model
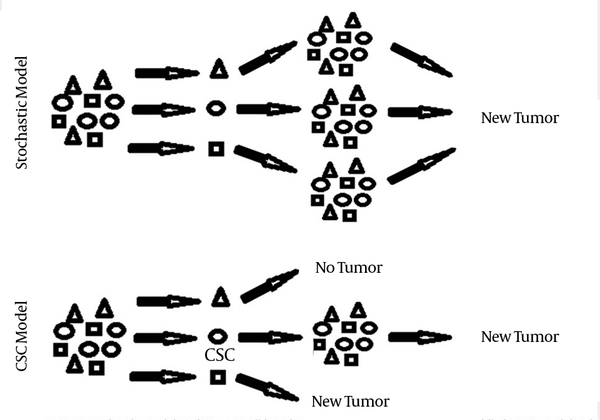
The first study on the identification of CSC was done by Bonnet and Dick, who identified a rare malignant subset with the ability to repopulate the original disease over serial transplantation. They showed that in human acute myeloid leukemia (AML) the self-renewal capacity was found only in CD34+ /CD38- subpopulation and their work represented a foundation for CSC research in both hematologic and solid tumors (3). To date, CSCs have been isolated in a variety of solid tumors such as breast cancer, glioblastoma, osteosarcoma, prostate cancer, ovarian cancer, gastric cancer and lung cancer (4). In addition, a great body of evidences has focused on the application of CSC for clinical purposes such as therapeutic, prognostic and diagnostic implications (4-6). However, application of CSC for clinical purposes has been slowed down due to some complexities including CSC heterogeneity (7) and CSC similarity to normal stem cells (8), and unraveling such ambiguities will pave the way for the CSC clinical implications. Taking colorectal cancer as an example, several potential markers including CD133, CD66, CD24, Lgr5, Musashi1, Bmi1 and DCLK1 have been proposed as colorectal CSC markers. To complicate the matter further, the results of some studies contradict the results of other studies. For instance, several studies have suggested that colon CSC may be identified by the cell surface marker CD133. However, Shmelkov et al. have demonstrated that even the CD133- parenchymal tumor cells are able to initiate tumor in xenotransplantation model. Similarly, in glioblastoma multiform, some groups have enriched stem-like cells using CD133 as a candidate for brain tumor cancer stem cell surface marker while some other groups have proved tumorogenic properties in both CD133+ and CD133- cell population in some gliomas.
Similarities of CSC markers to normal stem cell markers have also been a significant challenge in developing targeted therapy for selective elimination of cancer stem cells with minimal toxicity to normal stem cells (9). Fortunately, the knowledge in this area is growing slowly but steadily. One of the most compelling evidence has been reported by Nakanishi et al. by using lineage-tracing experiments. They have shown that normal stem cells are not marked by Dclk1 in the intestine. However tumor stem cells that continuously produce tumor progeny in the polyps of ApcMin/+ mice are marked by Dclk1. Although this study has attempted to find a marker that specifically marks colon CSC and not normal stem cells, still raises some unanswered questions. As we showed in our previous study, DCLK1 expression is also seen in the blood circulation of tumor-free control samples, although at lower levels compared to colorectal cancer patient’s samples. It could be concluded that although DCLK1 is not being expressed by normal colon stem cells, there is still some other sources of DCLK1 expression in normal individuals that makes DCLK1 therapeutic implications complicated (10).
In spite of the mentioned challenges in CSC clinical applications, the variety of valuable potential CSC clinical implications is attracting an increasing number of scientists to overcome these challenges.
In this review, we discuss some previously proposed CSC potential clinical implications including therapeutic, prognostic and diagnostic applications and then discussed some theoretical potential CSC implications which have not been discussed before, including pan-specific cancer screening and therapy. It is of note that all of the following implications are consistent with CSC model and not stochastic model.
3. Results
3.1. Previously Considered Clinical Implications of CSCs:
3.1.1. CSC Potential Therapeutic Implications
According to CSC model, CSCs are the key players of the tumors and tumor initiation, growth, resistance to treatment, metastasis and recurrence all controlled by CSC subpopulation (11). While most anticancer agents employ anti-proliferative mechanisms for tumor cells elimination, CSC may remain quiescent for extended periods leading to resistance to anti-proliferative effects and retaining the capacity to regenerate the tumor (1, 12). In other words, while quiescent or slowly-dividing CSCs works as the tumor root, other rapidly proliferating and differentiated cells of tumor bulk work as the tumor foliage. In consequence, specific targeting of quiescent or slowly-dividing CSCs as the root of tumor tree instead of targeting rapidly proliferating non- CSCs, has recently attracted many interests.
AML, as the pioneer in CSC study, has taken the lead in CSC targeted therapy. The most widely used leukemic stem cell (LSC) marker in the study of AML treatment is CD33 (13, 14). It has also made it as far as to gain FDA approval and Anti-CD33 antibodies have become a significant aspect of CSC targeted therapy (15, 16). A CD33 based therapy, named Mylotarg, which combines calicheamicin (a cytotoxic antibiotic) with an anti-CD33 antibody, was approved by the FDA in 2000 (17). Another popular LSC marker is C-type lectin-like molecule or CLL-1 (18). As previously mentioned, one of the main drawbacks of CSC clinical implication is its similarity to normal stem cells. However, in-vitro study showed that CLL-1 is present on AML CD34+ /CD38- subset but is not present on normal bone marrow CD34+ /CD38- cells (18). Such exclusivity makes CLL-1 a desirable target for AML targeted therapy. Although some advances in therapeutic and diagnostic application of CLL-1 in AML patients have been observed, verification of CLL-1 targeting in AML will only depend on future additional studies. John Dick’s group also indicated that CD44, a highly expressed surface antigen of AML blasts with lower expression on normal bone marrow hematopoietic stem cells (BM HSCs), can selectively block engraftment of AML LSCs but not normal HSCs when the cells are pretreated with anti-CD44 antibody before transplantation (19).
Besides targeting surface CSC markers, blockage of various self-renewal pathways, including Notch, PTEN, and Hedgehog, can be used as alternative strategies (20, 21). Hoey et al. (22) have shown that inhibition of delta-like 4 ligand (DLL4), an important component of Notch pathway, leads to a decrease in tumor growth and CSC frequency in colorectal cancer (CRC). In spite of limited available data, many clinical trials are in progress to determine the application of Hedgehog (Hh) inhibitors for the treatment of cancers. For instance, GDC-0449, as a novel Hh inhibitor, has been applied in an open label clinical trial to examine its therapeutic effect in advanced basal cell carcinoma patients. The overall response rate of 50% in metastatic carcinoma and 60% in localized carcinoma were seen (23).
Cell therapy is another available option in targeting CSC for therapeutic purposes. Accordingly, Weng et al. (24) demonstrated that fusion of dendritic cells (DCs) and ovarian CSCs resulted in the activation of T cells expressing higher levels of IFN-γ, with an increased killing power against CSCs. Glioma CSCs can also be targeted by cytotoxic T lymphocytes (CTL) through a perforin-mediated mechanism. In this regard, Brown and his colleagues (25) demonstrated that CSCs derived from high-grade glioma may be targeted and eliminated by CD8+ CTLs.
Genetic targeting of CSC is also one of the potential CSC targeted therapies and RNA interference strategies are at the frontline of such therapies through targeting aberrant expression of different genes utilizing miRNAs. Yu et al. (26) were able to increase the expression of let-7 miRNA in breast CSC via a lentiviral vector, leading to a reduced CSC fraction and delayed tumor formation and metastasis. In leukemia, miRNA-17-92 was found to be up-regulated in LSCs and similar results can be achieved if we target this miRNA in leukemia (27).
Despite all CSC therapeutic potentials, several important issues still remain to be resolved. As the most important issue, most of the pathways important to CSCs are shared by normal stem cells and targeting these pathways may have a detrimental effect on normal stem cells leading to tissue or organ damages due to depletion of the reserve stem cells and off-target effects such as irreversible tissue failure. Therefore, before applying CSC for therapeutic implications, it is critical to define the molecular differences amongst CSCs and their counterpart tissue specific stem cells. To this aim, candidate genes and pathways that are more important for CSC survival but are not for normal stem cell function should be identified.
3.1.2. CSC Potential Prognostic Implications
Much more interests have been devoted to CSC prognostic and diagnostic implications compared to its therapeutic applications. While therapeutic application of CSC markers requires very demanding intense clinical trial processes to minimize unwanted off-target effects of such interventions, a clinically relevant CSC marker would easily enter the clinical utilization cycle without going through exhausting time-consuming clinical trial processes, leading to a more interest in investigators.
Considering CSC as the supplier source of tumor cells in the tumor bulk and according to its responsibility in unlimited tumor growth, maintenance, metastasis, recurrence, resistance to treatment, and utterly tumor biological behavior, evaluation of the presence and extent of this population rationally result in more clinical relevance than the other tumor proliferating or differentiated cells do. Generally, it is believed that a higher CSC proportion signifies a worse prognosis. For instance, in breast cancer, the most poorly differentiated tumors contain the highest burden of breast CSCs (28). Likewise, elevated CD133 expression in colon cancer is also a marker of poor prognosis and is associated to liver metastasis (29). In pancreatic cancer, high CD133 is an adverse prognostic factor for 5-year survival and is associated with lymph node invasion (30). CD133 expression is also associated with poor clinical outcome in ovarian cancer (31). Similarly, CD133-positive non- small cell lung cancer (NSCLC) had worse prognosis (32).
In addition, expression of ALDH, another CSC specific marker, is correlated with poor prognosis in a number of tumors including breast, head and neck, prostate, colon and AML. ABC transporter, as potential CSC associated marker, has also been reported to be an indicator of poor prognosis in AML patients (33).
CD44 is another proposed cancer stem cell marker which its prognostic value has also been shown in several studies. Mima et al. (34) showed that the over-expression of CD44 was associated with poor prognosis in hepatocellular carcinoma patients, including reduced disease-free and overall survival. Mulder et al. (35) also showed that CD44 has prognostic value independent of Dukes’ stage in colorectal cancer patients and it may predict the propensity to metastasis after curative surgery. CD44 expression was also correlated with decreased overall survival in pancreatic cancer (36).
Based on the presented body of evidences, patients with elevated CSC marker’s expression mostly tend to have a poorer prognosis.
3.1.3. CSC Potential Diagnostic Implications
According to the fact that diagnostic tests should be safe, easy, relatively non-invasive, cost-benefit and acceptable for patients and clinicians, taking tissue samples for diagnosis proposes could not be considered as an appropriate option. Fortunately, a large number of tumor cells known as circulating tumor cells are being released on a daily basis in to the bloodstream whose smallest fraction is believed to be circulating CSCs (CCSC) (37, 38). According to the classical metastasis cascade, an orderly sequence of steps, including local invasion, intravasation, survival in the blood circulation, extravasation, and colonization in new organ, is expected in order to complete the metastatic process (39). Based on CSC theory, CSCs, as the most invasive and qualified cells to form metastasis, will enter the blood stream to accomplish their metastasis mission. Their presence in blood is our fortunate opportunity to apply them in diagnostic implications. Therefore, tracing the floating cancer stem cells in blood pool using their specific markers would be regarded as a favorable approach which has been exploited in a considerable number of recent investigations and the results have been promising.
Yang et al. (40) in china in 2008 showed that identification of CD45-CD90+ CSCs in both tumor tissues and blood circulation of patients with liver cancer could be used as a target for diagnosis and therapy of these patients. Linuma et al. (41) in Japan evaluated the clinical importance of circulating tumor cells (CTCs) including CSCs as a prognostic factor in patients with colorectal cancer after curative surgery and found that detection of CEA/CK/CD133 mRNA in peripheral blood would be a useful tool for identification of patients who are at higher risk of recurrence and poor prognosis. Pilati et al. (42) studied the prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis and concluded that CD133+CTC may represent a suitable prognostic marker to stratify the risk of these patients. Valladares et al. (43) evaluated the adenocarcinoma-associated gene AGR2 and the intestinal stem cell marker LGR5 as biomarkers in colorectal cancer. Their findings indicated that assessment of AGR2 and LGR5 in peripheral blood might reflect the presence of CTCs and CSCs in colorectal cancer and increased AGR2 and LGR5 are associated with poor outcomes. Wang and his group studied the role of CTCs and CSCs of breast cancer patients as well as their clinical relevance using flow cytometry concluding that flow cytometric detection of CTC and CTSC could be used to diagnose disease at early stage, which would be beneficial for clinical therapy guidance or prognosis prediction (44). Recently, our research group has also evaluated circulating CSC markers, DCLK1 and Lgr5, in colorectal cancer patients and showed a significant over-expression in both markers compared to tumor-free control group (10).
Although these studies confirm the potential application of circulating cancer stem cell for diagnostic purposes, further studies are needed to more elucidate CCSC diagnostic potential and supportive evidences in this field needs to be broaden.
3.1.4. Preventive Implications of CSC
The presence of anti-cancer properties in dietary phytochemicals has been demonstrated in several studies. Hilwaki-Clark et al. (45) showed that administrated phytostrogens to pregnant mice diminished breast cancer development in their offspring. Phytostrogen protective events have also been shown when administered during adolescence (45). Given the proposed role of CSC in tumor initiation it could be concluded that such dietary elements probably interfere with tumor initiation components such as CSCs. This assumption has been evaluated in some investigations. It has been shown that chemo-preventive agents such as curcumim from turmeric may function through regulating self-renewal cascade of stem cells including Wnt and Notch (46, 47). Some other dietary polyphenoids including apple-derived quercetin and epigallocetechin-galleate have also been shown to modulate in the Wnt- β catenin and Notch pathways (48). In addition, involvement of vitamin D3 in stem cell differentiation has been shown and therefore its preventive applications aimed at CSC population should be considered (49). Besides, lots of other dietary elements with anti-tumor activities have been introduced which their anti-tumor mechanisms need to be investigated in future studies.
3.2. Theoretical CSC Potential Clinical Implications
3.2.1. Screening Several Cancers Simultaneously, Applying CSC Markers
Investing in cancer screening saves lives and reduces the costs through early detection which leads to less aggressive and less expensive treatments. Yet the high costs of screening programs are a major barrier of such interventions, especially in less developed countries. Although even the most industrialized countries may be unable to meet these costs, more judicious usage of such tests will improve cancer screening quality and makes it economically more feasible. In other words, reducing screening costs to the extent that even less developed countries can apply them routinely, needs to promote high-value, cost-conscious cancer screening tests. One potential strategy to make cancer screening more judicious would be the development of a pan-specific marker for screening several cancers simultaneously. Such screening test mainly fits in the definition “High-value, cost-conscious cancer screening tests” (50). For this purpose, we need to take all types of cancers as one disease and search for one marker. We can instead categorize tumors based on their origin such as cancers with epithelial origin or carcinomas and search for a relevant marker in all cancers with the same origin. This categorization may even get narrower, taking the tumors of the same tract such as gastrointestinal or urinary tract together. We here propose that CSC markers have the potential to be applied for such proposes.
Each organ contains a subset of stem cells whose main responsibility is to establish tissue homeostasis. Differentiation signals from different components of tissue microenvironment stimulate stem cells to enter the differentiation pathway and after transition from transient amplifying state, they generate differentiated tissue cells (51, 52). CSCs follow a relatively similar pathway, while they are able to divide autonomously due to the undesired genetic changes (53). In other words, tumors are caricatures of normal tissues and CSCs are also caricatures of normal stem cells. Interestingly, Wong et al. (54) showed a widespread activation of embryonic stem cell-like (ESC-like) expression pattern in human cancers and demonstrated that the degree of this activation is associated to poor prognosis, increased risk of metastasis and mortality in multiple types of tumors. As to determine whether this ESC-like gene expression activation is related to CSC fraction or not, they showed that ESC-like gene expression was significantly up-regulated in enriched CD44+ /CD24-/low breast CSC population. These results empower the CSC model of cancer initiation and its similarity to normal stem cell model.
Although recently a considerable effort has been invested in the detection and characterization of stem cell markers and steadily increasing number of such marker molecules have been identified, most of these markers are lineage-specific rather than being tissue-specific (55). In this regard, markers that distinguish totipotent stem cells are nearly similar between different tissues. In addition, as it can be drawn from their names, undifferentiated or less differentiated cells of each organ, especially tissue stem cells, demonstrate more similar characteristics which have been the core of many research investigations. Fatima et al. (56) showed that Abcg2 is a stem cell marker for blood, small intestine, testicular germ cells, and possibly for injured skeletal and/or cardiac muscle. LGR5 is another stem cell marker which is expressed in the eye, brain, hair follicle, mammary gland, reproductive organs, stomach and intestinal tract and based on its expression pattern; it probably marks stem cells in some other tissues (57). Furthermore, ALDH1 has been introduced as a universal stem cell markers for the identification and isolation of stem cells from multiple sources (58).
Considering undifferentiated cells such as tissue normal stem cells as the most similar cells amongst different organs and accounting CSCs as the most undifferentiated cells of cancers which favor drastic similarity to normal stem cell, it could be concluded that CSCs may also be the most similar cells of different tumors (Figure 2). Since screening several cancers simultaneously using one pan-specific marker needs the viability of a similar marker in different tumors, it can be presumed that this marker probably would be located on tumor undifferentiated cells that is categorized as cancer stem cell. Moreover, tumors that share same embryonic origin such as carcinomas may benefit from even more similarity in their CSC population. Besides, the discovery of the similar CSC markers in the interrelated epithelial organs, such as the organs of gastrointestinal tract including intestine, pancreas and liver, may lead to even more similarity in CSC subsets of the tumors of these organs.
Take a brief look at proposed CSC markers and you could easily found that several markers such as ALDH, CD133, CD44, etc. are common among different tumors and their screening can predict existence of CSCs of that tissues, making them a good candidate for screening several tumors, simultaneously (59).
The Least Differentiated Cells of Tumors, Named CSC, are the Most Similar Ones in Surface Markers
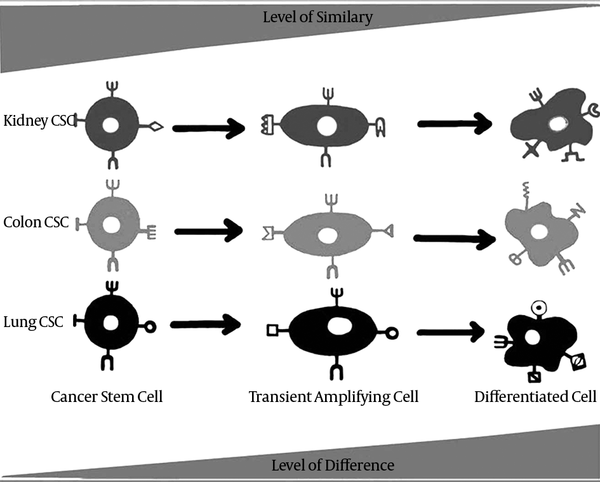
Since the advent of cancer stem cell hypothesis, ALDH1 has been widely mentioned as cancer stem cell marker in different cancers such as colon, liver, lung, breast, pancreas, prostate, bladder and some other cancers. Also, the effective role of CD133 as a common cancer stem cell has been demonstrated in several types of cancers including colon, liver, lung, prostate, pancreas, kidney, glioma and AML. Several other markers includingCD44, CD24 and CD90 whose putative role as cancer stem cell marker has been showed in more than one type of cancer may also accord the proposed application of CSC marker as pan-specific screening marker (Table 1) (60).
Similar Proposed CSC Markers in Different Tumor Types
| CD133 | ALDH1 | CD44 | CD24 | CD90 |
|---|---|---|---|---|
| Colon | Colon | Colon | Colon | Lung |
| Breast | Breast | Breast | Breast | Breast |
| Liver | Liver | Liver | Liver | Liver |
| Ovarian | ovarian | Ovarian | Ovarian | Glioma |
| Pancreatic | Pancreatic | Pancreatic | Pancreatic | |
| Melanoma | Melanoma | Glioma | ||
| Glioma | Glioma | Prostate | ||
| Prostate | Prostate | |||
| Lung | Lung |
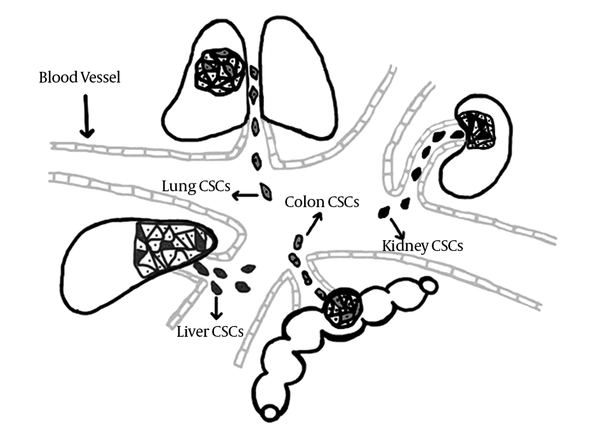
Whereas evaluation of different tumor applying extracted biopsy samples is regarded as an unpleasant clinical application, presence of circulating CSC of different tumors in blood circulation of affected parsons provides the opportunity of screening several cancers with one blood sample besides eliminating the repetitive sampling procedure of tissue markers’ assessment. Consequently, tracing CCSC’s of different tumors using their shared markers as a screening potential of CSC model, could be considered as a valuable opportunity that needs to be evaluated in future investigations (Figure 3).
Cancer Stem Cells of Different Tumors Will Be Released into the Blood stream in Order to Complete Metastatic Cascade
3.2.2. Simultaneous Targeting Of Different Cancers
Considering all cancers as one disease provides another potential opportunity. Beside screening possibility of several cancers using their shared CSC marker, such marker might be used to target several cancers, simultaneously. In addition to reduced treatment costs of such therapeutic potential, it also has the potential to be used for preventive purposes. Although the treatment of several cancers with one drug may seem ambitious, a closer look at nature may make it more believable. Nearly all dietaries with anti-tumor effects, apply their anti-tumor effects in more than one tumor. As an example, turmeric anti-cancer effects have been reported in several cancers such as hepatic cancer (61), breast cancer (46), head and neck carcinoma (62), colon cancer (63), lung cancer (64), prostate cancer (65), ovarian cancer (66) and pancreatic cancer (67). As a result, the presence of an anti-cancer drug which is applicable to more than one cancer is not an impossible achievement.
4. Conclusions
Despite all potential valuable clinical implications of CSC model of cancer development, two important aspects of the CSC still remain to be elucidated before applying into clinical implications. First of all, there is still no clear-cut agreement on a certain CSC marker and many reports on isolating CSCs from different and sometimes opposing cell fractions has been presented for each cancer.
The second complicating issue is the similarity of nearly all proposed CSC markers to normal tissue stem cells. This difficulty is even more challenging than CSC heterogeneity, seeing that a marker shared by CSC and normal stem cell would not be considered as a credible therapeutic option due to its off-target effects on normal stem cell. However, such markers may still be valuable for diagnostic purposes.
Ultimately, in spite of CSC model widespread acceptance, its application to clinical practices still remains to be elucidated in future advancements, focusing on a specific CSC marker not shared by other normal tissue markers.
Acknowledgements
References
-
1.
Lobo NA, Shimono Y, Qian D, Clarke MF. The biology of cancer stem cells. Annu Rev Cell Dev Biol. 2007;23:675-99. [PubMed ID: 17645413]. https://doi.org/10.1146/annurev.cellbio.22.010305.104154.
-
2.
Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17(3):313-9. [PubMed ID: 21386835]. https://doi.org/10.1038/nm.2304.
-
3.
Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730-7. [PubMed ID: 9212098]. https://doi.org/10.1038/nm0797-730.
-
4.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755-68. [PubMed ID: 18784658]. https://doi.org/10.1038/nrc2499.
-
5.
Gil J, Stembalska A, Pesz KA, Sasiadek MM. Cancer stem cells: the theory and perspectives in cancer therapy. J Appl Genet. 2008;49(2):193-9. [PubMed ID: 18436993]. https://doi.org/10.1007/BF03195612.
-
6.
Bonito MD, Cantile M, Malzone G, Liguori G, Botti G. The prognostic role of cancer stem cells in breast tumors. J Clin Med Res. 2013;5(5):325-6. [PubMed ID: 23976904]. https://doi.org/10.4021/jocmr1302w.
-
7.
Tang DG. Understanding cancer stem cell heterogeneity and plasticity. Cell Res. 2012;22(3):457-72. [PubMed ID: 22357481]. https://doi.org/10.1038/cr.2012.13.
-
8.
Shackleton M. Normal stem cells and cancer stem cells: similar and different. Seminars in cancer biology. Elsevier; 2010.
-
9.
Natarajan TG, FitzGerald KT. Markers in normal and cancer stem cells. Cancer Biomark. 2007;3(4-5):211-31. [PubMed ID: 17917151].
-
10.
Mirzaei A, Tavoosidana G, Modarressi MH, Rad AA, Fazeli MS, Shirkoohi R, et al. Upregulation of circulating cancer stem cell marker, DCLK1 but not Lgr5, in chemoradiotherapy-treated colorectal cancer patients. Tumour Biol. 2015;36(6):4801-10. [PubMed ID: 25631749]. https://doi.org/10.1007/s13277-015-3132-9.
-
11.
Chen K, Huang YH, Chen JL. Understanding and targeting cancer stem cells: therapeutic implications and challenges. Acta Pharmacol Sin. 2013;34(6):732-40. [PubMed ID: 23685952]. https://doi.org/10.1038/aps.2013.27.
-
12.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105-11. [PubMed ID: 11689955]. https://doi.org/10.1038/35102167.
-
13.
Hauswirth AW, Florian S, Printz D, Sotlar K, Krauth MT, Fritsch G, et al. Expression of the target receptor CD33 in CD34+/CD38-/CD123+ AML stem cells. Eur J Clin Invest. 2007;37(1):73-82. [PubMed ID: 17181570]. https://doi.org/10.1111/j.1365-2362.2007.01746.x.
-
14.
Sperr WR, Florian S, Hauswirth AW, Valent P. CD 33 as a target of therapy in acute myeloid leukemia: current status and future perspectives. Leuk Lymphoma. 2005;46(8):1115-20. [PubMed ID: 16085551]. https://doi.org/10.1080/10428190500126075.
-
15.
ten Cate B, de Bruyn M, Wei Y, Bremer E, Helfrich W. Targeted elimination of leukemia stem cells; a new therapeutic approach in hemato-oncology. Curr Drug Targets. 2010;11(1):95-110. [PubMed ID: 20017722]. https://doi.org/10.2174/138945010790031063.
-
16.
ten Cate B, Bremer E, de Bruyn M, Bijma T, Samplonius D, Schwemmlein M, et al. A novel AML-selective TRAIL fusion protein that is superior to Gemtuzumab Ozogamicin in terms of in vitro selectivity, activity and stability. Leukemia. 2009;23(8):1389-97. [PubMed ID: 19262596]. https://doi.org/10.1038/leu.2009.34.
-
17.
British Committee for Standards in H, Milligan DW, Grimwade D, Cullis JO, Bond L, Swirsky D, et al. Guidelines on the management of acute myeloid leukaemia in adults. Br J Haematol. 2006;135(4):450-74. [PubMed ID: 17054678]. https://doi.org/10.1111/j.1365-2141.2006.06314.x.
-
18.
van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, et al. The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood. 2007;110(7):2659-66. [PubMed ID: 17609428]. https://doi.org/10.1182/blood-2007-03-083048.
-
19.
Smadja F, Dick J, Kadouche J. Chimeric Anti Cd44 Antibodies and Their Use for Treating Acute Myeloid Leukemia. Google Patents; 2004.
-
20.
Pardal R, Clarke MF, Morrison SJ. Applying the principles of stem-cell biology to cancer. Nat Rev Cancer. 2003;3(12):895-902. [PubMed ID: 14737120]. https://doi.org/10.1038/nrc1232.
-
21.
Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226-35. [PubMed ID: 19265662]. https://doi.org/10.1016/j.stem.2009.01.007.
-
22.
Hoey T, Fischer M, Yen WC, Kapoun AM, Wang M, O'Young G, et al. Anti-DLL4 inhibits growth and reduces tumor-initiating cell frequency in colorectal tumors with oncogenic KRAS mutations. Cancer Res. 2011;71(5):1520-5. [PubMed ID: 21193546]. https://doi.org/10.1158/0008-5472.CAN-10-2817.
-
23.
Von Hoff DD, LoRusso PM, Rudin CM, Reddy JC, Yauch RL, Tibes R, et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N Engl J Med. 2009;361(12):1164-72. [PubMed ID: 19726763]. https://doi.org/10.1056/NEJMoa0905360.
-
24.
Weng D, Song B, Durfee J, Sugiyama V, Wu Z, Koido S, et al. Induction of cytotoxic T lymphocytes against ovarian cancer-initiating cells. Int J Cancer. 2011;129(8):1990-2001. [PubMed ID: 21154809]. https://doi.org/10.1002/ijc.25851.
-
25.
Brown CE, Starr R, Martinez C, Aguilar B, D'Apuzzo M, Todorov I, et al. Recognition and killing of brain tumor stem-like initiating cells by CD8+ cytolytic T cells. Cancer Res. 2009;69(23):8886-93. [PubMed ID: 19903840]. https://doi.org/10.1158/0008-5472.CAN-09-2687.
-
26.
Yu F, Yao H, Zhu P, Zhang X, Pan Q, Gong C, et al. let-7 regulates self renewal and tumorigenicity of breast cancer cells. Cell. 2007;131(6):1109-23. [PubMed ID: 18083101]. https://doi.org/10.1016/j.cell.2007.10.054.
-
27.
Jiang P, Rao EY, Meng N, Zhao Y, Wang JJ. MicroRNA-17-92 significantly enhances radioresistance in human mantle cell lymphoma cells. Radiat Oncol. 2010;5:100. [PubMed ID: 21040528]. https://doi.org/10.1186/1748-717X-5-100.
-
28.
Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell. 2010;140(1):62-73. [PubMed ID: 20074520]. https://doi.org/10.1016/j.cell.2009.12.007.
-
29.
Horst D, Kriegl L, Engel J, Kirchner T, Jung A. Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Invest. 2009;27(8):844-50. [PubMed ID: 19626493]. https://doi.org/10.1080/07357900902744502.
-
30.
Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, et al. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98(8):1389-97. [PubMed ID: 18349830]. https://doi.org/10.1038/sj.bjc.6604307.
-
31.
Zhang J, Guo X, Chang DY, Rosen DG, Mercado-Uribe I, Liu J. CD133 expression associated with poor prognosis in ovarian cancer. Mod Pathol. 2012;25(3):456-64. [PubMed ID: 22080056]. https://doi.org/10.1038/modpathol.2011.170.
-
32.
Qu H, Li R, Liu Z, Zhang J, Luo R. Prognostic value of cancer stem cell marker CD133 expression in non-small cell lung cancer: a systematic review. Int J Clin Exp Pathol. 2013;6(11):2644-50. [PubMed ID: 24228135].
-
33.
Guo Y, Kock K, Ritter CA, Chen ZS, Grube M, Jedlitschky G, et al. Expression of ABCC-type nucleotide exporters in blasts of adult acute myeloid leukemia: relation to long-term survival. Clin Cancer Res. 2009;15(5):1762-9. [PubMed ID: 19240178]. https://doi.org/10.1158/1078-0432.CCR-08-0442.
-
34.
Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, et al. CD44s regulates the TGF-beta-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res. 2012;72(13):3414-23. [PubMed ID: 22552294]. https://doi.org/10.1158/0008-5472.CAN-12-0299.
-
35.
Mulder JW, Kruyt PM, Sewnath M, Oosting J, Seldenrijk CA, Weidema WF, et al. Colorectal cancer prognosis and expression of exon-v6-containing CD44 proteins. Lancet. 1994;344(8935):1470-2. [PubMed ID: 7526103]. https://doi.org/10.1016/S0140-6736(94)90290-9.
-
36.
Gotoda T, Matsumura Y, Kondo H, Saitoh D, Shimada Y, Kosuge T, et al. Expression of CD44 variants and its association with survival in pancreatic cancer. Jpn J Cancer Res. 1998;89(10):1033-40. [PubMed ID: 9849582]. https://doi.org/10.1111/j.1349-7006.1998.tb00493.x.
-
37.
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559-64. [PubMed ID: 21436443]. https://doi.org/10.1126/science.1203543.
-
38.
Rao GC, Larson C, Repollet M, Rutner H, Terstappen LW, O'hara SM. Analysis of circulating tumor cells, fragments, and debris. Google Patents; 2012.
-
39.
Pantel K, Brakenhoff RH. Dissecting the metastatic cascade. Nat Rev Cancer. 2004;4(6):448-56. [PubMed ID: 15170447]. https://doi.org/10.1038/nrc1370.
-
40.
Yang ZF, Ngai P, Ho DW, Yu WC, Ng MN, Lau CK, et al. Identification of local and circulating cancer stem cells in human liver cancer. Hepatology. 2008;47(3):919-28. [PubMed ID: 18275073]. https://doi.org/10.1002/hep.22082.
-
41.
Iinuma H, Watanabe T, Mimori K, Adachi M, Hayashi N, Tamura J, et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J Clin Oncol. 2011;29(12):1547-55. [PubMed ID: 21422427]. https://doi.org/10.1200/JCO.2010.30.5151.
-
42.
Pilati P, Mocellin S, Bertazza L, Galdi F, Briarava M, Mammano E, et al. Prognostic value of putative circulating cancer stem cells in patients undergoing hepatic resection for colorectal liver metastasis. Ann Surg Oncol. 2012;19(2):402-8. [PubMed ID: 22071867]. https://doi.org/10.1245/s10434-011-2132-2.
-
43.
Valladares-Ayerbes M, Blanco-Calvo M, Reboredo M, Lorenzo-Patino MJ, Iglesias-Diaz P, Haz M, et al. Evaluation of the adenocarcinoma-associated gene AGR2 and the intestinal stem cell marker LGR5 as biomarkers in colorectal cancer. Int J Mol Sci. 2012;13(4):4367-87. [PubMed ID: 22605983]. https://doi.org/10.3390/ijms13044367.
-
44.
Wang N, Shi L, Li H, Hu Y, Du W, Liu W, et al. Detection of circulating tumor cells and tumor stem cells in patients with breast cancer by using flow cytometry: a valuable tool for diagnosis and prognosis evaluation. Tumour Biol. 2012;33(2):561-9. [PubMed ID: 22241087]. https://doi.org/10.1007/s13277-011-0303-1.
-
45.
Hilakivi-Clarke L, de Assis S. Fetal origins of breast cancer. Trends Endocrinol Metab. 2006;17(9):340-8. [PubMed ID: 16997567]. https://doi.org/10.1016/j.tem.2006.09.002.
-
46.
Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503-13. [PubMed ID: 16628653]. https://doi.org/10.1002/cncr.21904.
-
47.
Jaiswal AS, Marlow BP, Gupta N, Narayan S. Beta-catenin-mediated transactivation and cell-cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene. 2002;21(55):8414-27. [PubMed ID: 12466962]. https://doi.org/10.1038/sj.onc.1205947.
-
48.
Pahlke G, Ngiewih Y, Kern M, Jakobs S, Marko D, Eisenbrand G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J Agric Food Chem. 2006;54(19):7075-82. [PubMed ID: 16968065]. https://doi.org/10.1021/jf0612530.
-
49.
Nagler A, Riklis I, Kletter Y, Tatarsky I, Fabian I. Effect of 1,25 dihydroxyvitamin D3 and retinoic acid on normal human pluripotent (CFU-mix), erythroid (BFU-E), and myeloid (CFU-C) progenitor cell growth and differentiation patterns. Exp Hematol. 1986;14(1):60-5. [PubMed ID: 3510890].
-
50.
Owens DK, Qaseem A, Chou R, Shekelle P, Clinical Guidelines Committee of the American College of P. High-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174-80. [PubMed ID: 21282697]. https://doi.org/10.7326/0003-4819-154-3-201102010-00007.
-
51.
Sylvester KG, Longaker MT. Stem cells: review and update. Arch Surg. 2004;139(1):93-9. [PubMed ID: 14718284]. https://doi.org/10.1001/archsurg.139.1.93.
-
52.
Avasthi S, Srivastava R, Singh A. Stem cell: past, present and future-a review article. Int J Med Update. 2008;3(1).
-
53.
Soltysova A, Altanerova V, Altaner C. Cancer stem cells. Neoplasma. 2005;52(6):435-40. [PubMed ID: 16284686].
-
54.
Wong DJ, Liu H, Ridky TW, Cassarino D, Segal E, Chang HY. Module map of stem cell genes guides creation of epithelial cancer stem cells. Cell Stem Cell. 2008;2(4):333-44. [PubMed ID: 18397753]. https://doi.org/10.1016/j.stem.2008.02.009.
-
55.
Karsten U, Goletz S. What makes cancer stem cell markers different? Springerplus. 2013;2(1):301. [PubMed ID: 23888272]. https://doi.org/10.1186/2193-1801-2-301.
-
56.
Fatima S, Zhou S, Sorrentino BP. Abcg2 expression marks tissue-specific stem cells in multiple organs in a mouse progeny tracking model. Stem Cells. 2012;30(2):210-21. [PubMed ID: 22134889]. https://doi.org/10.1002/stem.1002.
-
57.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003-7. [PubMed ID: 17934449]. https://doi.org/10.1038/nature06196.
-
58.
Douville J, Beaulieu R, Balicki D. ALDH1 as a functional marker of cancer stem and progenitor cells. Stem Cells Dev. 2009;18(1):17-25. [PubMed ID: 18573038]. https://doi.org/10.1089/scd.2008.0055.
-
59.
Medema JP. Cancer stem cells: the challenges ahead. Nat Cell Biol. 2013;15(4):338-44. [PubMed ID: 23548926]. https://doi.org/10.1038/ncb2717.
-
60.
Madka V, Rao CV. Cancer stem cell markers as potential targets for epithelial cancers. Indian J Exp Biol. 2011;49(11):826-35. [PubMed ID: 22126013].
-
61.
Notarbartolo M, Poma P, Perri D, Dusonchet L, Cervello M, D'Alessandro N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005;224(1):53-65. [PubMed ID: 15911101]. https://doi.org/10.1016/j.canlet.2004.10.051.
-
62.
Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. [PubMed ID: 21299897]. https://doi.org/10.1186/1476-4598-10-12.
-
63.
Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255(2):170-81. [PubMed ID: 17448598]. https://doi.org/10.1016/j.canlet.2007.03.005.
-
64.
Chen HW, Lee JY, Huang JY, Wang CC, Chen WJ, Su SF, et al. Curcumin inhibits lung cancer cell invasion and metastasis through the tumor suppressor HLJ1. Cancer Res. 2008;68(18):7428-38. [PubMed ID: 18794131]. https://doi.org/10.1158/0008-5472.CAN-07-6734.
-
65.
Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47(4):293-303. [PubMed ID: 11398177]. https://doi.org/10.1002/pros.1074.
-
66.
Shi M, Cai Q, Yao L, Mao Y, Ming Y, Ouyang G. Antiproliferation and apoptosis induced by curcumin in human ovarian cancer cells. Cell Biol Int. 2006;30(3):221-6. [PubMed ID: 16376585]. https://doi.org/10.1016/j.cellbi.2005.10.024.
-
67.
Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14(14):4491-9. [PubMed ID: 18628464]. https://doi.org/10.1158/1078-0432.CCR-08-0024.