Abstract
Background:
Cervical cancer is one of the main causes of women’s death in the world. Human papilloma virus (HPV) types 16 and 18 have been known as a cause of more than 2/3 of cervical cancers. New combination treatment strategies based on immune responses against viral antigens are likely to be beneficial. Considering the important role of angiogenesis in nutrition and oxygenation of tumor cells, the inhibition of this process can help tumor clearance.Objectives:
In this study, co-administration of a plasmid encoding immune stimulatory epitopes of E6-E7-L1 genes of HPV with an anti-angiogenic peptide derived from Endostatin in tumor mice model was examined.Methods:
C57BL/6 mice were injected subcutaneously with TC-1 tumor cells and monitored for tumor progression. At exact time points, tumor size was determined. Then they were injected by pIRES-E6/E7/L1 as DNA vaccine, and anti angiogenic peptide. Relative tumor volume measurements and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was carried out in order to investigate therapeutic antitumor effects of vaccine and peptide.Results:
Based on the results, the group of mice that received vaccine and vaccine-peptide had significant inhibition rate of tumor growth in comparison with control groups (P < 0.05). Lymphocyte proliferation responses against the vaccine in the related groups were significantly higher than negative control group (P < 0.05).Conclusions:
In general, it could be concluded that the co-administration of DNA vaccine due to the good immunogenicity and suitable inhibitory effect of peptide in reduction of tumor size, shows higher efficacy and can be considered as a new therapeutic strategy.Keywords
1. Background
Cervical cancer is the third most common malignancy in women worldwide (1) and the second cause of cancer death in women in developing countries (2). Due to health importance, several solutions have been proposed for the treatment of cervical cancer. The initial treatment of this cancer is hysterectomy, followed by chemotherapy and radio therapy. Since the human papilloma viruses (HPV), especially types 16 and 18, are known as the main causes of cervical cancer, new treatment strategies are provided based on the literature findings (3). L1 protein encoded by HPVs is the major capsid protein and stimulates the production of neutralizing antibodies in serum. This protein is an appropriate candidate for production of preventive vaccines (4). On the other hand, the viral E6 and E7 oncoproteins are constantly expressed in the tumor cells.
Some studies have shown that the HPV E7 gene alone can stimulate the immune system at intermediate levels; it may be due to a nonhuman codon bias leading to inefficient expression of viral proteins (5). It seems that in cervical cancer cells, most of produced E7 proteins are degraded rapidly by the ubiquitin-proteasome pathway; therefore, they are detectable hardly using common detection protein methods such as immunostaining, immunoprecipitation, or western blot analysis (6). On the other hand, some other studies showed that using optimized codons can improve susceptibility of target cells to E6-drived CTL (cytotoxic T lymphocyte) recognition (7). For induction of HPV-specific T lymphocytes in cervical cancer model, vaccination against determined epitopes is an attractive treatment option. Up to now, various MHC class I-restricted CTL epitopes of HPV-16 E6 and E7 have been tested in early phase clinical trials (8).
Angiogenesis inhibitors can now be considered as the fourth modality of cancer therapy (9). Endostatin is a 20-kDa C-terminal fragment derived from collagen XVIII and one of the angiogenesis inhibitors that can target more than 12% of the angiogenesis regulatory human genes. Endostatin has known as an anti-cancer drug that has neither resistance nor toxicity in humans (10). It seems that the whole endostatin protein or its derivation could be considered as a model for angiogenesis inhibitor and could be administrated with other kinds of therapeutic strategies (11, 12).
Objectives: In the current study, we examined the co-administration of a synthetic peptide derived from N-terminal loop of Endostatin and DNA vaccine containing the antigenic cellular epitopes of E6, E7 and L1 genes in cervical cancer tumor model.
2. Methods
2.1. Design of Recombinant Gene
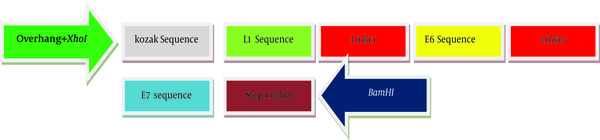
For the generation of the recombinant gene construct encoding HLA class-I (human leukocyte antigen class-I) restricted CTL epitopes, the immunogenicity of different epitops of HPV-16 E7 (49 - 57), E6 (41 - 50, 81 - 90, 98 - 107) and L1 (165 - 173) linked by a spacer of (glutamic acid, alanine, alanine, alanine, lysine) were assessed and optimized using computer-based modeling. Kozak sequence with the start codon (ACCATG) was added at the 5’-end of the DNA sequence. At both sides of sequence, two restricted enzyme sites (XhoI and BamHI) were predicted. Also, a stop codon (TAG) was added at the 3’-end of target sequences. The final DNA sequences were designed and synthesized. Artificial gene sequences encoding target immunogenic epitopes of E6, E7 and L1 create 397 bp. The scheme of recombinant DNA construct produced in this study has been shown in Figure 1. Validity of synthesized sequences was confirmed using automated sequencing. The construct was subcloned into pIRES and named as pIRES-E6/E7/L1.
The Schematic Map of Artificial Sequence That Was Produced in This Study
2.2. Cells Line, Mice and Media
The TC-1 cells were purchased from Pasteur Institute, Tehran, Iran. The original cells were prepared by transformation of C57BL/6 mouse primary lung cells with the HPV16 E6/E7 oncogenes and human activated H-ras (13). The cells were cultured in Roswell park memorial institute (RPMI) 1640 cell-culture medium (Gibco Invitrogen; Paisley, Scotland, UK), supplemented with 10% fetal bovine serum (FBS) (Gibco BRL), 100 IU/mL penicillin,100 μg/mL streptomycin, 2 mM glutamine, 1 mM sodium pyruvate, and 2 mM nonessential amino acids, at 37°C incubator with 5% CO2.
Female C57BL/6 mice (3 - 4 weeks old) were purchased from the Pasteur institute, Tehran, Iran (n = 25) and were used for experimental purposes. All procedures were performed according to approved protocols and in accordance with recommendations for the proper use and care of animal’s laboratory of Tarbiat Modares University. Before the experiment, mice were housed in standard condition with free access of food and water.
2.3. Vaccine Challenge
To evaluate anti-tumor activity of pIRES-E6/E7/L1 and peptide in vivo, TC-1 cancer cells (1 × 106) were subcutaneously (S.C.) injected in the right flank of each mouse. After 5 days, when tumors were palpable (about 100 mm3), the mice were randomly divided into 5 groups of 7 animals each. Tumor size was measured using caliper, and the weight of each mouse was measured by a scale. Tumors were monitored 4 times per week and their volumes were calculated by the equation “Volume = length × width2 / 2”.
The expression vector that contained pIRES-E6/E7/L1 was transformed in E. coli DH5α strain cultured in Luria Broth Medium and purified by the plasmid maxi kit (Gene All, Korea).
Two weeks after TC-1 cells injection, mice were immunized subcutaneously with 100 μL phosphate buffered saline (PBS; negative control), 100 μg naked DNA vaccine encoding pIRES (negative plasmid control) and pIRES-E6/E6/L1 in PBS, on days 0 and 10. Mice were also injected intraperitoneally with a dose of 1 mg/kg /day of peptide. Negative control mice groups were used for elimination of nonspecific responses and other environmental interferences. Finally, animals were sacrificed on day 21 after the final immunization, their spleens were isolated aseptically for in vitro splenocytes culture.
2.4. MTT Proliferation Assay
MTT assay actually determines the metabolic activity of mitochondria and correlates well with the number of viable cells. This assay has been previously used to evaluate endothelial cell proliferation (14). Since the proliferation of immune cells in the spleen represent responses to the immune stimulators, an MTT proliferation assay was used to evaluate the stimulating effect of E6, E7 and L1 proteins on proliferation of spleenocytes. Briefly, cells (300,000/well) were seeded onto 96-well culture plates and were treated with 10 mg/mL of each protein for 72 hours. Then, cells were incubated with MTT yellow dye (5 mg/mL in PBS) at 37°C incubator with 5% CO2 for 3 hours. The purple formazan product was dissolved in DMSO, and the absorbance was measured at 570 nm using an ELISA reader. The MTT assay was done in triplicate.
3. Results
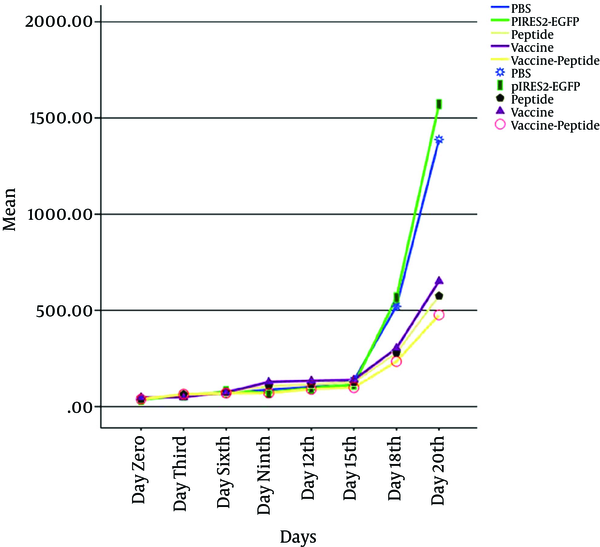
Mice were injected subcutaneously by TC-1 tumor cells, monitored for tumor formation, and the tumor was observed after 5 days post inoculation. Then to investigated therapeutic antitumor effects of vaccine, peptide and vaccine-peptide, the mice were injected by pIRES-E6/E7/L1 and peptide as described above and were followed for tumor development. Evidence of therapeutic effects of vaccine and peptide was investigated at the final days in vaccine and vaccine-peptide groups with significant rate inhibition of tumor growth (P < 0.05). There was no significant difference in tumor size between the test groups (Figure 2).
Assessment of Vaccine and Peptide on Tumor Size after the Tumor Inoculation
In order to determine how pIRES-E/E7/L1 stimulates spleen’s lymphocytes, MTT assay was performed to evaluate the proliferation of spleenocytes in response to stimulation with the E6 and E7 and L1. So the spleenocytes were extracted and harvested for 48 hours (Figure 3) and formation of formazan crystal was determined by solving the crystals in DMSO. Lymphocyte proliferation in the pIRES-E6/E7/L1 group, as vaccine group, and pIRES-E6/E7/L1-peptide group was significantly higher than in the other groups especially the negative control group (P < 0.05). (Figure 4). As seen in Table 1, the stimulation indexes of these groups were higher than those of the control groups. (Table 1).
Extracted Lymphocytes of spleen
The Stimulation Calculated Indexes for Each Groups

The Results of the Lymphocyte Stimulation Test
| Group | The Stimulation Index |
|---|---|
| PBS | 0.153 ± 0.09 |
| pIRES2-EGFP | 0.141 ± 0.07 |
| Peptide | 0.145 ± 0.04 |
| Vaccine | 0.288 ± 0.13 |
| Vaccine-Peptide | 0.286 ± 0.11 |
4. Discussion
Human papilloma virus type 16 (HPV16) is the most common HPV type that is associated with severe cervical dysplasia and cancers (15). Many strategies have been proposed for treatment of cervical cancer and one of them is immunotherapy against the HPV. In recent studies, it has been found that E6/E7/L1 antigens of HPV are suitable candidates to generate DNA vaccines as shown previously (4, 16, 17).
Recently preventive vaccines based on L1 antigens have been produced and have been successful in prophylactics goals, but these vaccines have shown no therapeutic effects and are expensive for many developing countries (18).
As mentioned in previous studies, the expression vector containing of E6/E7/L1 genes were evaluated beneficial (19). In this study we used their cellular epitopes in a single vector and the reduction effect of the construct in tumor size and proliferation of immune cells in the spleenwas shown. Based on our experiment the optimized truncated epitopes of HPV tumor antigens (E6/E7/L1) in mammalian expression vector had therapeutic effects in comparison to the peptide alone.
It seems that the therapeutic effects of DNA vaccine can be tolerated by the amount of inoculated tumor cells and the age of mice, which was similar to other related studies. Diniz et al. showed that therapeutic effects of DNA vaccine can be higher if 5 × 104 cells were injected instead of 5 × 105 cells and it seems that using the older mice is better maybe for having more developed immune systems (20, 21).
Despite the reduction in tumor size in the group that received peptide alone, it was not statistically significant by using 50 µg/kg of the peptide (12). Maybe higher doses response of inoculums or other related differences in susceptibility must be considered in future studies.
4.1. Conclusions
In conclusion, our data suggest that although combination targeted therapies are more preferable strategies, in some circumstances, combination therapies can affect the immune response and need more detailed pilot studies. The dosage and intervals of target therapies and detailed specifications of animal species, sex, age, microbiological status and weight to assess their effect on specific tumor models should be investigated properly.
References
-
1.
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893-917. [PubMed ID: 21351269]. https://doi.org/10.1002/ijc.25516.
-
2.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74-108. [PubMed ID: 15761078].
-
3.
Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine. 2009;27(5):684-9. [PubMed ID: 19056449]. https://doi.org/10.1016/j.vaccine.2008.11.042.
-
4.
Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27(3):431-40. [PubMed ID: 19022315]. https://doi.org/10.1016/j.vaccine.2008.10.078.
-
5.
Steinberg T, Ohlschlager P, Sehr P, Osen W, Gissmann L. Modification of HPV 16 E7 genes: correlation between the level of protein expression and CTL response after immunization of C57BL/6 mice. Vaccine. 2005;23(9):1149-57. [PubMed ID: 15629358]. https://doi.org/10.1016/j.vaccine.2004.08.027.
-
6.
Cid-Arregui A, Juarez V, zur Hausen H. A synthetic E7 gene of human papillomavirus type 16 that yields enhanced expression of the protein in mammalian cells and is useful for DNA immunization studies. J Virol. 2003;77(8):4928-37. [PubMed ID: 12663798].
-
7.
Samorski R, Gissmann L, Osen W. Codon optimized expression of HPV 16 E6 renders target cells susceptible to E6-specific CTL recognition. Immunol Lett. 2006;107(1):41-9. [PubMed ID: 16949679]. https://doi.org/10.1016/j.imlet.2006.07.003.
-
8.
Riemer AB, Keskin DB, Zhang G, Handley M, Anderson KS, Brusic V, et al. A conserved E7-derived cytotoxic T lymphocyte epitope expressed on human papillomavirus 16-transformed HLA-A2+ epithelial cancers. J Biol Chem. 2010;285(38):29608-22. [PubMed ID: 20615877]. https://doi.org/10.1074/jbc.M110.126722.
-
9.
Folkman J. Antiangiogenesis in cancer therapy--endostatin and its mechanisms of action. Exp Cell Res. 2006;312(5):594-607. [PubMed ID: 16376330]. https://doi.org/10.1016/j.yexcr.2005.11.015.
-
10.
Huang X, Wong MK, Zhao Q, Zhu Z, Wang KZ, Huang N, et al. Soluble recombinant endostatin purified from Escherichia coli: antiangiogenic activity and antitumor effect. Cancer Res. 2001;61(2):478-81. [PubMed ID: 11212235].
-
11.
O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88(2):277-85. [PubMed ID: 9008168].
-
12.
Chamani R, Asghari SM, Alizadeh AM, Eskandari S, Mansouri K, Khodarahmi R, et al. Engineering of a disulfide loop instead of a Zn binding loop restores the anti-proliferative, anti-angiogenic and anti-tumor activities of the N-terminal fragment of endostatin: Mechanistic and therapeutic insights. Vascul Pharmacol. 2015;72:73-82. [PubMed ID: 26187352]. https://doi.org/10.1016/j.vph.2015.07.006.
-
13.
Lin KY, Guarnieri FG, Staveley-O'Carroll KF, Levitsky HI, August JT, Pardoll DM, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21-6. [PubMed ID: 8548765].
-
14.
Yoon SS, Eto H, Lin CM, Nakamura H, Pawlik TM, Song SU, et al. Mouse endostatin inhibits the formation of lung and liver metastases. Cancer Res. 1999;59(24):6251-6. [PubMed ID: 10626820].
-
15.
Meshkat Z, Soleimanjahi H, Mahmoudi M, Hassan ZM, Mirshahabi H, Meshkat M, et al. CTL responses to a DNA vaccine encoding E7 gene of human papillomavirus type 16 from an Iranian isolate. Iran J Immunol. 2008;5(2):82-91. [PubMed ID: 18523353].
-
16.
Farzanehpour M, Soleimanjahi H, Hassan ZM, Amanzadeh A, Ghaemi A, Fazeli M. HSP70 modified response against HPV based tumor. Eur Rev Med Pharmacol Sci. 2013;17(2):228-34. [PubMed ID: 23377813].
-
17.
Mirshahabi H, Soleimanjahi H, Pourpak Z, Meshkat Z, Hassan ZM. Production of human papilloma virus type 16 e6 oncoprotein as a recombinant protein in eukaryotic cells. Iran J Cancer Prev. 2012;5(1):16-20. [PubMed ID: 25780534].
-
18.
Schiller JT, Castellsague X, Villa LL, Hildesheim A. An update of prophylactic human papillomavirus L1 virus-like particle vaccine clinical trial results. Vaccine. 2008;26 Suppl 10:K53-61. [PubMed ID: 18847557]. https://doi.org/10.1016/j.vaccine.2008.06.002.
-
19.
Fazeli M, Soleimanjahi H, Dadashzadeh S. Further Stimulation of Cellular Immune Responses through Association of HPV-16 E6, E7 and L1 Genes in order to produce more Effective Therapeutic DNA Vaccines in Cervical Cancer Model. Iran J Cancer Prev. 2015;8(1):18-23. [PubMed ID: 25821567].
-
20.
Diniz MO, Lasaro MO, Ertl HC, Ferreira LC. Immune responses and therapeutic antitumor effects of an experimental DNA vaccine encoding human papillomavirus type 16 oncoproteins genetically fused to herpesvirus glycoprotein D. Clin Vaccine Immunol. 2010;17(10):1576-83. [PubMed ID: 20739505]. https://doi.org/10.1128/CVI.00264-10.
-
21.
Hattis D, Goble R, Chu M. Age-related differences in susceptibility to carcinogenesis. II. Approaches for application and uncertainty analyses for individual genetically acting carcinogens. Environ Health Perspect. 2005;113(4):509-16. [PubMed ID: 15811844].