Abstract
Context:
Breast cancer has been considered as one of the most common types of cancer among the women worldwide, and patients with breast neoplasms have been reported with high prevalence of low serum 25-hydroxyvitamin D levels.Objectives:
Our aim was to evaluate the correlation of plasma 25-hydroxyvitamin D deficiency with breast neoplasms risk among women.Data Sources:
PubMed database was searched with MeSH (medical subject headings) keywords "vitamin D AND breast neoplasms" which was restricted by original articles written only in English and published from January 1, 2014.Study Selection:
To find the articles that met eligibility criteria, titles and abstracts were examined.Data Extraction:
This systematic review was conducted according to PRISMA (preferred reporting items for systematic reviews and meta-analyses) statement. Critical appraising of evidence was performed, using the study quality assessment tools of national institutes of health, national heart, lung and blood institute (NHLBI).Results:
Overall, 76 potential articles were identified and after screening, 13 articles met eligible criteria for inclusion. Definition of low vitamin D levels varied greatly among studies, making comparisons difficult, but most of them have defined deficiency as 25(OH)D < 20 ng/mL. Evidence was mainly of fair quality.Conclusions:
This study has provided evidence that vitamin D deficiency has been very prevalent in patients with breast neoplasms, more than comparable matched control population, and risk of breast cancer has increased with low vitamin D levels, suggesting the need for high quality studies that assessed the health consequences attributable to vitamin D deficiency employing standard definitions.Keywords
1. Context
Breast cancer has been considered as the most common type of cancer among the women, within 161 countries ,and the most common cause for cancer deaths, within 98 countries (1).
Vitamin D (25-hydroxyvitamin D) deficiency has been known as a worrying public health problem for its association with musculoskeletal, immune system, cardiovascular and mental health (2-4). Reports have shown that breast cancer patients have a high prevalence of vitamin D deficiency (5-7). Vitamin D is influenced by many factors such as old age, high body mass index (BMI), high latitude, cold seasons, low sunlight exposure, and dark skin pigmentation which are associated with hypovitaminosis D (8). Vitamin D enters the body either from sunlight exposure or through both diet and dietary supplements. Ultraviolet B (UVB) irradiation (290-315 nm) through the skin, converts 7-dehydrocholesterol to pre-vitamin D3, which is converted to vitamin D3 and released into the circulation where the majority is quickly hydroxylated in the liver by cytochrome P-450-dependent enzyme. The product of this enzymatic modification, 25-hydroxyvitamin D3 is the major circulating D3 derivative that is used to measure serum vitamin D status. In the renal proximal convoluted tubule, 25-hydroxyvitamin D is hydroxylated to its biologically active metabolite, 1, 25-dihydroxyvitamin D (Calcitriol) (2, 9). Calcitriol exerts its actions by binding to a nuclear receptor protein, the vitamin D receptor (VDR) (10). VDR is active in virtually all tissues including breast and also in cancer cells (11). That has been the reason that suboptimal vitamin D levels might lead to cancer development through impairment of cell proliferation, differentiation, apoptosis, and angiogenesis (12). Biological and epidemiological data have revealed the protective functions of vitamin D against different cancers especially breast cancer (13-15) and the potential role of VDR gene polymorphisms and risk of cancer (16-18). Interestingly, it was found that people with higher vitamin D levels have shown reduced incidence of breast cancer (19, 20).
The association of vitamin D deficiency with the risk of breast cancer has been described among breast cancer patients, although most of the study groups were insufficient and definitions were heterogeneous.
2. Objectives
We have aimed to evaluate articles that have assessed the prevalence of vitamin D deficiency among women with breast cancer in comparison with healthy population, adjusted the deficiency definitions, and to specify whether it is correlated with increased risk of breast neoplasms.
3. Data Sources
The initial search was performed with the medical subject headings (MeSH) keywords "vitamin D AND breast neoplasms" using PubMed database to identify articles eligible for this review. The limitations were: 1, English language; 2, publication date from January 1, 2014. In the screening process, review articles and case-reports were excluded. We have also examined the reference list of all relevant articles.
4. Study Selection
To find the articles that met eligibility criteria, titles and abstracts were examined. Those articles which have evaluated serum 25-hydroxyvitamin D in females with breast neoplasms were included. The following data for each eligible study were summarized; design, age, sample size, the outcomes studied, definition of vitamin D deficiency, and the applicable results.
5. Data Extraction
This systematic review was performed in accordance with the PRISMA (Preferred reporting items for systematic reviews and meta-analyses) statement (21).
Critical appraising of evidence was performed, using the study quality assessment tools of national institutes of health, national heart, lung and blood institute (NHLBI). Studies with low risk of bias were determined as good quality, high risk of bias as poor quality, and moderate risk of bias as fair quality.
6. Results
6.1. Study Selection
Overall, 76 records were identified through database searching using the MeSH keywords as previously described. Two additional records were identified through from reference list but they were already in our identified articles list. After duplicates removal, 76 records were screened. After screening 56 records were excluded, including three articles which were published in other languages besides English, one case-report, twenty nine review articles and twenty three experimental studies. Eventually, 20 full-text articles have assessed for eligibility. Four articles evaluated vitamin D receptor polymorphisms but did not measure vitamin D levels, and three articles did not study breast cancer. At last, 13 studies were included that measured the association of vitamin D deficiency with breast cancer (Figure 1).
Association of Vitamin D Deficiency With Breast Cancer
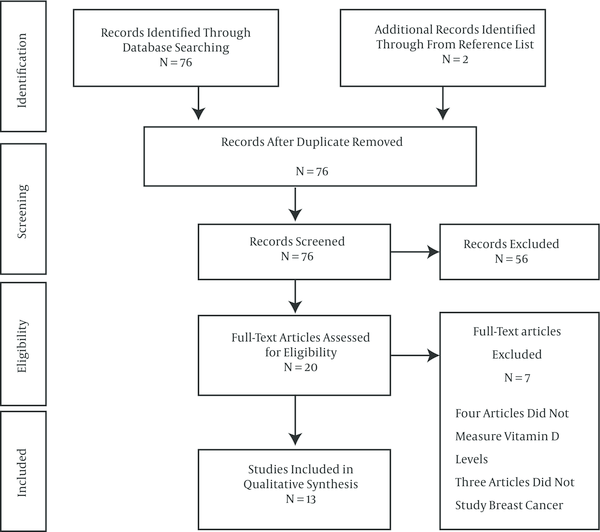
6.2. Study Characteristics
Four studies were conducted in the United States (6, 22-24), three in Iran (7, 25, 26). The remaining studies were conducted in Egypt (27), Thailand (28), Korea (29), Pakistan (30), China (31) and Turkey (7). Eleven studies were case-control (6, 22-27, 29-32) and two cross-sectional (7, 28). Measuring serum 25-hydroxyvitamin D was the primary outcome of most of the papers (6, 7, 22, 24, 26-32). Most of the articles were conducted among premenopausal/postmenopausal population (6, 7, 22, 23, 25-31), one article has studied postmenopausal women (24) and one article has studied premenopausal women (32). Ten of studies have compared vitamin D levels with control groups (6, 22-27, 29, 30, 32) and three of them have studied prevalence of vitamin D deficiency/insufficiency among breast cancer patients (7, 28, 31). In majority of researches vitamin D deficiency was defined as 25(OH)D < 20 ng/mL and insufficiency as 20 to 39 ng/mL. Evidence was mainly of fair quality.
6.3. Results of Individual Studies
A study by Abdelgawad et al. (27), assessed 25(OH)D, calcium, phosphorus, magnesium, and parathormone levels in 98 newly diagnosed adult female patients with breast cancer, ages 30 to 80 years, who have presented to the medical oncology department Cairo university, Cairo, Egypt. Forty nine age-matched healthy female volunteers have enrolled as the control group. Vitamin D deficiency was defined as serum level < 20 ng/mL, but the insufficiency as 20 - 39 ng/mL. Vitamin D deficiency was seen in 67% of cases while 49% of the control group were deficient with the median level significantly lower in the breast cancer group (P = 0.044).
A study in Iran by Colagar et al. (25), has assessed vitamin D receptor polymorphism and 25(OH)D status in 134 breast cancer cases and a control group consisting of 127 healthy women. The mean ages for the case and control groups were almost similar (48.72 ± 9.60 vs. 47.04 ± 12.07 years). Researchers have found that lower levels of the 25(OH)D (< 14 ng/mL), were associated with susceptibility to breast cancer (P < 0.001). The secondary outcome has revealed that poly(A)L allele in the presence of low 25-hydroxyvitamin D status were more susceptible to breast cancer.
Park et al. (29), have studied 3,634 breast cancer cases and 17,133 controls, ages 20 to more than 80 years, in Korea. Vitamin D was defined as deficient if serum 25-hydroxyvitamin D < 20 ng/mL. The mean serum 25-hydroxyvitamin D of cases was significantly less than controls (15.1 ± 7.2 vs. 17.1 ± 6.3 ng/mL). Women with vitamin D deficiency had 27% increased risk of breast cancer, in comparison with women by sufficient levels of serum 25-hydroxyvitamin D.
In a study of 25(OH)D status and mammographic density among women living in the United States, Bertrand et al. (22) have assessed 493 newly diagnosed breast cancer patients and 835 matched controls, ages 32 to 58 years. They have found that Women in the highest tertile of percent mammographic density and lowest quartile of 25(OH)D (1.95 - 19.8 ng/mL), had a > 60% increased risk of breast cancer, in comparison with the women with low mammographic density and high 25(OH)D.
A study by Reimers et al. (23), has evaluated the association of vitamin D-related genetic polymorphisms and plasma 25-hydroxyvitamin D with risk of breast cancer among 967 incident breast cancer cases and 993 controls, mean age of 58.6 years, in the United States. Plasma 25(OH) D was divided into two categories (< 19.1 and ≥ 19.1 ng/mL), based on the lowest quartile of 25(OH)D versus all above. Authors have found that breast cancer risk was reduced among women with the homozygous common allele with plasma 25-hydroxyvitamin D ≥ 19.1 ng/mL in comparison with those with 25-hydroxyvitamin D < 19.1 ng/mL.
Imtiaz et al. (30), have conducted a study of vitamin D levels in 90 newly diagnosed breast cancer patients (mean age 47.5 ± 9.8 years) and 90 age-matched healthy females in Lahore, Pakistan. The mean serum 25(OH)D level in cases was significantly lower than control group (9.3 ng/mL vs 14.9 ng/mL) (P < 0.001). Vitamin D deficiency (< 20 ng/mL) was reported among 95.6% of cases and 77% of control group (P < 0.001). Vitamin D insufficiency (20 - 39 ng/mL) was seen in 4.4% of the patients and 18.9% of healthy females (P < 0.001).
A report by Alipour et al. (26), has compared 25(OH)D levels in 308 women with benign and malignant breast tumors (mean age 44.2 years) and 364 controls in Iran. Serum levels between 25 - 35 ng/mL, 12.5 - 25 ng/mL and < 12.5 ng/mL were considered as mild, moderate and severe vitamin D deficiency, respectively. In this study the median serum 25(OH)D level in the case group was 7.7 ng/mL and in control group was 8.7 ng/mL. The secondary outcome has demonstrated that the median serum levels of 25(OH)D were higher in benign, in comparison with malignant cases (7.9 ng/mL vs. 7 ng/mL).
In a study of five race/ethnic groups from the United States conducted by Kim et al. (24), vitamin D levels were examined among 707 postmenopausal breast cancer cases (mean age 68.5 years) and matched controls. In white population, 25-hydroxyvitamin D deficiency (< 20 ng/mL) has caused a 7.5 times greater risk of breast cancer, in comparison with the females with plasma 25(OH)D ≥ 20 ng/mL.
Bidgoli et al. (32), has studied 25(OH)D levels in 60 newly diagnosed premenopausal females with breast cancer (mean age 36.45 ± 7.02 years) and 116 normal women as control group in Sabzevar, Iran. The mean concentrations of 25(OH)D was 15.17 ± 8.15 ng/mL in cases and 15.47 ± 7.45 ng/mL in control group and more than 95% of individuals in each group had vitamin D deficiency. Though, authors have detected that lack of vitamin D supplements intake might increase the risk of premenopausal breast cancer.
Wang et al. (6), have evaluated serum 25(OH)D level, vitamin D binding protein and risk of breast cancer in 584 cases (mean age 45.1 years) and matched controls in the United States. calculated free 25-hydroxyvitamin D were 62.6 nmol/L in cases and 61.4 nmol/L in controls, in which no association was observed between free 25(OH)D and risk of breast cancer.
The final three studies have assessed the prevalence of vitamin D deficiency, but have not evaluated the association of vitamin D levels with breast cancer risk. A report by Thanasitthichai et al. (28), has examined 200 cases of newly diagnosed breast cancer patients (105 patients aged ≤ 50 and 95 patients > 50 years) at national cancer institute of Thailand. Low 25-hydroxyvitamin D levels (< 32 ng/mL) have been detected in 93% of cases. The secondary outcome has shown that 25(OH)D < 32 ng/mL has significantly detected in cases with poor prognosis and higher stage of the disease (P = 0.036), positive nodal involvement (P = 0.030) and larger tumor size (P = 0.038).
In a study in Turkey by Alco et al. (7), the prevalence of low 25-hydroxyvitamin D levels in 186 women with breast cancer, ages 27 to 79 years, has been assessed. Serum 25(OH)D levels were categorized as deficient (< 10 ng/ mL) and insufficient (10 - 24 ng/mL). Authors have reported that 25% of patients were deficient and 45% had insufficient 25(OH)D level.
A study by Shi et al. (31), has examined 25-Hydroxyvitamin D among 1,940 Chinese breast cancer patients, ages 22 to 77 years. Vitamin D status was categorized as deficient (< 30 nmol/L) and insufficient (30 – 50 nmol/L). Approximately 23% of patients were vitamin D deficient and 48% were vitamin D insufficient.
7. Discussion
7.1. Summary of Evidence
This study has suggested that vitamin D deficiency would be very prevalent in patients with breast neoplasms, more than comparable matched control population, and risk of breast cancer has increased with low Vitamin D levels.
The main findings have been summarized and shown in (Table 1). Serum 25-hydroxyvitamin D concentration has been known as a significant predictor of breast cancer risk. Park et al. (29) have found that serum 25-hydroxyvitamin D < 20 ng/mL was associated with 27% increased risk of breast cancer. Similar results have been reported by Colagar et al., Bertrand et al., Reimers et al. and Kim et al. (22-25). The prevalence of vitamin D deficiency in breast cancer population has ranged from 23% to 95.6%.
Summary of Evidence
| Author(s) | Year | Design | Cases, N | Controls, N | Age, Y | Outcomes Studied | Vitamin D Deficiency Definitions | Results | Quality |
|---|---|---|---|---|---|---|---|---|---|
| 2015 | Case-Control | 98 | 49 | 30 to 80 | 25-OH vitamin D, calcium, phosphorus, magnesium, parathormone | Deficiency: < 20 ng/mL | Vitamin D deficiency was seen in 67% of cases and 49% of controls (P = 0.044) | Fair | |
| 2015 | Case-Control | 134 | 127 | Mean (Range) = 48.7 (39.1 to 59.3) | VDR gene polymorphism, 25-OH vitamin D | Deficiency: < 14 ng/mL | lower levels of vitamin D was associated with susceptibility to breast cancer (P < 0.001) | Fair | |
| 2015 | Case-Control | 3634 | 17133 | 20 to 80 | 25-OH vitamin D | Deficiency: < 20 ng/mL | Mean serum 25(OH)D of cases was 15.1 ± 7.2 and in controls was 17.1 ± 6.3 ng/mL. Women with Vitamin D deficiency had 27% increased risk of breast cancer. | Good | |
| 2015 | Case-Control | 493 | 835 | 32 to 58 | 25-OH vitamin D, mammographic density | Deficiency: < 19.8 ng/mL | Women in the highest tertile of percent mammographic density and lowest quartile of 25(OH)D (1.95 - 19.8 ng/mL), had a > 60% increased risk of breast cancer | Good | |
| 2015 | Case-Control | 967 | 993 | Mean= 58.6 | vitamin D-related genetic polymorphisms, 25-OH vitamin D | Deficiency: < 19.1 ng/mL | Reduced risk of breast cancer has seen with the homozygous common allele with plasma 25(OH)D ≥ 19.1 ng/mL. | Fair | |
| 2015 | Case-Control | 90 | 90 | Mean (Range) = 47.5 (37.7 to 57.3) | 25-OH vitamin D | Deficiency: < 20 ng/mL | Vitamin D deficiency was reported in 95.6% of cases and 77% of the control (P < 0.001). | Good | |
| 2014 | Case-Control | 308 | 364 | Mean = 44.2 | 25-OH vitamin D | Deficiency: mild 25 – 35; moderate 12.5 – 25; severe < 12.5 ng/mL | Median serum vitamin D level in the case group was 7.7 ng/mL and 8.7 ng/mL in the control group. | Fair | |
| 2014 | Case-Control | 707 | 707 | Mean = 68.5 | 25-OH vitamin D | Deficiency: < 20 ng/mL | Vitamin D deficiency caused a 7.5 times greater risk of breast cancer. | Good | |
| 2014 | Case-Control | 60 | 116 | Mean (Range) = 36.45 (29.43 to 43.47) | 25-OH vitamin D | Deficiency: < 20 ng/mL | 95% of both groups were Vitamin D deficient. | Fair | |
| 2014 | Case-Control | 584 | 584 | Mean= 45.1 | 25-OH vitamin D, vitamin D binding protein | Mean 25(OH)D was 62.6 nmol/Lin cases vs 61.4 nmol/L in controls. | Fair | ||
| 2015 | Cross-Sectional | 200 | 105 patients ≤ 50; 95 patients > 50 | 25-OH vitamin D | Deficiency: < 32 ng/mL | 93% of patients were vitamin D deficient. 25(OH)D < 32 ng/mL was significantly detected in cases with poor prognosis and higher stage of the disease (P = 0.036), positive nodal involvement (P = 0.030) and larger tumor size (P = 0.038). | Fair | ||
| 2014 | Cross-Sectional | 186 | 27 to 79 | 25-OH vitamin D | Deficiency: < 10 ng/mL; insufficiency: 10 - 24 ng/mL | 70% of patients were vitamin D deficient/insufficient. | Poor | ||
| 2014 | Case-Control | 1940 | 22 to 77 | 25-OH vitamin D | Deficiency: < 30 nmol/L; insufficiency: 30 - 50 nmol/L | 72% of patients were vitamin D deficient/insufficient. | Fair |
Two studies have not supported the key role of vitamin D in breast cancer. First study by Wang et al. (6), detected no significant differences between mean serum 25(OH)D levels in cases and controls (62.6 nmol/L vs 61.4 nmol/L) which has not supported by other evidence. The second by Bidgoli et al. (32), has reported 95% of both cases and controls had vitamin D deficiency. Although, the latter found that lack of vitamin D supplements intake might increase the risk of premenopausal breast cancer. Lack of association between vitamin D deficiency and breast cancer in the study by Bidgoli et al. (32), was probably due to great prevalence of vitamin D deficiency in that area.
7.2. Limitations
We have only identified 13 studies which mainly had small sample sizes and were highly variable in quality. There was insufficient evidence to accurately determine prevalence rates of vitamin D deficiency/insufficiency and risk assessment in the breast cancer population. There were variable definitions of deficiency and insufficiency used in these studies making comparisons difficult.
7.3. Conclusions
This systematic review has represented evidence for the prevalence of low vitamin D levels in breast cancer population worldwide, and investigated its association with cancer risk. We have observed the possibility of a high prevalence of vitamin D deficiency and insufficiency in patients with breast cancer and increased risk of cancer with vitamin D deficiency. There is a need for future high-quality studies that assess the health consequences attributable to vitamin D deficiency employing standard definitions.
Acknowledgements
References
-
1.
Global Burden of Disease Cancer C, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1(4):505-27. [PubMed ID: 26181261]. https://doi.org/10.1001/jamaoncol.2015.0735.
-
2.
Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266-81. [PubMed ID: 17634462]. https://doi.org/10.1056/NEJMra070553.
-
3.
Meems LM, de Borst MH, Postma DS, Vonk JM, Kremer HP, Schuttelaar ML, et al. Low levels of vitamin D are associated with multimorbidity: results from the LifeLines Cohort Study. Ann Med. 2015;47(6):474-81. [PubMed ID: 26340085]. https://doi.org/10.3109/07853890.2015.1073347.
-
4.
Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, et al. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality-a review of recent evidence. Autoimmun Rev. 2013;12(10):976-89. [PubMed ID: 23542507]. https://doi.org/10.1016/j.autrev.2013.02.004.
-
5.
Obaidi J, Musallam E, Al-Ghzawi HM, Azzeghaiby SN, Alzoghaibi IN. Vitamin D and its relationship with breast cancer: an evidence based practice paper. Glob J Health Sci. 2015;7(1):261-6. [PubMed ID: 25560331]. https://doi.org/10.5539/gjhs.v7n1p261.
-
6.
Wang J, Eliassen AH, Spiegelman D, Willett WC, Hankinson SE. Plasma free 25-hydroxyvitamin D, vitamin D binding protein, and risk of breast cancer in the Nurses' Health Study II. Cancer Causes Control. 2014;25(7):819-27. [PubMed ID: 24748579]. https://doi.org/10.1007/s10552-014-0383-5.
-
7.
Alco G, Igdem S, Dincer M, Ozmen V, Saglam S, Selamoglu D, et al. Vitamin D levels in patients with breast cancer: importance of dressing style. Asian Pac J Cancer Prev. 2014;15(3):1357-62. [PubMed ID: 24606465].
-
8.
Mithal A, Wahl DA, Bonjour JP, Burckhardt P, Dawson-Hughes B, Eisman JA, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807-20. [PubMed ID: 19543765]. https://doi.org/10.1007/s00198-009-0954-6.
-
9.
Henry HL. Regulation of vitamin D metabolism. Best Pract Res Clin Endocrinol Metab. 2011;25(4):531-41. [PubMed ID: 21872796]. https://doi.org/10.1016/j.beem.2011.05.003.
-
10.
Racz A, Barsony J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J Biol Chem. 1999;274(27):19352-60. [PubMed ID: 10383447].
-
11.
Tagliabue E, Raimondi S, Gandini S. Vitamin D, Cancer Risk, and Mortality. Adv Food Nutr Res. 2015;75:1-52. [PubMed ID: 26319903]. https://doi.org/10.1016/bs.afnr.2015.06.003.
-
12.
de Lyra EC, da Silva IA, Katayama ML, Brentani MM, Nonogaki S, Goes JC, et al. 25(OH)D3 and 1,25(OH)2D3 serum concentration and breast tissue expression of 1alpha-hydroxylase, 24-hydroxylase and Vitamin D receptor in women with and without breast cancer. J Steroid Biochem Mol Biol. 2006;100(4-5):184-92. [PubMed ID: 16828283]. https://doi.org/10.1016/j.jsbmb.2006.04.009.
-
13.
Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, Mohr SB, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252-61. [PubMed ID: 16380576]. https://doi.org/10.2105/AJPH.2004.045260.
-
14.
Ness RA, Miller DD, Li W. The role of vitamin D in cancer prevention. Chin J Nat Med. 2015;13(7):481-97. [PubMed ID: 26233839]. https://doi.org/10.1016/S1875-5364(15)30043-1.
-
15.
Holick MF. Vitamin D: its role in cancer prevention and treatment. Prog Biophys Mol Biol. 2006;92(1):49-59. [PubMed ID: 16566961]. https://doi.org/10.1016/j.pbiomolbio.2006.02.014.
-
16.
Touvier M, Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, et al. Meta-analyses of vitamin D intake, 25-hydroxyvitamin D status, vitamin D receptor polymorphisms, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2011;20(5):1003-16. [PubMed ID: 21378269]. https://doi.org/10.1158/1055-9965.EPI-10-1141.
-
17.
Mun MJ, Kim TH, Hwang JY, Jang WC. Vitamin D receptor gene polymorphisms and the risk for female reproductive cancers: A meta-analysis. Maturitas. 2015;81(2):256-65. [PubMed ID: 25882760]. https://doi.org/10.1016/j.maturitas.2015.03.010.
-
18.
Johnson AL, Zinser GM, Waltz SE. Vitamin D3-dependent VDR signaling delays ron-mediated breast tumorigenesis through suppression of beta-catenin activity. Oncotarget. 2015;6(18):16304-20. [PubMed ID: 26008979]. https://doi.org/10.18632/oncotarget.4059.
-
19.
Garland CF, Gorham ED, Mohr SB, Garland FC. Vitamin D for cancer prevention: global perspective. Ann Epidemiol. 2009;19(7):468-83. [PubMed ID: 19523595]. https://doi.org/10.1016/j.annepidem.2009.03.021.
-
20.
Boeke CE, Tamimi RM, Berkey CS, Colditz GA, Giovannucci E, Malspeis S, et al. Adolescent dietary vitamin D and sun exposure in relation to benign breast disease. Cancer Causes Control. 2015;26(8):1181-7. [PubMed ID: 26084210]. https://doi.org/10.1007/s10552-015-0612-6.
-
21.
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-12. [PubMed ID: 19631508]. https://doi.org/10.1016/j.jclinepi.2009.06.005.
-
22.
Bertrand KA, Rosner B, Eliassen AH, Hankinson SE, Rexrode KM, Willett W, et al. Premenopausal plasma 25-hydroxyvitamin D, mammographic density, and risk of breast cancer. Breast Cancer Res Treat. 2015;149(2):479-87. [PubMed ID: 25543181]. https://doi.org/10.1007/s10549-014-3247-5.
-
23.
Reimers LL, Crew KD, Bradshaw PT, Santella RM, Steck SE, Sirosh I, et al. Vitamin D-related gene polymorphisms, plasma 25-hydroxyvitamin D, and breast cancer risk. Cancer Causes Control. 2015;26(2):187-203. [PubMed ID: 25421379]. https://doi.org/10.1007/s10552-014-0497-9.
-
24.
Kim Y, Franke AA, Shvetsov YB, Wilkens LR, Cooney RV, Lurie G, et al. Plasma 25-hydroxyvitamin D3 is associated with decreased risk of postmenopausal breast cancer in whites: a nested case-control study in the multiethnic cohort study. BMC Cancer. 2014;14:29. [PubMed ID: 24438060]. https://doi.org/10.1186/1471-2407-14-29.
-
25.
Colagar AH, Firouzjah HM, Halalkhor S. Vitamin D Receptor Poly(A) Microsatellite Polymorphism and 25-Hydroxyvitamin D Serum Levels: Association with Susceptibility to Breast Cancer. J Breast Cancer. 2015;18(2):119-25. [PubMed ID: 26155287]. https://doi.org/10.4048/jbc.2015.18.2.119.
-
26.
Alipour S, Hadji M, Hosseini L, Omranipour R, Saberi A, Seifollahi A, et al. Levels of serum 25-hydroxy-vitamin d in benign and malignant breast masses. Asian Pac J Cancer Prev. 2014;15(1):129-32. [PubMed ID: 24528013].
-
27.
Abdelgawad IA, El-Mously RH, Saber MM, Mansour OA, Shouman SA. Significance of serum levels of vitamin D and some related minerals in breast cancer patients. Int J Clin Exp Pathol. 2015;8(4):4074-82. [PubMed ID: 26097595].
-
28.
Thanasitthichai S, Chaiwerawattana A, Prasitthipayong A. Association of Vitamin D Level with Clinicopathological Features in Breast Cancer. Asian Pac J Cancer Prev. 2015;16(12):4881-3. [PubMed ID: 26163608].
-
29.
Park S, Lee DH, Jeon JY, Ryu J, Kim S, Kim JY, et al. Serum 25-hydroxyvitamin D deficiency and increased risk of breast cancer among Korean women: a case-control study. Breast Cancer Res Treat. 2015;152(1):147-54. [PubMed ID: 26037255]. https://doi.org/10.1007/s10549-015-3433-0.
-
30.
Imtiaz S, Siddiqui N. Vitamin-D status at breast cancer diagnosis: correlation with social and environmental factors and dietary intake. J Ayub Med Coll Abbottabad. 2014;26(2):186-90. [PubMed ID: 25603674].
-
31.
Shi L, Nechuta S, Gao YT, Zheng Y, Dorjgochoo T, Wu J, et al. Correlates of 25-hydroxyvitamin D among Chinese breast cancer patients. PLoS One. 2014;9(1). e86467. [PubMed ID: 24466109]. https://doi.org/10.1371/journal.pone.0086467.
-
32.
Bidgoli SA, Azarshab H. Role of vitamin D deficiency and lack of sun exposure in the incidence of premenopausal breast cancer: a case control study in Sabzevar, Iran. Asian Pac J Cancer Prev. 2014;15(8):3391-6. [PubMed ID: 24870727].