Abstract
Context:
Proton therapy has currently used to treat brain, spinal and prostate cancers, as well as Breast cancer. Proponents have cited the modality’s ability to spare healthy tissue, but critics have claimed the benefit gained from its use has not validated its cost in comparison with photon therapy. The aim of this study was evaluation of proton therapy cost effectiveness versus photon therapy, in the Breast Cancer management through a literature survey.Evidence Acquisition:
Standard search strategies covering the querying of available online databases (MEDLINE®, PubMed, EMBASE and Cochrane) have been applied. Database searches have conducted in an iterative manner during June–September 2015 to retrieve articles related to our policy. No specific key words have required as inclusion criteria; a relatively small number of studies exist on this topic, so a “bottom-up” search strategy has required.Results:
Three studies have modeled the costs and cost-effectiveness of PBT in breast cancer. The ICER has lied below the threshold for women at high risk of cardiac disease and so it could be judged cost-effective whereas it was above this threshold for other patients. The cost per QALY has gained would, however, be considerably lower if a population with high-risk of developing cardiac disease has treated.Conclusions:
It has concluded that proton therapy for breast cancer could be cost-effective if appropriate risk groups have chosen as targets for the therapy. Also the number of patient whose radiation therapy has considered in their treatment schedule, was other important factor which could affect the decision on PBT cost effectiveness.Keywords
1. Context
It has been reported that the risk of ischemic heart disease has increased in women with left breast cancer who have treated with radiotherapy (1-3). For women with left breast cancer and receiving post-mastectomy radiotherapy by X-ray, it would be a great challenge to reduce the dose to the heart if the internal mammary chain has included in the treatment field. The dosimetric comparisons between X-ray or X-ray/electron and proton beam have shown that proton is cardiac- and pulmonary-sparing (4) a report of early clinical outcome in 12 patients has shown that this application of proton therapy was feasible and well tolerated (5).
Protons were positively-charged subatomic particles that have been in clinical use as a form of external beam radiotherapy for over 60 years. In comparison with the photon X-ray energy that has used in conventional radiotherapy, proton beams have physically attributed that have been potentially appealing. Specifically, protons have deposited radiation energy at or around the target, at the end of the range of beam penetration, a phenomenon has known as the Bragg peak (6). In contrast, photons deliver radiation across tissue had depth on the way toward the target tumor. The total radiation dose for proton therapy has delivered in the “spread out Bragg peak” (SOBP) region from multiple proton beams; proton radiation has delivered to the target tumor as well as to shallow tissue depths before the target, but not to deeper tissue depths beyond the target (7). Initial use of proton beam therapy (PBT) has focused on conditions where sparing very sensitive adjacent normal tissues has felt to be of utmost importance, such as cancers or noncancerous malformations of the brain stem, eye, or spinal cord. More recently, however, the use of PBT has expanded in many settings to treat more common cancers such as breast. Proton therapy has offered a number of other compelling benefits in the management of breast cancer: treatment was noninvasive and painless, proton therapy was effective for treating early stage breast cancer, treatment has quickly offered recovery times with minimal side effects, caused less cosmetic damage in comparison with the burn marks caused by regular radiation, it was more accurate and precise than other kinds of radiation and treatment has provided in an outpatient. Regarding the above mentioned advantages, the construction of proton centers has grown substantially over the world. However, while enthusiasm for using PBT has grown in recent years, there have remained uncertainties regarding its cost-effectiveness under question. The aim of this study was evaluation of proton therapy cost effectiveness versus photon therapy, in the Breast Cancer management through a literature survey.
2. Evidence Acquisition
We have focused primary attention on studies that have involved explicit in comparisons with PBT to one or more treatment alternatives and measures cost effectiveness. Based on input from our expert advisors and practical considerations, we have developed literature review methods that included: inclusion and exclusion criteria to identify potentially relevant articles, search strategies to retrieve articles, abstract review protocols, and a system of scoring published studies for completeness.
This article has provided evidences regarding the key questions above, the article had to address one of the predictor variables. The objective of our search strategy was to identify all published papers and all ongoing research concerning the cost and cost-effectiveness of PBT in the management of patient suffering from Breast cancer. For the literature review, we have used standard search strategies involving the querying of available online databases (MEDLINE®, PubMed, EMBASE and Cochrane). Database searches have conducted in an iterative manner during June–September 2015 to retrieve articles related to our policy. Search terms included “Proton Therapy,” “Breast Cancer,” “Cost,” and “Cost-effectiveness.” No specific key words have required as inclusion criteria; a relatively small number of studies have existed on this topic, so a “bottom-up” search strategy has required. The reference lists of each article have reviewed in details to find additional articles. Each article have reviewed independently in full text, the relevance of retrieved articles have evaluated, and recorded the main findings of each study in a table. Finally, published studies of the economic impact of PBT have summarized in response to the question of this study regarding the costs and cost-effectiveness of PBT in the management of patient suffering from Breast cancer.
3. Results
Due to the higher capital investment have needed for the construction of a proton facility and operating costs, an average course of proton therapy has estimated to be about 2 - 3 times the cost of IMRT (8-12). With the more popular use of proton treatment, the cost-effectiveness comparisons between proton and X-ray treatment have attracted a lot of attention, especially on some specific sites such as left side breast cancer (13).
Three studies have modeled the costs and cost-effectiveness of PBT in breast cancer. One U.S.-based study has examined reimbursement for treatment with 3D-conformal partial breast irradiation using protons or photons vs. traditional whole breast irradiation (14). Payments have included those of treatment planning and delivery as well as patient time and transport. Total per-patient costs had substantially higher for PBT vs. photon partial irradiation ($13,200 vs. $5,300) but only modestly increased relative to traditional whole breast irradiation ($10,600), as the latter incurred higher professional service fees and involved a greater amount of patient time.
Two additional studies from the same group have assessed the cost-effectiveness of PBT vs. photon radiation among women with left-sided breast cancer in Sweden (15, 16). In the first of these, the cost-effectiveness of proton therapy in the treatment of women with left-sided breast cancer has assessed. A Markov cohort simulation model has used to simulate the life of patients diagnosed with breast cancers and treated with radiation. Cost and quality has adjusted life years (QALYs) were the primary outcome measures. In this study photon radiation has assumed to increase the risk of ischemic and other cardiovascular disease as well as Pneumonitis relative to PBT (15); clinical effectiveness has assumed to be identical. Reductions in adverse events have led to a gain in quality-adjusted life years (QALYs) (One QALY equates to one year in perfect health). Equivalent to approximately one month (12.35 vs. 12.25 for photon). Costs of PBT were nearly triple those of photon therapy, however ($11,124 vs. $4,950), leading to an incremental cost-effectiveness ratio (ICER) (ICER is synonymous with the cost per quality-adjusted life year (QALY) gained. It is defined by the difference in cost between two possible interventions, divided by the difference in their effect) of $65,875 per QALY gained.
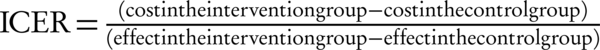
The other study has used essentially the same model but has focused attention only on women at high risk of cardiac disease (43% higher than general population) (16). In this instance, a lower ICER has observed ($33,913 per QALY gained). National Institute for Health and Care Excellence (NICE) has adopted a nominal cost-per-QALY (ICER) threshold of £20,000 to £30,000 equal to 30800 to 46200 (GBP/USD has assumed approximately 1.54). The ICER lies below the threshold for women at high risk of cardiac disease and so it could be judged cost-effective whereas it was above this threshold for other patients. The cost per QALY gained would, however, be considerably lower if a population with high-risk of developing cardiac disease has treated.
4. Conclusions
Proton beam therapy (PBT) has used for clinical purposes for over 50 years, and has delivered to tens of thousands of patients with a variety of cancers and noncancerous conditions. The clinical benefits of proton therapy have recognized in reducing side effects when in comparison with photon therapy, but the significant expense of building and maintaining proton facilities and the high treatment costs have been areas of concern. The study’s results have demonstrated that by avoiding years of costly side effects, proton therapy could be cost-effective for Breast cancer providing the yearly cost for treating radiation related side effects was noticeable. Using current risk has estimated the data on required capital investments, proton therapy for breast cancer treatment was only cost-effective in the situation that standard photon radiation leaded to significant side effects e.g. women at high risk of cardiac disease. It could be concluded that proton therapy for breast cancer could be cost-effective if appropriate risk groups have chosen as targets for the therapy. Also the number of patient who radiation therapy has considered in their treatment schedule was other important factor which could affect the decision on PBT cost effectiveness.
Acknowledgements
References
-
1.
Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Bronnum D, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368(11):987-98. [PubMed ID: 23484825]. https://doi.org/10.1056/NEJMoa1209825.
-
2.
Giordano SH, Kuo YF, Freeman JL, Buchholz TA, Hortobagyi GN, Goodwin JS. Risk of cardiac death after adjuvant radiotherapy for breast cancer. J Natl Cancer Inst. 2005;97(6):419-24. [PubMed ID: 15770005]. https://doi.org/10.1093/jnci/dji067.
-
3.
Chargari C. The issue of radiation-induced cardiovascular toxicity: preclinical highlights and perspectives on preventive strategies. Biomed J. 2013;36(3):150-1. [PubMed ID: 23806886]. https://doi.org/10.4103/2319-4170.113235.
-
4.
MacDonald SM, Jimenez R, Paetzold P, Adams J, Beatty J, DeLaney TF, et al. Proton radiotherapy for chest wall and regional lymphatic radiation; dose comparisons and treatment delivery. Radiat Oncol. 2013;8:71. [PubMed ID: 23521809]. https://doi.org/10.1186/1748-717X-8-71.
-
5.
MacDonald SM, Patel SA, Hickey S, Specht M, Isakoff SJ, Gadd M, et al. Proton therapy for breast cancer after mastectomy: early outcomes of a prospective clinical trial. Int J Radiat Oncol Biol Phys. 2013;86(3):484-90. [PubMed ID: 23523326]. https://doi.org/10.1016/j.ijrobp.2013.01.038.
-
6.
Larsson B, Leksell L, Rexed B, Sourander P, Mair W, Andersson B. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182(4644):1222-3. [PubMed ID: 13590280].
-
7.
Levin WP, Kooy H, Loeffler JS, DeLaney TF. Proton beam therapy. Br J Cancer. 2005;93(8):849-54. [PubMed ID: 16189526]. https://doi.org/10.1038/sj.bjc.6602754.
-
8.
Goitein M, Jermann M. The relative costs of proton and X-ray radiation therapy. Clin Oncol (R Coll Radiol). 2003;15(1):S37-50. [PubMed ID: 12602563].
-
9.
Lievens Y, Pijls-Johannesma M. Health economic controversy and cost-effectiveness of proton therapy. Semin Radiat Oncol. 2013;23(2):134-41. [PubMed ID: 23473691]. https://doi.org/10.1016/j.semradonc.2012.11.005.
-
10.
Mailhot Vega RB, Kim J, Bussiere M, Hattangadi J, Hollander A, Michalski J, et al. Cost effectiveness of proton therapy compared with photon therapy in the management of pediatric medulloblastoma. Cancer. 2013;119(24):4299-307. [PubMed ID: 24105630]. https://doi.org/10.1002/cncr.28322.
-
11.
Peeters A, Grutters JP, Pijls-Johannesma M, Reimoser S, De Ruysscher D, Severens JL, et al. How costly is particle therapy? Cost analysis of external beam radiotherapy with carbon-ions, protons and photons. Radiother Oncol. 2010;95(1):45-53. [PubMed ID: 20106540]. https://doi.org/10.1016/j.radonc.2009.12.002.
-
12.
Pijls-Johannesma M, Pommier P, Lievens Y. Cost-effectiveness of particle therapy: current evidence and future needs. Radiother Oncol. 2008;89(2):127-34. [PubMed ID: 18707784]. https://doi.org/10.1016/j.radonc.2008.07.015.
-
13.
Epstein K. Is spending on proton beam therapy for cancer going too far, too fast? BMJ. 2012;344. e2488. [PubMed ID: 22511301]. https://doi.org/10.1136/bmj.e2488.
-
14.
Taghian AG, Kozak KR, Katz A, Adams J, Lu HM, Powell SN, et al. Accelerated partial breast irradiation using proton beams: Initial dosimetric experience. Int J Radiat Oncol Biol Phys. 2006;65(5):1404-10. [PubMed ID: 16730137]. https://doi.org/10.1016/j.ijrobp.2006.03.017.
-
15.
Lundkvist J, Ekman M, Ericsson SR, Isacsson U, Jonsson B, Glimelius B. Economic evaluation of proton radiation therapy in the treatment of breast cancer. Radiother Oncol. 2005;75(2):179-85. [PubMed ID: 15885828]. https://doi.org/10.1016/j.radonc.2005.03.006.
-
16.
Lundkvist J, Ekman M, Ericsson SR, Jonsson B, Glimelius B. Proton therapy of cancer: potential clinical advantages and cost-effectiveness. Acta Oncol. 2005;44(8):850-61. [PubMed ID: 16332592]. https://doi.org/10.1080/02841860500341157.