Abstract
Background:
Antibodies might protect against low doses of environmental carcinogens by decreasing systemic uptake, activation of metabolic pathways, and redistribution of carcinogens within the organism. The features of antibody formation in relation to environmental carcinogens and sex steroids under natural conditions should be determined to identify breast cancer risk, then to develop cancer immune prevention strategies.Objectives:
The purpose of this study was to investigate antibodies specifications to benzo(a)pyrene, estradiol and progesterone in postmenopausal women with invasive breast cancer.Patients and Methods:
A semi-quantitative non-competitive immunoassay of IgG antibodies to benzo(a)pyrene (IgG-Bp), estradiol (IgG-Es), and progesterone (IgG-Pg) has conducted. The assay has performed on 322 serum samples from patients with breast cancer and 179 serum samples from healthy postmenopausal women by using low-molecular-weight Bp, Es, and Pg conjugated with bovine serum albumin. ROC analysis has also conducted to determine the odds ratio (OR).Results:
Combination of the high levels of IgG-Bp and IgG-Es without IpG-Pg was more frequent in breast cancer patients than that in healthy women, and the OR has increased to 3.8. Combination of the high levels of IgG-Pg with high levels of both IgG-Bp and IgG-Es were significantly more frequent in breast cancer patients (36.9%) than that in healthy women (5.6%), and the OR increased to 11.7. These differences have peculiarly expressed in breast cancer patients with hormone status ER+/PR- (OR = 26.7). The minimum OR (0.4) has obtained at low levels of the three antibodies.Conclusions:
Immunoassay of antibodies against environmental carcinogens and sex steroid hormones could use to detect breast cancer risk. Induction of antibodies against Bp for cancer immunoprevention could lead to antibody formation against steroid hormones, thereby increasing breast cancer risk.Keywords
Antibody Formation Benzo(a)pyrene Estradiol Progesterone Breast Neoplasms
1. Background
Breast cancer risk has increased among the women continuously. The potential factors for primary chemoprevention of breast cancer have included the following: selective estrogen receptor modulators (tamoxifen, raloxifene, arzoxifene, and lazofoxifene), aromatase inhibitors (exemestane and anastrozole), isoflavones, retinoids, rexinoids, and deltanoids, polyamine synthesis inhibitors, and tyrosine kinase inhibitors (1, 2). All these agents have influenced the intracellular pathways of breast cancer development.
As tumors of mammary gland could induce by polycyclic aromatic hydrocarbons (3-5) and phytoestrogens (6, 7), scholars have developed immunoprophylactic strategies with carcinogen-specific antibodies (Abs) (8-11). They have demonstrated that Abs might protect against low doses of environmental carcinogens by decreasing systemic uptake, activation of metabolic pathways, and redistribution of carcinogens within the organism. Studies on animal models with estrogen-sensitive tumors have shown that active immunization against estrogens could alter the concentration of the hormone in blood serum (12, 13), reduced tumor growth and increased survival time (14).
The features of antibody formation related to environmental carcinogens and sex steroids under natural conditions should determine to develop cancer immunoprevention strategies. Previous studies have revealed the presence of Abs against polycyclic aromatic hydrocarbons and their DNA adducts in human blood serum (15-21). Abs to carcinogens and sex steroids in breast cancer patients (BCP) have only described in a few studies (22-24).
2. Objectives
In the present study, Abs specific to benzo(a)pyrene (Bp), estradiol (Es), and progesterone (Pg) have evaluated to detect breast cancer risk in postmenopausal women.
3. Patients and Methods
3.1. Patients
A total of 501 serum samples have obtained from postmenopausal women, of which 322 women have primarily diagnosed with invasive breast cancer in the regional clinical oncology hospital (Kemerovo, Russia). Each case diagnosis has confirmed morphologically and radiographically in the oncology hospital. Data related to tumor size, histological type, grade, and stage have collected from surgical pathology reports. Hormone receptor status (positive or negative) has determined immunohistochemically (25) with anti-human ER antibody (clone 1D5) and anti-human PR antibody (clone PR-2C5), obtained from Dako Corp. (Carpinteria, USA). The following groups have identified based on the hormone receptor status: positive (ER+PR+), negative (ER-PR-), and mixed (ER+PR- or ER-PR+). Healthy women (n = 189) without breast pathology have included for comparing. The median ages of participants were 61 (ranging from 42 to 85) for BCP and 58 (ranging from 39 to 80) for healthy women. The study protocol has conformed to the ethical guidelines of the 1975 declaration of Helsinki and has approved by ethics committee of institute of human ecology SB RAS (protocol No. 12/1). All women have provided informed consents.
3.2. Immunoassay of Abs to Bp, Es, and Pg
Abs to Bp, Es, and Pg have tested through solid-phase indirect enzyme-linked immunosorbent assays with minor modifications (20). Microtiter wells have coated with 2 μg/mL Bp (Es or Pg) (Sigma-Aldrich, Germany) conjugated with bovine serum albumin BSA (Amresco, USA) in 100 μL phosphate-buffered saline PBS (Amresco, USA) at room temperature overnight. The hapten-BSA conjugates have synthesized according to a previously reported method (21, 26). Coated wells have blocked for 30 minutes with incubate buffer (PBS with 0.05% Tween 20 and 0.5% BSA). The serum samples have diluted at 1/100 incubate buffer, and have incubated 100 μL/well for 1 hour at 37°C. Bound Abs have detected with goat anti-human IgG antibody labeled with horseradish peroxidase (1/10000 dilution, Sigma-Aldrich, Germany). After each assay steps wells have washed 250 μL/well of PBS with 0.05% Tween 20 (Amresco, USA). The amounts of bound Abs have determined through enzymatic reaction with the chromogenic substrate TMB (Bector Corp., USA). The reaction has then terminated by addition of 2 N HCl and absorbance has measured at 450 nm in duplicate.
The levels of Abs to Bp, Es, and Pg have expressed in arbitrary units and calculated based on the following Formula:
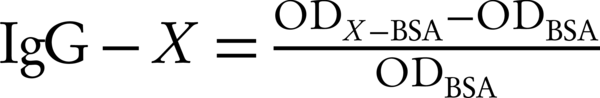
Where X is Bp, Es, or Pg; ODx-BSA is the absorbance of the binding to hapten-BSA conjugate and hapten-BSA and ODBSA is the absorbance of the binding to BSA.
3.3. Statistical Analysis
All statistical analyses have conducted using STATISTICA (StatSoft Inc, USA, version 6.0). Parametric normally distributed data has statistically analyzed using Shapiro-Wilk’s W-test, and non-parametric data have analyzed by Mann-Whitney U-test and χ2 with Yates’ correction. Statistical significance has based on two-sided P values, P < 0.05 have considered statistically significant. The prognostic value of markers has assessed through ROC analysis (27), and odds ratio (OR) has determined with 95% confidence interval (95% CI).
4. Results
The significant border levels of IgG-Bp, IgG-Es, and IgG-Pg between healthy women and BCP have determined through ROC analysis. High Abs levels (> 4) were more frequent in BCP than that in healthy donors, and the difference was statistically significant (Table 1, positions 1 and 2). The ORs for the BPC group were 3.5, 4.1, and 2.6 in relation to high Abs levels against Bp, Es, and Pg, respectively. The frequency of IgG-Pg (> 4) in the BCP PR+ subgroup (39%) was lower than that in the BCP PR- subgroup (50%), but the difference was not statistically significant. High levels of IgG-Pg have rarely found in the BCP ER+/PR+ subgroup (38%) in comparison with those in the BCP ER+/PR- subgroup (59%), and the difference was statistically significant (p < 0.05). No differences have found between the other subgroups in terms of the frequency of the high levels of the three Abs among the sex hormone receptors. The highest ORs have found in ER+/PR+ patients (5.5, 5.2, and 4.8).
Cases Quantity (n) and Frequency (%) With High Levels of Antibodies to Benzo[a]Pyrene (IgG-Bp), Estradiol (IgG-Es) and Progesterone
| Group | N | IgG-Bp > 4 | IgG-Es > 4 | IgG-Pg > 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | χ2 | P | OR (95%CI) | No. (%) | χ2 | P | OR (95%CI) | No. (%) | χ2 | P | OR (95%CI) | ||
| 179 | 49 (27) | 51 (28) | 41 (23) | ||||||||||
| 322 | 184 (57) | 39.8 | 0.0005 | 3.5 (2.3 - 5.4) | 199 (62) | 49.7 | 0.0005 | 4.1 (2.7 - 6.1) | 139 (43) | 19.6 | 0.0005 | 2.6 (1.7 - 3.9) | |
| 234 | 131 (56) | 32.6 | 0.0005 | 3.4 (2.2 - 5.2) | 147 (63) | 46.5 | 0.0005 | 4.2 (2.7 - 6.6) | 99 (42) | 16.2 | 0.0006 | 2.5 (1.6-3.9) | |
| 88 | 53 (60) | 25.6 | 0.0005 | 4.0 (2.3-7.1) | 52 (59) | 22.0 | 0.0005 | 3.6 (2.1 - 6.4) | 40 (45) | 8.9 | 0.004 | 2.3 (1.3 - 4.1) | |
| 198 | 107 (54) | 26.5 | 0.0005 | 3.1 (1.9-4.9) | 123 (62) | 41.4 | 0.0005 | 4.1 (2.6 - 6.5) | 77 (39) | 10.4 | 0.002 | 2.1 (1.3 - 3.4) | |
| 124 | 77 (62) | 34.9 | 0.0005 | 4.3 (2.6-7.3) | 76 (61) | 31.0 | 0.0005 | 2.9 (2.4 - 6.7) | 62 (50) | 22.8 | 0.0005 | 3.4 (1.9 - 5.7) | |
| 78 | 46 (59) | 21.9 | 0.0005 | 3.8 (2.1 - 6.9) | 45 (58) | 18.6 | 0.0005 | 3.4 (1,9 - 6,2) | 35 (45) | 11.6 | 0.002 | 2.7 (1.5 - 5.0) | |
| 46 | 31 (67) | 23.9 | 0.0005 | 5.5 (2.6 - 11.7) | 31 (67) | 22.0 | 0.0005 | 5.2 (2.5 - | 27 (59a) | 20.6 | 0.0005 | 4.8 (2.3 - 10.0) | |
| 188 | 100(53) | 24.3 | 0.0005 | 3.0 (1.9 – 4.8) | 116 (62) | 39.5 | 0,0005 | 11.1) 4,0 (2.6 - 6.4) | 72 (38a) | 9.5 | 0.003 | 2.1 (1.3 - 3.3) | |
Considering the possibility of different individual combinations of high and low Abs levels to Bp, Es, and Pg (Table 2), we have separated all the women into eight groups. The absence or low Abs levels to the three haptens (combination 1) have rarely found in the BCP group in comparison with that in healthy donors (27.9% vs. 49.7%). The OR in this case was 0.4. By contrast, high Abs levels to the three haptens (combination 8) have more frequently found in BCP than that in healthy women (36.9% vs. 5.6%). In this case, the OR has increased to 11.7. This value was higher than the OR calculated through separate Abs analysis of each hapten (Table 1).
(IgG-Pg) and Breast Cancer Risks (OR) at the Postmenopausal Women Allowing for Sex Hormone Receptors (ER/PR)
| Condition | Healthy Women, N = 179 | BCP, N = 322 | BCP ER+, N = 234 | BCP ER- N = 88 | BCP PR+, N = 198 | BCP PR-, N = 124 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | ||
| 89 (49.7) | 90 (27.9) | 0.0005 | 0.4 (0.3 - 0.6) | 63 (26.9) | 0.0005 | 0.4 (0.2 - 0.6) | 27 (30.7) | 0.006 | 0.4 (0.2 - 0.8) | 57 (28.8) | 0.0006 | 0.4 (0.3 - 0.6) | 33 (26.6) | 0.0007 | 0.4 (0.2 - 0.6) | |
| 18 (10.1) | 20 (6.2) | 0.9 | 1.1 (0.5 - 2.3) | 16 (6.8) | 0.7 | 1.3 (0.6 - 2.8) | 4 (4.5) | 0.8 | 0.7 (0.2 – 2.6) | 14 (7.1) | 0.8 | 1.2 (0.5 - 2.8) | 6 (4.8) | 1.0 | 0.9 (0.3 – 2.7) | |
| 21 (11.7) | 36 (11.2) | 0.1 | 1.7 (0.9 - 3.3) | 29 (12.4) | 0.06 | 1.9 (0.9 - 3.9) | 7 (8.0) | 1.0 | 1.1 (0.4 – 3.1) | 26 (13.1) | 0.07 | 1.9 (0.9 – 3.9) | 10 (8.1) | 0.7 | 1.3 (0.5 – 3.2) | |
| 10 (5.6) | 5 (1.6) | 0.3 | 0.5 (0.1 - 1.7) | 5 (2.1) | 0.7 | 0.7 (0.2 - 2.4) | 0 (0) | 0.2 | 0.0 (0.9 – 1.9) | 3 (1.5) | 0.4 | 0.4 (0.1 – 1.9) | 2 (1.6) | 0.7 | 0.5 (0.1 – 2.8) | |
| 10 (5.6) | 38 (11.8) | 0.001 | 3.8 (1.7 - 8.6) | 28 (11.9) | 0.002 | 3.9 (1.7 - 9.4) | 10 (11.4) | 0.03 | 3.3 (1.1 – 9.7) | 24 (12.1) | 0.003 | 3.7 (1.6 – 9.1) | 13 (10.5) | 0.01 | 3.5 (1.3 – 9.7) | |
| 11 (6.1 ) | 8 (2.5) | 0.7 | 0.7 (0.3 - 2.0) | 3 (1.3) | 0.7 | 0.7 (0.3 - 2.0) | 5 (5.7) | 0.7 | 1.5 (0.4 – 5.2) | 1 (0.5) | 0.07 | 0.1 (0.01 - 1.1) | 7 (5.6) | 0.4 | 1.7 (0.5 – 5.3) | |
| 10 (5.6) | 7 (2.2) | 0.6 | 0.7 (0.2 - 2.1) | 6 (2.6) | 0.9 | 0.8 (0.3 - 2.7) | 1 (1.1) | 0.5 | 0.3 (0.02 – 7.7) | 5 (2.5) | 0.9 | 0.8 (0.2 - 2.6) | 2 (1.6) | 0.7 | 0.5 (0.1 – 2.8) | |
| 10 (5.6) | 118 (36.6) | 0.0005 | 11.7 (5.5 - 23.4) | 84 (35.9) | 0.0005 | 11.9 (5.5 - 26.5) | 34 (38.6) | 0.0005 | 11.2 (4.6 – 28.0) | 68 (34.3) | 0.0005 | 10.6 (4.8 – 24.0) | 51 (41.1) | 0.0005 | 13.8 (5.9 – 32.8) | |
High levels of IgG-Bp or Ig-Es only without Abs to other haptens (combinations 2 and 3) presented similar frequency between BCP and healthy women. Abs formation to Bp only or to Es only has not increased BC risk. High levels of IgG-Pg only in the absence of IgG-Bp and IgG-Es (combination 4) have rarely found in BCP in comparison with that, among healthy women (1.6% vs. 5.6%), but the difference was not statistically significant. Combination 5 (high levels of both IgG-Bp and IgG-Es without IgG-Pg) has more frequently found in BCP than that in healthy women (11.5% vs. 5.6%), and the difference was statistically significant and the OR increased to 3.8. By contrast, combinations 6 and 7 (high levels of IgG-Pg with IgG-Bp or with Ig-Es) have rarely found in BCP compared with that in healthy women and the OR approached 0.7.
The frequency of high levels of IgG-Bp and IgG-Pg without IgG-Es (combination 6) was lower than those of IgG-Bp and IgG-Es without IgG-Pg (combination 5) in the BCP group (2.5% vs. 11.8%, P = 0.009). The increased ORs (3.8) at high levels of IgG-Bp and IgG-Es (combination 5) were statistically significant in all BCP subgroups allowing for sex hormone receptors without any differences between them. There were no any differences between healthy women and BCP as well as between subgroups of BCP when high levels of IgG-Pg have combined with IgG-Bp and with IgG-Es (combinations 6 and 7).
The quantity and frequency of Abs combinations in BCP suitable for sex hormone receptor combinations (ER/PR) have shown in Table 3. The results have determined that the minimum OR (0.2) at low levels of Abs to the three haptens (combination 1) and maximum OR (26.7) at high levels of Abs to the three haptens (combination 8) have found in BCP ER+/PR-.
The statistically significant high ORs (2.4) at high levels of IgG-Bp with IgG-Es (combination 5) have found in all three subgroups of BCP. The statistically significant low OR (0.02) at high levels of IgG-Bp with IgG-Pg (combination 6) have found in BCP ER+/PR+ only.
Cases Quantity and Frequency (No. %) of Antibodies Levels to Benzo[a]Pyrene (IgG-Bp). Estradiol (IgG-Es) and Progesterone (IgG-Pg) Combinations and Breast Cancer Risks (OR) at the Postmenopausal Women Allowing for Hormone Receptors Existence (ER/PR)
| Condition | Healthy Women, N = 179 | BCP, ER-/PR-, N = 78 | BCP, ER+/PR-, N = 46 | BCP, ER+/PR+, N = 188 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | No. (%) | P | OR (95%CI) | ||
| 89 (49.7) | 26(33.4) | 0.02 | 0.5 (0.3 - 0.9) | 7 (15.2) | 0.0006 | 0.2 (0.1 - 0.5) | 56 (29.8) | 0.008 | 0.4 (0.3 - 0.7) | |
| 18 (10.1) | 3 (3.8) | 0.6 | 0.6 (0.1 - 2.3) | 3 (6.5) | 0.5 | 2.1 (0.4 - 10.5) | 13 (6.9) | 0.9 | 1.1 (0.5 - 2.7) | |
| 21 (11.7) | 5 (6.4) | 0.9 | 0.8 (0.2 - 2.6) | 5 (10.9) | 0.1 | 3.0 (0.7 - 12.1) | 24 (12.8) | 0.1 | 1.8 (0.9 - 3.8) | |
| 10 (5.6) | 0 (0) | 0.2 | 0.0 (0.9 - 1.9) | 2 (4.3) | 0.6 | 2.5 (0.3 - 16.6) | 3 (1.6) | 0.4 | 0.5 (0.1 - 1.9) | |
| 10 (5.6) | 9 (11.5) | 0.046 | 3.1 (1.0 - 9.4) | 4 (8.7) | 0.045 | 5.1(1.03-24.7) | 23 (12.2) | 0.003 | 3.7 (1.5 - 8.9) | |
| 11 (6.1) | 4 (5.1) | 0.9 | 1.2 (0.3 - 4.7) | 3 (6.5) | 0.2 | 3.5 (0.6 - 18.5) | 0 (0) | 0.02 | (0.0 - 0.8) | |
| 10 (5.6) | 1 (1.3) | 0.5 | 0.3 (0.02 - 2.8) | 1 (2.2) | 1.0 | 1.3 (0.1 - 12.5) | 5 (2.7) | 0.9 | 0.8 (0.2 - 2.7) | |
| 10 (5.6) | 30 (38.5) | 0.0005 | 10.3 (4.1 - 26.1) | 21 (45.7) | 0.0005 | 26.7 (8.2 - 92.3) | 64 (34.0) | 0.0005 | 10.2 (4.6 - 23.1) | |
5. Discussion
The modern system for clinical prevention of BC (1, 2) has required the use of laboratory methods to determine BC risk; such methods included analysis of steroid-binding globulins in the blood serum (28). Cancer immunoprevention strategies have developed by some scholars which have based on induction of Abs specific to environmental carcinogens (8-11). However, induction of Abs against estradiol reduced the growth of estrogen-sensitive tumors (14). Thus, analysis of Abs against environmental carcinogens (including phytoestrogens) and endogenous steroid hormones could be used to determine cancer risks for the humans and develop cancer immunoprevention strategies.
In this study, the risks of postmenopausal BC have calculated using immunoanalysis of Abs to Bp, Es, and Pg. The results have revealed that high levels of IgG-Bp only or IpG-Es only have rarely found (6% to 10 %) in BCP and in healthy women. Combination of the high levels of IgG-Bp and IgG-Es without IpG-Pg was more frequent in BCP than that in healthy women, and the OR increased to 3.8.Combination of the high levels of IgG-Pg with high levels of both IgG-Bp and IgG-Es were significantly more frequent in BCP (36.9%) than that in healthy women (5.6%), and the OR increased to 11.7. These differences have peculiarly expressed in BCP ER+/PR- (OR = 26.7).
Previous studies have shown a negative association or absence of strong association between sex-hormone binding globulin and BC risk (28). In a large study that have analyzed data from nine prospective trials involving women who develop or not develop BC, the risk of BC statistically significantly have increased with increasing concentrations of all sex hormones examined, whereas sex-hormone binding globulin has associated with decreased BC risk. In a case-controlled study of the European prospective investigation into cancer and nutrition, sex-hormone binding globulin levels in postmenopausal women who have developed BC have confirmed to be significantly lower than those in the control (29). Future studies should further investigate why Abs to Es and Pg, as the sex-hormone binding immunoglobulins, with Abs to Bp have shown a strong positive association with BC risk. Interrelations of Abs to environmental carcinogens with steroid hormones and steroid hormone receptors should also be investigated.
Although some scholars have speculated that mucosal and serum Abs reduce carcinogenesis by preventing high local carcinogen concentration (11), other scientists have revealed that the mechanism could be induction of mucosal, not systemic, immune reaction in environmental carcinogens (10). A previous study has also shown that active immunization using Bp conjugated to protein has significantly increased the titers of Bp Abs and tumor formation when mice have treated with Bp (30).
This study has confirmed that high levels of serum Ig-Bp with Ig-Es and Ig-Pg corresponding to high BC risk. In this case, induction of systemic immune reactions against environmental carcinogens could stimulate Abs-formation against steroid hormones and breast carcinogenesis. However, the molecular and cellular effects of Abs specific to environmental carcinogens and endogenous steroids on the mammary gland, have remained unknown.
Acknowledgements
References
-
1.
Kahan Z, Thurzo L. [Breakthrough in breast cancer chemoprevention]. Orv Hetil. 2003;144(13):597-603. [PubMed ID: 12728784].
-
2.
Sestak I. Preventative therapies for healthy women at high risk of breast cancer. Cancer Manag Res. 2014;6:423-30. [PubMed ID: 25378950]. https://doi.org/10.2147/CMAR.S55219.
-
3.
el-Bayoumy K. Environmental carcinogens that may be involved in human breast cancer etiology. Chem Res Toxicol. 1992;5(5):585-90. [PubMed ID: 1445997].
-
4.
Jeffy BD, Chirnomas RB, Romagnolo DF. Epigenetics of breast cancer: polycyclic aromatic hydrocarbons as risk factors. Environ Mol Mutagen. 2002;39(2-3):235-44. [PubMed ID: 11921194].
-
5.
Rundle A, Tang D, Hibshoosh H, Schnabel F, Kelly A, Levine R, et al. Molecular epidemiologic studies of polycyclic aromatic hydrocarbon-DNA adducts and breast cancer. Environ Mol Mutagen. 2002;39(2-3):201-7. [PubMed ID: 11921190].
-
6.
Lorand T, Vigh E, Garai J. Hormonal action of plant derived and anthropogenic non-steroidal estrogenic compounds: phytoestrogens and xenoestrogens. Curr Med Chem. 2010;17(30):3542-74. [PubMed ID: 20738246].
-
7.
Albini A, Rosano C, Angelini G, Amaro A, Esposito AI, Maramotti S, et al. Exogenous hormonal regulation in breast cancer cells by phytoestrogens and endocrine disruptors. Curr Med Chem. 2014;21(9):1129-45. [PubMed ID: 24304271].
-
8.
Moolten FL, Capparell NJ, Boger E, Mahathalang P. Induction of antibodies against carcinogenic polycyclic aromatic hydrocarbons. Nature. 1978;272(5654):614-6. [PubMed ID: 417266].
-
9.
Peck RM, Peck EB. Inhibition of chemically induced neoplasia by immunization with antigenic carcinogen-protein conjugate. Cancer Res. 1971;31(11):1550-4. [PubMed ID: 5121659].
-
10.
Silbart LK, Rasmussen MV, Oliver AR. Immunoprophylactic intervention in chemical toxicity and carcinogenicity. Vet Hum Toxicol. 1997;39(1):37-43. [PubMed ID: 9004467].
-
11.
Cernohorska H, Klimesova S, Lepsa L, Jinoch P, Milcova A, Schmuczerova J, et al. Influence of immunization with non-genotoxic PAH-KLH conjugates on the resistance of organisms exposed to benzo(a)pyrene. Mutat Res. 2012;742(1-2):2-10. [PubMed ID: 22138421]. https://doi.org/10.1016/j.mrgentox.2011.10.016.
-
12.
Elsaesser F. Effects of active immunization against oestradiol-17 beta, testosterone or progesterone on receptivity in the female rabbit and evaluation of specificity. J Reprod Fertil. 1980;58(1):213-8. [PubMed ID: 7359480].
-
13.
Rosenberg M, Amir D, Folman Y. The effect of active immunization against progesterone on plasma concentrations of total and free progesterone, estradiol-17beta and LH in the cyclic ewe. Theriogenology. 1987;28(4):417-26. [PubMed ID: 16726324].
-
14.
Caldwell BV, Tillson SA, Esber H, Thorneycroft IH. Survival of tumours after immunization against oestrogens. Nature. 1971;231(5298):118-9. [PubMed ID: 4930090].
-
15.
Petruzzelli S, Celi A, Pulera N, Baliva F, Viegi G, Carrozzi L, et al. Serum antibodies to benzo(a)pyrene diol epoxide-DNA adducts in the general population: effects of air pollution, tobacco smoking, and family history of lung diseases. Cancer Res. 1998;58(18):4122-6. [PubMed ID: 9751623].
-
16.
Galati R, Zijno A, Crebelli R, Falasca G, Tomei F, Iecher F, et al. Detection of antibodies to the benzo(a)pyrene diol epoxide-DNA adducts in sera from individuals exposed to low doses of polycyclic aromatic hydrocarbons. J Exp Clin Cancer Res. 2001;20(3):359-64. [PubMed ID: 11718215].
-
17.
Verdina A. Carcinogen-modified DNA and specific humoral immunity toward carcinogen-DNA adducts. A review. Ann Ist Super Sanita. 2006;42(2):189-94. [PubMed ID: 17033140].
-
18.
Pauk N, Klimesova S, Kara J, Topinka J, Labaj J. The relevance of monitoring of antibodies against the polycyclic aromatic hydrocarbon (PAH) and PAH-DNA adducts in serum in relation to lung cancer and chronic obstructive pulmonary disease (COPD). Neoplasma. 2013;60(2):182-7. [PubMed ID: 23259787].
-
19.
Ustinov VA, Matveeva VA, Kostyanko MA, Glushkov AN. Antibodies against benzo[a]pyrene in immunized mouse and in lung cancer patients. Exp Oncol. 2013;35(3):207-10. [PubMed ID: 24084460].
-
20.
Glushkov AN, Polenok EG, Anosova TP, Savchenko YA, Bakanova ML, Minina VI. Serum antibodies to benzo [a] pyrene and chromosomal aberration in lymphocytes peripheral blood at the workers of coal processing enterprise. Rus J Immunol. 2011;5:39-44.
-
21.
Glushkov AN, Polenok EG, Verzbitskaya NE, Varin IA, Ragozina SE. Antibodies to chemical carcinogens and steroid hormones in the lung cancer patients. Russ J Immunol. 2014;8(2):219-27.
-
22.
Chagnaud JL, Faiderbe S, Geffard M. Identification and immunochemical characterization of IgA in sera of patients with mammary tumors. Int J Cancer. 1992;50(3):395-401. [PubMed ID: 1735608].
-
23.
Glushkov AN, Anosova TP, Nebesnaya NG, Zheleznova LY. Isotipical special features of antibodies to polycyclic aromatic hydrocarbons in patients with cancer of mammary gland, stomach, colon and rectum. Exp Oncol. 1996;18:426-8.
-
24.
Anosova TP, Polenok EG, Anosov MP, Krasilnikova KS, Kostyanko MV, Verzbitskaya NE. Antibodies to xeno- and endobiotics at the patients with breast cancer. Izvestiya Samara Science Center RAS. 2012;5(2):440-3.
-
25.
Almasri NM, Al Hamad M. Immunohistochemical evaluation of human epidermal growth factor receptor 2 and estrogen and progesterone receptors in breast carcinoma in Jordan. Breast Cancer Res. 2005;7(5):R598-604. [PubMed ID: 16168103]. https://doi.org/10.1186/bcr1200.
-
26.
Glushkov AN, Kostyanko MV, Cherno SV, Vasilchenko IL. Synthesis of polycyclic aromatic hydrocarbon-protein conjugates for preparation and immunoassay of antibodies. Russ J Immunol. 2002;7(1):42-6. [PubMed ID: 12687265].
-
27.
Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39(4):561-77. [PubMed ID: 8472349].
-
28.
Zeleniuch-Jacquotte A, Shore RE, Koenig KL, Akhmedkhanov A, Afanasyeva Y, Kato I, et al. Postmenopausal levels of oestrogen, androgen, and SHBG and breast cancer: long-term results of a prospective study. Br J Cancer. 2004;90(1):153-9. [PubMed ID: 14710223]. https://doi.org/10.1038/sj.bjc.6601517.
-
29.
Kaaks R, Rinaldi S, Key TJ, Berrino F, Peeters PH, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071-82. [PubMed ID: 16322344]. https://doi.org/10.1677/erc.1.01038.
-
30.
Curtis GL, Ryan WL, Stenback F. Antibody stimulation of benzo(a)pyrene carcinogenesis. Cancer Lett. 1978;4(4):223-8. [PubMed ID: 647663].