Abstract
Background:
The association between human papillomavirus (HPV) infections and cervical cancer has suggested the design of prophylactic and therapeutic vaccines against genital warts. The HPV capsid has made of two L1 and L2 coat proteins that have produced late in viral infections. Regarding to the recent studies, two commercial prophylactic vaccines have based on L1 viral like particles (VLPs) could strongly induce antibody responses, and protect human body from HPV infections. However, the use of these HPV vaccines has hindered due to their high cost and some limitations. Currently, among various vaccination strategies, live vector-based vaccines have attracted a great attention.Objectives:
Herein, a non-pathogenic strain of the protozoan organism known as Leishmania tarentolae has utilized to induce potent humoral immunity in mice model.Materials and Methods:
At first, cloning of HPV16 L1 gene into Leishmania expression vector has performed and confirmed by PCR and digestion with restriction enzymes. The promastigotes of Leishmania tarentolae (L.tar) have transfected with linearized DNA construct by electroporation. Protein expression has analyzed by SDS-PAGE and western blotting. Then, the immunogenicity of leishmania expressing L1 protein (L.tar-L1) has assessed in mice model.Results:
Our data has indicated that subcutaneous immunization of mice with the recombinant L.tar-L1 has led to enhance the levels of IgG1 and lgG2a in comparison with control groups. Furthermore, there was no significant increase in antibody levels between two and three times of immunizations.Conclusions:
The recombinant live vector was able to induce humoral immunity in mice without need of any adjuvant. However, further studies have required to increase its efficiency.Keywords
1. Background
Cervical cancer, as the most common malignancies in women, has associated with human papillomavirus (HPV) infection (1, 2). The HPV genotypes have accompanied by genital infection included low-risk types (e.g. HPV 6 and 11), and high-risk types (e.g. HPV 16 and 18) generating ~ 90% of anogenital warts, and ~ 70% of cervical cancers, respectively (1, 3). Epidemiological studies have shown that primary prevention of cervical cancer by a prophylactic vaccine against HPV infections could significantly decrease the incidence of cervical cancer (1). HPV genome has coded eight early proteins (E1-E8) and two late coat proteins (L1 and L2) (1, 4). One of the best strategies for generating a preventive HPV vaccine has included the use of VLPs (virus-like particles) composed of the major capsid L1 protein, alone (5). Two prophylactic VLP-based vaccines have commercialized worldwide known as Cervarix® and Gardasil® (3, 6). The main disadvantages of the VLP vaccines were the high cost of preparation, and the existence of suitable cold chain. These drawbacks have hampered their extensive use in developing or resource-poor countries which had 80% cervical cancer cases (5, 7). Thus, the studies have focused on other approaches such as protein or live vector-based vaccines (7-10). Among them, live vaccines could open an efficient way for prevention of infectious diseases. For instance, intracellular bacteria, such as Salmonella, Shigella, Listeria, and Lactococci as well as Rubella or Vaccinia viruses, have broadly used to develop HPV vaccine (11-14). However, the pathogenicity, re-infection, and toxicity of these live vectors have prevented their application as vaccine candidates in humans (7, 8).
Recently, a trypanosomatid protozoan parasite, Leishmania tarentolae, has used as an efficient gene expression system for production of the recombinant proteins (15-17). In particular, Leishmania tarentolae, as a live vector was able to efficiently target the antigen-presenting cells (APCs), and stimulate immune has responded without replication within APCs (18, 19). For example, the recombinant Leishmania tarentolae expressing the HIV-1 Gag protein has elicited a potent cellular immune response, leading to a reduction of HIV replication ex vivo (19).
2. Objectives
In current study, a recombinant Leishmania tarentolae expressing the HPV16 L1 protein (L.tar-L1) has generated and its immunogenicity has assessed in C57BL/6 mice model. Our results have shown that the recombinant L.tar-L1 could significantly increase both levels of IgG1 and lgG2a antibodies without the differences between two and three times of immunizations.
3. Materials and Methods
3.1. Preparation of Plasmid DNA Constructs
For the production of HPV16 L1-expressing vector, the L1 gene was subcloned from pcDNA-L1 into the XhoI and KpnI cloning sites of pLEXSY-I-blecherry3 expression vector (Jena bioscience, Germany). The obtained pLEXSY-I-L1 construct has purified in large-scale using Midi-kit (Qiagen). The accuracy of pLEXSY-I-L1 has confirmed using DNA sequencing. Then, pLEXSY-I-L1 has linearized by SwaI enzyme for integration into the chromosomal odc locus of the LEXSY host (T7-TR).
3.2. Parasite Transfection
The promastigotes of Leishmania tarentolae (L. tar) have grown in brain-heart infusion medium (BHI, Sigma) at pH = 7.2 and 26°C. For transfection, the linearized pLEXSY-I-L1 (8 μg) was electroporated into 4 × 107 log phase parasites at 450 V and 500 μF using Bio-Rad Gene PulserEcell. The electroporated parasites have cultured on the BHI-agar plate containing 50 μg/mL of bleomycin for selection of recombinant transfectants (Jena Bioscience, Germany).
3.3. Genomic Analysis
The genomic DNA of recombinant L.tar-L1 has extracted using the DNeasy® blood and tissue kit (GF-1). Integration of pLEXSY-L1 into the genome has confirmed by diagnostic PCR using L1 primers and also odc forward/ aprt reverse primers as mentioned in Jena bioscience manual.
3.4. Protein Analysis
The protein expression has induced by tetracycline according to Jena bioscience manual. Parasite promastigotes have harvested by centrifugation at 3000 rpm for 10 minutes and washed in PBS. The pellets have lysed in 2X SDS-PAGE sample buffer on ice and then boiled for 5 minutes. The samples have loaded on a 12.5% SDS-PAGE. After that, western blot analysis has performed using an anti-HPV16 L1 monoclonal antibody (MD2H11, kindly provided by Professor Martin Muller, German Cancer Research Center; 1:5000 v/v) under standard procedures. In order to detect the expected band of L1 protein, 3, 3'-diaminobenzidine (DAB, Sigma) has used as a substrate.
3.5. Mice Immunization
Five groups of 6 - 8-week-old female C57BL/6 mice (n = 5) have obtained from breeding stock maintained at the Pasteur Institute of Iran. All mice have maintained under specific pathogen-free conditions and all procedures have performed according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals. Mice have immunized two or three times with two-week intervals as: group 1 (L.tar-L1/L.tar-L1), group 2 (L.tar-L1/L.tar-L1/ L.tar-L1), group 3 (L.tar/L.tar: control), group 4 (L.tar/L.tar/ L.tar: control), and group 5 (PBS: control), respectively. Total number of 2 × 107 stationary phase recombinant L. tar or L.tar-L1 promastigotes, have injected subcutaneously into groups 1 - 4.
3.6. Assessment of Humoral Immune Responses
Mice sera from each group have collected two weeks after the last injections. The levels of IgG1 and IgG2a have assessed using ELISA. Briefly, a 96-well flat-bottom ELISA plate (Greiner) has coated overnight at 4°C with killed L.tar-L1 protein as an antigen (prepared in 0.1% formalin (20), 10μg/mL) diluted in PBS (pH = 7.2). Then, the plate has rinsed with washing buffer (0.5% (v/v) Tween-20 in PBS), incubated with blocking buffer (1% BSA in PBS) for 2 hours at 37°C. The pooled sera have diluted 1:50 in dilution buffer (0.5% (v/v) Tween-20 in blocking buffer), added to the plate and incubated for 2 hours at 37°C. After rinsing with washing buffer, the plate has incubated with biotin-conjugated goat anti-mouse IgG1 or IgG2a (diluted 1:1000 in 1% BSA/PBS-Tween, Southern biotechnology Association Inc, USA) for 2 hours at 37°C. Then, the plates have washed and incubated with streptavidin-horseradish peroxidase diluted in PBS (1:100,000; Sigma) at 37°C for 1 hour. Detection has done with 100 μL of O-Phenylenediamine (OPD, Sigma) as the substrate in citrate phosphate buffer (pH = 4.5), followed by incubation for 30 minutes at 37°C. The enzyme reaction has stopped by 1 M H2SO4 and the absorbance has measured at 492 nm.
3.7. Statistical Analysis
Statistical analysis has performed using Prism 5.0 software (GraphPad, San Diego, California, USA). One-way ANOVA has performed to analyze humoral immune responses. For all comparisons, P < 0.05 has considered statistically significant. Data have presented as mean ± standard deviation (SD).
4. Results
4.1. Generation of Leishmania tarentolae Expressing L1 Protein (L.tar-L1)
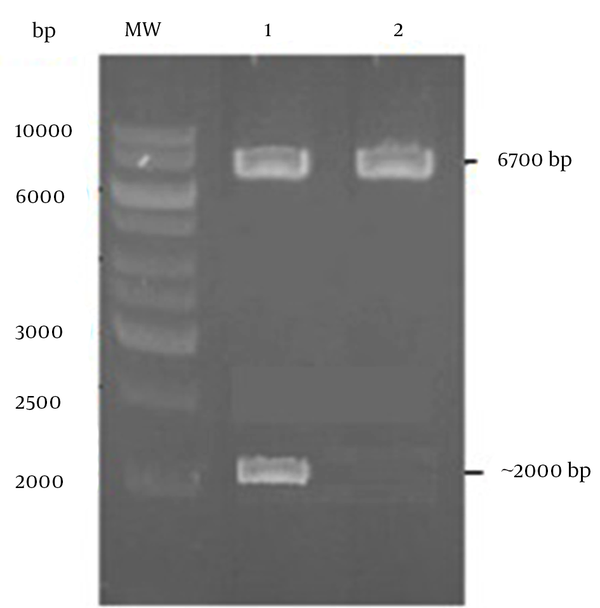
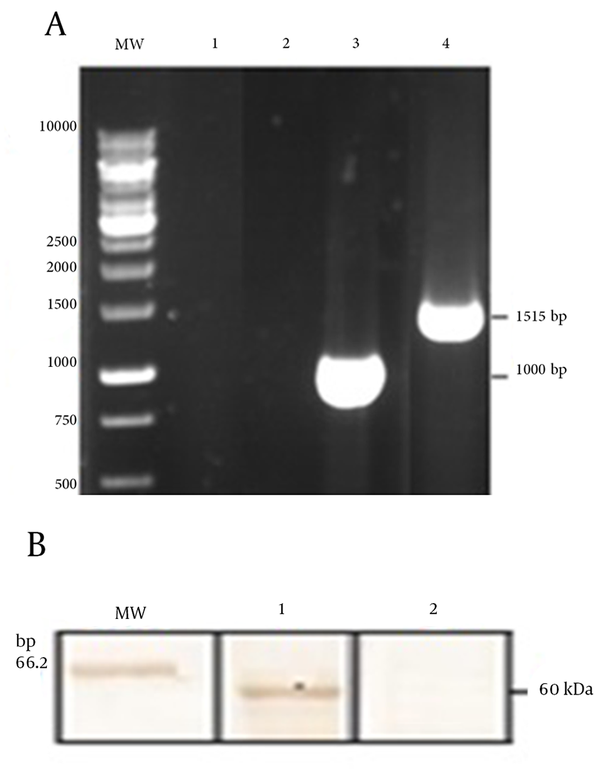
To generate L.tar-L1, firstly, the 1515 bp fragment encoding the L1 has cloned into pLEXSY vector. Then, the linearized pLEXSY-L1 has electroporated into L. tar.Figure 1 has shown the linearized pLEXSY-L1 with SwaI and separation of ~ 2 kb band for formation of 5’odc and 3’odc regions (~ 6700 bp) and homologous recombination into the host chromosome. After obtaining the recombinant clones, integration of gene in the genomic DNA has confirmed by PCR analysis. The expected 1 kb band has only observed from transgenic cells suggesting the correct integration of linearized plasmid into the genome (Figure 2 A). The PCR product of L1 has also appeared as ~ 1515 bp fragment for L1 positive clones (Figure 2 A). Furthermore, western blotting by an anti-L1 antibody has indicated the expression of L1 protein (~ 60 kDa) for L.tar-L1 versus wild type L.tar (Figure 2 B).
Generation of 5’odc and 3’odc Regions for Homologous Recombination
A) Confirmation of the Correct Integration of Linearized pLEXSY-I-L1 Into the Genome: the Amplification of Wild Type L.tar Genome With odc/aprt Primers (Line 1) and Also L1 Primers (Line 2); the Amplification of L.tar-L1 With odc/aprt Primers (Line 3) and Also L1 Primers (Line 4); B) Western Blot Analysis for L1 Protein has Been Using the Anti-HPV16 L1 Monoclonal Antibody: The 60 kDa Band of the L1 Protein (Line 1) Against no Band for L. tar as a Negative Control (Line 2)
4.2. L.tar-L1 Induces Both IgG1 and IgG2a Isotypes in Mice
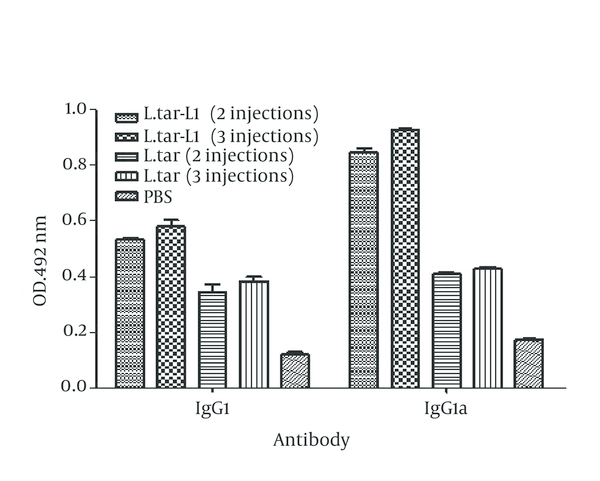
C57BL/6 mice have subcutaneously immunized two and three times at 2-weeks interval with 2 × 107L.tar-L1 and L.tar. The levels of IgG1 and IgG2a in the mice sera have determined by ELISA two weeks after third immunization. Vaccination with L.tar-L1 has generated the mixture of IgG2a and IgG1 antibodies against killed L.tar-L1 with high intensity toward IgG2a response in comparison with control groups after two and three immunizations (P < 0.05). It was interesting that antibody levels have not changed with increasing the number of injections as observed in groups 2 and 4 in comparison with groups 1 and 3, respectively (Figure 3).
Analysis of the Levels of IgG2a and IgG1 With Respect to L.tar-L1 per Group Using ELISA
5. Discussion
In this article, we have represented the immunological properties of a novel live vector expressing the L1 protein in mice model. Herein, the linearized pLEXSY-L1 construct has generated and transfected in Leishmania tarentolae for integration into the genome. PCR technique and western blot analysis of the recombinant L.tar-L1 has confirmed L1 integration and its expression in the parasite as a 60 kDa fragment. Since, the use of bacterial and viral vectors for development of HPV vaccine have shown an important problem for human safety, live non-pathogenic L. tarentolae has suggested as a live vaccine without the risk of infection (19). The studies indicated that live vaccines mainly have greater immunogenicity in comparison with subunit vaccines (11, 16). In current study, the humoral immune responses have induced by the recombinant live Leishmania tarentolae expressing HPV16 L1 (L. tar-L1) has evaluated and compared with controls (L.tar, and PBS). In addition, we have compared the immunological effects of two and three immunizations with L.tar-L1 and also L.tar in C57BL/6 mice. Our results have indicated high levels of IgG1 and IgG2a Ievels with high intensity toward IgG2a response in comparison with control groups after two and three immunizations. Furthermore, the number of immunizations has not shown any changes in enhancement of their potency. Our previous studies have also shown that a novel live vaccine using recombinant Leishmania tarentolae expressing HPV16 E7 protein could generate the protection of mice against HPV-associated tumors. Indeed, the immunization with live recombinant L.tar-E7 induced high levels of IgG2a in mice. In contrast with our recent data for L.tar-L1, no significant levels of IgG1 have observed for L.tar-E7 (8). In addition, subcutaneous administration of mice with both the recombinant L.tar-E7-NT (gp96) and L.tar-E7-CT (gp96) fusion proteins have led to enhance the levels of IgG2a before and after challenge with TC-1 tumor cells. But, the levels of IgG1 have not increased in these groups (21). These results have shown that the antigen could influence type of immune responses, e.g. L1 was able to induce both IgG1 and IgG2a significantly against L.tar or PBS, while E7, E7-NT (gp96) and E7-CT (gp96) were able to elicit IgG2a, alone. For this reason, the L1 has suggested as a target antigen inducing high antibody levels for prophylactic vaccines and E7 has proposed as a target antigen inducing potent cellular immune response for therapeutic vaccines.
Except to Leishmania tarentolae has used as live vector, the studies have shown that Toxoplasma gondii and Neospor acaninum (22, 23) have closely related apicomplexan parasites. The surface antigen of T. gondii (TgSAG1) was a major immunodominant antigen and, therefore, has considered being a good candidate for the development of an effective recombinant vaccine against toxoplasmosis. In a study, N. caninum stably expressing the TgSAG1 gene (Nc/TgSAG1) has induced TgSAG1-specific Th1-dominant immune responses and protected the mice from a lethal challenge infection with T. gondii. These results have indicated that N. caninum might be used for the production of a live recombinant vector vaccine against toxoplasmosis in animals (22). Generally, the non-pathogenic live parasites could stimulate antigen-specific immune responses. They have possessed the high adjuvant properties for increasing the potency of a vaccine. However, it was necessary to optimize these systems and enhancement of antigen expression.
Our study has demonstrated that live Leishmania tarentolae could be used as an appropriate carrier to deliver HPV16 L1 antigen and induce potent humoral immune responses. Moreover, this live vector would be safe for human, as a potent vaccination strategy against HPV and other intracellular pathogens. The live Leishmania tarentolae has been capable of stimulating humoral immunity in mice without the need of any adjuvant.
Acknowledgements
References
-
1.
Stanley M, Lowy DR, Frazer I. Chapter 12: Prophylactic HPV vaccines: underlying mechanisms. Vaccine. 2006;24 Suppl 3:S3/106-13. [PubMed ID: 16949996]. https://doi.org/10.1016/j.vaccine.2006.05.110.
-
2.
Giannini SL, Hanon E, Moris P, Van Mechelen M, Morel S, Dessy F, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33-34):5937-49. [PubMed ID: 16828940]. https://doi.org/10.1016/j.vaccine.2006.06.005.
-
3.
Goncalves AK, Cobucci RN, Rodrigues HM, de Melo AG, Giraldo PC. Safety, tolerability and side effects of human papillomavirus vaccines: a systematic quantitative review. Braz J Infect Dis. 2014;18(6):651-9. [PubMed ID: 24780368]. https://doi.org/10.1016/j.bjid.2014.02.005.
-
4.
Bolhassani A, Mohit E, Rafati S. Different spectra of therapeutic vaccine development against HPV infections. Hum Vaccin. 2009;5(10):671-89. [PubMed ID: 19684468].
-
5.
Thones N, Muller M. Oral immunization with different assembly forms of the HPV 16 major capsid protein L1 induces neutralizing antibodies and cytotoxic T-lymphocytes. Virology. 2007;369(2):375-88. [PubMed ID: 17822733]. https://doi.org/10.1016/j.virol.2007.08.004.
-
6.
Kwak K, Yemelyanova A, Roden RB. Prevention of cancer by prophylactic human papillomavirus vaccines. Curr Opin Immunol. 2011;23(2):244-51. [PubMed ID: 21185706]. https://doi.org/10.1016/j.coi.2010.11.009.
-
7.
Xu D, Wang D, Yang X, Cao M, Yu J, Wang Y. Fusion of HPV L1 into Shigella surface IcsA: a new approach in developing live attenuated Shigella-HPV vaccine. Antiviral Res. 2014;102:61-9. [PubMed ID: 24333518]. https://doi.org/10.1016/j.antiviral.2013.12.003.
-
8.
Salehi M, Taheri T, Mohit E, Zahedifard F, Seyed N, Taslimi Y, et al. Recombinant Leishmania tarentolae encoding the HPV type 16 E7 gene in tumor mice model. Immunotherapy. 2012;4(11):1107-20. [PubMed ID: 23194361]. https://doi.org/10.2217/imt.12.110.
-
9.
Fraillery D, Baud D, Pang SY, Schiller J, Bobst M, Zosso N, et al. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin Vaccine Immunol. 2007;14(10):1285-95. [PubMed ID: 17687110]. https://doi.org/10.1128/CVI.00164-07.
-
10.
Echchannaoui H, Bianchi M, Baud D, Bobst M, Stehle JC, Nardelli-Haefliger D. Intravaginal immunization of mice with recombinant Salmonella enterica serovar Typhimurium expressing human papillomavirus type 16 antigens as a potential route of vaccination against cervical cancer. Infect Immun. 2008;76(5):1940-51. [PubMed ID: 18332214]. https://doi.org/10.1128/IAI.01484-07.
-
11.
Bermudez-Humaran LG, Cortes-Perez NG, Lefevre F, Guimaraes V, Rabot S, Alcocer-Gonzalez JM, et al. A novel mucosal vaccine based on live Lactococci expressing E7 antigen and IL-12 induces systemic and mucosal immune responses and protects mice against human papillomavirus type 16-induced tumors. J Immunol. 2005;175(11):7297-302. [PubMed ID: 16301635].
-
12.
Baud D, Ponci F, Bobst M, De Grandi P, Nardelli-Haefliger D. Improved efficiency of a Salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J Virol. 2004;78(23):12901-9. [PubMed ID: 15542642]. https://doi.org/10.1128/JVI.78.23.12901-12909.2004.
-
13.
Xu W, Liu J, Gong W, Chen J, Zhu S, Zhang L. Protective immunity against Chlamydia trachomatis genital infection induced by a vaccine based on the major outer membrane multi-epitope human papillomavirus major capsid protein L1. Vaccine. 2011;29(15):2672-8. [PubMed ID: 21324344]. https://doi.org/10.1016/j.vaccine.2010.12.132.
-
14.
Yoon SW, Lee TY, Kim SJ, Lee IH, Sung MH, Park JS, et al. Oral administration of HPV-16 L2 displayed on Lactobacillus casei induces systematic and mucosal cross-neutralizing effects in Balb/c mice. Vaccine. 2012;30(22):3286-94. [PubMed ID: 22426329]. https://doi.org/10.1016/j.vaccine.2012.03.009.
-
15.
Basile G, Peticca M. Recombinant protein expression in Leishmania tarentolae. Mol Biotechnol. 2009;43(3):273-8. [PubMed ID: 19779853]. https://doi.org/10.1007/s12033-009-9213-5.
-
16.
Basak A, Shervani NJ, Mbikay M, Kolajova M. Recombinant proprotein convertase 4 (PC4) from Leishmania tarentolae expression system: purification, biochemical study and inhibitor design. Protein Expr Purif. 2008;60(2):117-26. [PubMed ID: 18485734]. https://doi.org/10.1016/j.pep.2008.03.013.
-
17.
Breitling R, Klingner S, Callewaert N, Pietrucha R, Geyer A, Ehrlich G, et al. Non-pathogenic trypanosomatid protozoa as a platform for protein research and production. Protein Expr Purif. 2002;25(2):209-18. [PubMed ID: 12135552].
-
18.
Breton M, Tremblay MJ, Ouellette M, Papadopoulou B. Live nonpathogenic parasitic vector as a candidate vaccine against visceral leishmaniasis. Infect Immun. 2005;73(10):6372-82. [PubMed ID: 16177308]. https://doi.org/10.1128/IAI.73.10.6372-6382.2005.
-
19.
Breton M, Zhao C, Ouellette M, Tremblay MJ, Papadopoulou B. A recombinant non-pathogenic Leishmania vaccine expressing human immunodeficiency virus 1 (HIV-1) Gag elicits cell-mediated immunity in mice and decreases HIV-1 replication in human tonsillar tissue following exposure to HIV-1 infection. J Gen Virol. 2007;88(Pt 1):217-25. [PubMed ID: 17170454]. https://doi.org/10.1099/vir.0.81995-0.
-
20.
Mutiso JM, Macharia JC, Mutisya RM, Taracha E. Subcutaneous immunization against Leishmania major - infection in mice: efficacy of formalin-killed promastigotes combined with adjuvants. Rev Inst Med Trop Sao Paulo. 2010;52(2):95-100. [PubMed ID: 20464130].
-
21.
Hosseinzadeh S, Bolhassani A, Rafati S, Taheri T, Zahedifard F, Daemi A, et al. A non-pathogenic live vector as an efficient delivery system in vaccine design for the prevention of HPV16 E7-overexpressing cancers. Drug Deliv. 2013;20(3-4):190-8. [PubMed ID: 23745741]. https://doi.org/10.3109/10717544.2013.801534.
-
22.
Zhang G, Huang X, Boldbaatar D, Battur B, Battsetseg B, Zhang H, et al. Construction of Neospora caninum stably expressing TgSAG1 and evaluation of its protective effects against Toxoplasma gondii infection in mice. Vaccine. 2010;28(45):7243-7. [PubMed ID: 20832493]. https://doi.org/10.1016/j.vaccine.2010.08.096.
-
23.
Zou J, Huang XX, Yin GW, Ding Y, Liu XY, Wang H, et al. Evaluation of Toxoplasma gondii as a live vaccine vector in susceptible and resistant hosts. Parasit Vectors. 2011;4:168. [PubMed ID: 21871123]. https://doi.org/10.1186/1756-3305-4-168.