Abstract
Background:
Head and Neck cancer (HNC) is the 6th most common cancer worldwide. Its recurrence probability is known as the greatest obstacle to prolong survival rate.Objectives:
This study was conducted to assess survival and recurrence rate of HNC and their associated risk factors.Methods:
This retrospective cohort study was conducted on 179 HNC patients, who were diagnosed from April 2007 to November 2013 in Tehran, Iran. Two outcomes were simultaneously analyzed: time between diagnosis and disease recurrence or inter-recurrences; the time between diagnosis and death or end of the study. Kaplan-Meier curve, log-rank test, and general joint frailty model were utilized to data analysis, using Stata 11.0 and R.Results:
From 179 patients, 52.5% experienced relapse at least once and 15.6% of cases deceased. The survival rate in 12-, 24-, and 60- month were 94.4, 83.1%, and 55.4%, respectively. The median of survival time was 60.92 (1.1 - 72.9) months, which was longer in patients with relapse (63.62 versus 24.16). Advanced stage and the age older than 50 significantly increased the risk of death about 4-fold and 3-fold (P = 0.007, P = 0.014). Moreover, the initial treatment of surgery + radiotherapy + chemotherapy had significantly raised the hazard of relapse (P < 0.001).Conclusions:
The percentage of deceased patients in relapse group was more than non-relapses, but the median of survival time in them was longer. Early detection can prevent recurrent events and the premature death of HNC patients.Keywords
Head and Neck Neoplasms Survival Recurrence Joint frailty model
1. Background
Head and neck cancer (HNC), including epithelial malignancies, is developed in the paranasal sinuses, nasal cavity, oral cavity, pharynx, larynx, and parotid gland (1). This cancer with more than a half a million new cases in each year is the 6th most common cancer in the world (2, 3). In 2012, about 375000 people died from HNC throughout the world, which was 4.6% of total cancer mortality (2).
HNC is associated with firmly, specific environmental and lifestyle risk factors such as tobacco, cigarette, smoking, addicting to wine, human papillomavirus, and ultraviolet radiation (4). The American Cancer Society has reported the 5-year survival rate of HNC patients about 40% to 50% in United States (5). This rate is affected by multiple factors, like the stage of disease at the time of diagnosis, treatment methods, primary tumor site, and some other factors (6). Beside the mentioned potential risk factors, the greatest obstacle to prolong survival rate in HNC is the probability of recurrence of disease, which varies between 25% and 50%, depending on the location of cancer (7, 8). Thus, to determine the risk indicators of the patients’ survival, the occurrence of the relapse should be accounted for. Although the reasons for continued high rates of recurrence in HNC patients have not been identified yet, performance status of patients, tumor stage, the location of cancer, and the treatment type (surgery, chemotherapy, and radiotherapy) were known as the important prognostic factors for relapse (8).
Assessing prognostic factors associated with the occurrences of relapse and death requires especial models called joint frailty models. Initially, these models have been proposed by Lancaster and Intrator (9), which induced the dependence between the two rates by an unobserved shared frailty, but various studies have shown that the shared frailty did not apply for the two rates equivalently (10-12). Rondeau et al. (12) have proposed a kind of joint frailty model that considered a dependence between the two rates, but it was not able to deduce the origin of this dependence (the dependence between recurrences or the dependence between recurrent and terminal events). Mazroui et al. (13) in 2012 have developed a general joint frailty model for recurrent events in the presence of a dependent terminal event with two frailty components. These frailties distinguish between the inter-recurrences dependence and the dependence between the two survival end points. Also, using general joint frailty model, compared to separate models for recurrent and death events, lead to avoid biased estimations of regression coefficients.
In recent years, numerous studies have been conducted to assess prognostic risk factors on survival (6, 14) and recurrence rate of HNC patients (8, 15). According to the literature reviewed, we found few papers investigating the survival rate of HNC patients after recurrence (16) or considering the recurrent event as a prognostic factor on survival rate (17). Masoudi et al. in 2017 assessed the risk of local and metastatic recurrence and death in HNC patients (18). We also found no published manuscript about joint modeling of relapse and death occurrence. Regarding the geographical location of Iran in the second high-risk area in the world for oral cavity cancer (3), assessing the survival and recurrence rate of HNC in our country seems to be necessary. The main focus of most studies in this field was epidemiological characteristics of Iranian patients and providing a map of relative risk for this cancer (19-22) and little information is available about the survival rate of this cancer (23).
2. Objectives
Therefore, the present study was conducted to detect prognostic factors associated with both recurrent and death events for HNC patients in Tehran (capital of Iran). Also, we aimed at assessing the inter-recurrences dependency and the relationship between delayed disease recurrences and prolonged survival in HNC patients, using a general joint frailty model.
3. Methods
This retrospective cohort study was conducted from April 2007 to November 2013 in Tehran, the capital of Iran. All HNC patients, who were referred to the Taleghani Hospital and diagnosed based on pathology biopsy in this period of time, were enrolled; 179 patients were eligible according to the inclusion criteria and nobody excluded. Data gathering was done from patients’ medical records by a predetermined checklist including gender, age at diagnosis (older or younger than 50 years), smoking history, consumption of alcohol and other opiate, wearing dentures, primary complaint such as ulcer, swelling-mass, pain, type of cancer (squamous cell carcinoma [SCC] or Non-SCC), stage of disease at diagnosis (primary [stage I & II], advanced [stage III & IV]), clinical view at diagnosis (nasopharynx + pharynx + larynx, tongue and others), duration between the first noticing symptoms and the first visit of physician (less or more than 6 months), type of initial treatment after diagnosis (surgery, radiotherapy + chemotherapy and surgery + radiotherapy + chemotherapy), date of cancer diagnosis, and date of recurrent events to HNC. Also, the endpoint status (whether alive or death) of the patients was obtained by phone interview with the permission of both the patients and the hospital. The “alcohol use” variable was excluded from the analysis because of a low sample size.
In this study, two interested outcomes were simultaneously analyzed in the joint model. First, the duration between diagnosis time and the initial recurrence of disease or between repeated recurrences; second, the time between diagnosis and death or the end of the study. The patients with no relapse or lost to follow-up were respectively censored for relapse and survival time.
To study the prognostic factors associated with both recurrent events and death in HNC patients, some descriptive statistics were carried out such as life tables, Kaplan-Meier curve, and log-rank test. Moreover, the general joint frailty model was fitted to obtain unbiased estimates of the model parameters and identify risk factors of recurrent events and death in HNC patients. In this model, two proportional hazards (PH) survival models are combined: the relapse time of HNC and the time to occurrence of death. These two models are linked through two frailty components (vi, ui) to distinguish the inter-recurrences dependence and the dependence between two survival endpoints (occurrence of relapse or death). This model can be written as:
Where r0(t) and λ0(t) are the recurrent and terminal event baseline hazard functions. In addition, β1 and β2 are the regression coefficient vectors for occurrences of relapse and death associated with the covariate vector Zi(t). It is assumed that ui and vi are two independent frailties with gamma distribution and variances η and θ. These variances, respectively, indicate dependencies between recurrent times and dependence between occurrences of recurrences and death (13). A high value of θ means strong dependence between recurrent and death events and θ = 0 illustrates independency between death and recurrent times. A high value of η illustrates strong dependence between recurrent events and when η = 0, the recurrent times for the same individual are independent of each other (13).
In this model, the assumption of hazard proportionality was checked for the time of relapses and death. Then, we fitted univariate models for every single variable and selected variables with P value less than 0.3 to include in the joint analysis (results were not shown here). After fitting general joint, frailty model with remaining variables, the variables with P value more than 0.3 that their elimination leads to better fitting, were removed from the model. All analyses were performed at 0.05 significance level, using Stata 11 (Stata Corp, College Station, TX, USA) software and “frailtypack”, a freely available package from the Comprehensive R Archive Network (CRAN).
This study was approved by the Ethics Committees of the Shahid Beheshti University of Medical Science, Tehran, Iran. Also, we obtained permission from the Taleghani Hospital to use the data and get informed consent from all patients during follow-up by phone.
4. Results
In this retrospective cohort study, 179 HNC patients were followed after diagnosis from 2007 to 2013. Out of 179 patients, there were 94 men (52.5%), the age of diagnosis for 100 (55.9%) patients were more than 50 years, the original diagnosis of 133 patients were SCC (81.1%), 70 patients had advanced stage (39.1%), and 40 patients visited physician after passing 6 months from the first noticing symptoms (22.4%). Among these patients, 48 (26.8%) and 22 (12.3%) cases had consumption of smoke and other-opiate, respectively. The primary complaint of 70 (39.1%), 114 (63.7%), and 145 (81.1%) patients were ulcer, swelling-mass, and pain, respectively. The initial type of treatment for 103 cases was surgery (57.5%), 32 cases were radiotherapy + chemotherapy (17.8%), and 44 cases were surgery + radiotherapy + chemotherapy (24.7%). The first clinical view of 59 patients was nasopharynx + pharynx + larynx (33%), 37 patients were tongue (20.7%), and 83 patients were others (46.4%).
The mean (SD) duration of follow-up time for patients under study was 13.76 (19.41) months. The number of patients, who experienced 1, 2, and up to 5 relapse (s), was 64 (35.7%), 21 (11.7%), and 9 (5.1%) cases, respectively. Moreover, 28 cases of death (15.6%) occurred during the follow-up period. As shown in Figure 1. and life tables, the survival rate of patients with HNC in 12-, 24-, 60-, and 74-month were 94.4%, 83.1%, 55.4%, and 29.8%, respectively.
Survival rate in head and neck cancer patients from the time of diagnosis to the time of death using Kaplan-Meier curve with 95% confidence interval
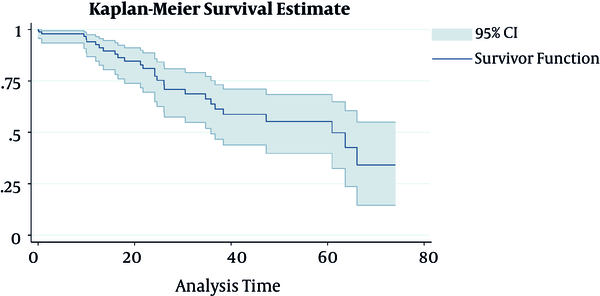
Table 1 presents the median times of the HNC recurrence events during 74 months and means (SD) of the gap times between recurrences of the disease. As shown in this table, a decreasing trend can be observed in gap times from the first to the last event, which means that the previous relapse had an impact on the succeeding relapses.
Descriptive Statistics for the HNC Event and Gap Times Between Recurrent HNC
| Recurrent | Number | Median Time to Event, Month | Gap Time, Montha |
|---|---|---|---|
| 1st event | 94 | 12.99 | 19.70 (3.55) |
| 2nd event | 30 | 14.01 | 17.74 (3.94) |
| ≥ 3rd event | 14 | 22.82 | 14.44 (3.90) |
The frequency and percentage of potential indicators in patients who deceased and patients who experienced relapse at least once are listed in Table 2. Also, the mean (SD) of OS (overall survival) and survival at the first event in terms of these factors are reported and compared, using the log rank test. Despite the non-significant association between characteristics and curves of survival at the first event, 3 variables including stage, type of treatment, and occurrence of relapse were statistically significant in OS curves, so that patients with primary stage and those who underwent surgery at diagnosis had higher survival mean than the others. Although the median of OS time was longer in patients with relapse compared to non-relapse patients (63.62 vs. 24.16), the number of patients, who deceased in relapse group, was more than non-relapse.
| Characteristics | Number | Overall Survival | Survival to First Event | ||||
|---|---|---|---|---|---|---|---|
| Number of Death | Mean of Survival Time, Month | P Value | Number of Relapse | Mean of Survival Time at the First Event, Month | P Value | ||
| Sex | 0.452 | 0.852 | |||||
| Female | 85 (47.5) | 15 (17.7) | 46.35 ± 4.23 | 42 (49.4) | 18.01 ± 2.91 | ||
| Male | 94 (52.5) | 13 (13.8) | 52.88 ± 4.74 | 52 (55.3) | 16.36 ± 2.72 | ||
| Age, y | 0.289 | 0.558 | |||||
| < 50 | 79 (44.1) | 12 (15.2) | 53.53 ± 4.69 | 46 (58.2) | 18.50 ± 2.87 | ||
| ≥ 50 | 100 (55.9) | 16 (16.0) | 45.66 ± 4.63 | 48 (48.0) | 15.82 ± 2.79 | ||
| Type | 0.667 | 0.635 | |||||
| Not SCC | 46 (25.7) | 9 (19.6) | 53.25 ± 5.38 | 28 (60.9) | 19.35 ± 3.65 | ||
| SCC | 133 (74.3) | 19 (14.3) | 47.71 ± 4.20 | 66 (49.6) | 16.01 ± 2.39 | ||
| Stage | < 0.001 | 0.149 | |||||
| Primary | 109 (60.9) | 13 (11.9) | 56.82 ± 3.49 | 64 (58.7) | 18.83 ± 2.59 | ||
| Advance | 70 (39.1) | 15 (21.4) | 34.78 ± 5.76 | 30 (42.9) | 12.94 ± 2.45 | ||
| Smoke | 0.239 | 0.380 | |||||
| No | 131 (73.2) | 23 (17.6) | 47.23 ± 3.78 | 66 (50.4) | 16.59 ± 2.38 | ||
| Yes | 48 (26.8) | 5 (10.4) | 58.69 ± 5.66 | 28 (58.3) | 18.35 ± 3.65 | ||
| Other-opiate | 0.359 | 0.665 | |||||
| No | 157 (87.7) | 24 (15.3) | 50.44 ± 3.54 | 87 (55.4) | 17.15 ± 2.14 | ||
| Yes | 22 (12.3) | 4 (18.2) | 39.37 ± 7.66 | 7 (31.8) | 16.16 ± 2.93 | ||
| Denture | 0.471 | 0.282 | |||||
| No | 161 (89.9) | 26 (16.1) | 49.16 ± 3.64 | 84 (52.2) | 18.02 ± 2.21 | ||
| Yes | 18 (10.1) | 2 (11.1) | 59.83 ± 7.26 | 10 (55.6) | 10.45 ± 2.58 | ||
| Ulcer | 0.423 | 0.983 | |||||
| No | 109 (60.9) | 20 (18.3) | 47.83 ± 4.11 | 61 (56.0) | 17.48 ± 2.67 | ||
| Yes | 70 (39.1) | 8 (11.4) | 52.86 ± 5.56 | 33 (47.1) | 16.89 ± 3.02 | ||
| Swelling-mass | 0.547 | 0.683 | |||||
| No | 65 (36.3) | 8 (12.3) | 50.76 ± 5.31 | 32 (49.2) | 17.72 ± 3.03 | ||
| Yes | 114 (63.7) | 20 (17.5) | 48.58 ± 4.22 | 62 (54.4) | 17.06 ± 2.65 | ||
| Pain | 0.413 | 0.873 | |||||
| No | 34 (18.9) | 7 (20.6) | 45.30 ± 7.46 | 17 (50.0) | 16.81 ± 4.62 | ||
| Yes | 145 (81.1) | 21 (14.5) | 50.88 ± 3.76 | 77 (53.1) | 17.34 ± 2.25 | ||
| Treatment | 0.019 | 0.134 | |||||
| Surgery | 103 (57.5) | 10 (9.7) | 56.69 ± 4.40 | 47 (45.6) | 20.33 ± 3.09 | ||
| Radiotherapy + chemotherapy | 32 (17.8) | 6 (18.8) | 46.67 ± 7.56 | 17 (53.1) | 16.80 ± 4.62 | ||
| Radiotherapy + chemotherapy + surgery | 44 (24.7) | 12 (27.3) | 36.99 ± 5.17 | 30 (68.2) | 12.61 ± 2.99 | ||
| Clinical view | 0.921 | 0.700 | |||||
| Other | 83 (46.4) | 10 (12.0) | 43.72 ± 4.41 | 39 (47.0) | 16.27 ± 2.77 | ||
| Nasopharynx + pharynx + larynx | 59 (33.0) | 11 (18.6) | 52.14 ± 5.38 | 35 (59.3) | 16.51 ± 3.71 | ||
| Tongue | 37 (20.7) | 7 (18.9) | 47.72 ± 6.76 | 20 (54.1) | 20.27 ± 4.31 | ||
| Duration | |||||||
| ≤ 6 month | 139 (77.6) | 24 (17.3) | 46.48 ± 3.57 | 0.163 | 73 (82.8) | 15.85 ± 2.23 | 0.140 |
| > 6 month | 40 (22.4) | 4 (10.0) | 58.97 ± 6.42 | 21 (38.0) | 21.41 ± 4.44 | ||
| Relapse | 0.013 | - | |||||
| No | 85 (47.5) | 6 (7.1) | 28.23 ± 4.77 | - | - | ||
| Yes | 94 (52.5) | 22 (23.4) | 52.20 ± 3.49 | - | - | ||
Regarding the described association between the occurrence of relapse and death and dependence between gap times (as shown in Table 1), general joint frailty model was applied. The results of this model were shown in Table 3; the effect of potential risk factors on recurrence rate and death are assessed and reported in the right and left panels, respectively. Accordingly, age older than 50 years and advanced stage were two factors that significantly increased the risk of death 2.93-fold (P = 0.014) and 3.97-fold (P = 0.007), respectively. Based on these findings, the only factor that significantly increased the risk of relapse was the type of treatment, so that the risk of relapse in patients with initial treatment of surgery + radiotherapy + chemotherapy was about two times of patients who had only surgery (P < 0.001). According to the results of this study, other factors including denture, other-opiate, ulcer, swelling mass, and duration had no significant effect on the survival or recurrence rate.
Assessing the Effect of Various Factors on Death and Recurrence Rate Using General Joint Model
| Variables | Death | Recurrence | ||||
|---|---|---|---|---|---|---|
| HR | (95% CI) | P Value | HR | (95% CI) | P Value | |
| Age, y | ||||||
| < 50 | 1 | - | - | - | - | - |
| ≥ 50 | 2.93 | (1.24, 6.94) | 0.014 | - | - | - |
| Stage | ||||||
| Primary | 1 | - | - | - | - | - |
| Advanced | 3.97 | (1.44, 10.93) | 0.007 | - | - | - |
| Denture | ||||||
| No | 1 | - | - | - | - | - |
| Yes | 0.35 | (0.05, 2.44) | 0.291 | - | - | - |
| Other-opiate | ||||||
| No | - | - | - | 1 | - | - |
| Yes | - | - | - | 0.66 | (0.28, 1.53) | 0.330 |
| Ulcer | ||||||
| No | 1 | - | - | 1 | - | - |
| Yes | 3.06 | (0.60, 15.61) | 0.179 | 1.70 | (0.54, 5.41) | 0.367 |
| Swelling-mass | ||||||
| No | 1 | - | - | 1 | - | - |
| Yes | 3.02 | (0.63, 14.39) | 0.165 | 1.77 | (0.55, 5.72) | 0.339 |
| Duration | ||||||
| ≤ 6 month | 1 | - | - | 1 | - | - |
| > 6 month | 0.65 | (0.32, 1.29) | 0.215 | 0.87 | (0.62, 1.23) | 0.428 |
| Treatment | ||||||
| Surgery | 1 | - | - | 1 | - | - |
| Radiotherapy + chemotherapy | 1.70 | (0.49, 5.89) | 0.215 | 1.04 | (0.55, 1.97) | 0.910 |
| Radiotherapy + chemotherapy + surgery | 2.34 | (0.76, 7.2) | 0.403 | 1.97 | (1.07, 3.63) | 0.030 |
| Estimate | SD | |||||
| Theta | 0.46 | 0.21 | 0.014 | - | - | - |
| Eta | 0.005 | 0.0005 | < 0.001 | - | - | - |
Regarding the results in Table 3, the estimate of the frailty parameters was significantly different from zero. The estimated η represents a small but significant inter-recurrences dependence between the relapse gap times, which means that the duration between two consecutive relapses depends slightly on the previous duration intervals. This significance is confirmed according to result of Table 1, so that increasing the number of relapse decreases mean gap time of consecutive relapses.
Moreover, the estimate of θ shows a significant dependence between the occurrences of relapse and death, which means that the non-observed indicator risk factors concurrently weaken or strengthen these both events. Also, increasing the rate of recurrence increases the death hazard.
5. Discussion
The purpose of this study was assessing the influence of different indicators on relapses and death among HNC patients, using a general joint frailty model. Using this model have two important advantages. Firstly, this model enables us to estimate the effect of explanatories on two survival endpoints concurrently. So, we found that the age older than 50 years and advanced stage were two significant variables to increase the risk of death, and surgery + radiotherapy + chemotherapy as initial treatment was the only significant indicator that raised the risk of relapse. Secondly, this model estimates two significant dependencies between the relapse gap times and dependence between relapse and death occurrences. Accounting for these dependencies lead to avoid biased estimations of regression coefficients compared to separate models for recurrent and death events.
The findings of this research presented that the 1-, 2-, and 5-year survival rates were 94%, 83%, and 55%, respectively. Also, more than half of the patients (52.5%) experienced relapse at least once. Based on the report of WHO in 2014, the 5-year survival rate for HNC patients was reported about 40% to 50% (24). The findings of the current study consistently indicated that the median of survival time for these patients was approximately 5 years (60.92 months). Tiwana et al. conducted a study in a Canadian province on 1 657 HNC patients and assessed primary site specific and long-term survival. They found that 2-, 5-, 15-, and 25-year OS rates were 64%, 46%, 21%, and 11%, respectively (14). Pruegsanusak et al. studied 1 186 Thai patients and reported that the 5-year OS rate was 24.1% (6). In Netherlands, Braakhuis et al. reported 2-year survival rate about 72% for patients under study 25. Also, Novin et al. who studied 119 Iranian patients, reported the 28 months OS about 61.2% (23). Despite similarities between this research and many other studies, some inconsistencies in survival rate exist, too. It can be attributed to the various socioeconomic status of patients, which cause different access to health care facilities in various areas. This assess may lead to earlier/later diagnosis and treatment of cancer and, consequently, more/fewer chances to survive (23, 25).
According to previous studies, the rate of recurrence varied from 25% to 50% based on the various sites of cancer (7, 8, 26). Sakashita et al. conducted a study in Japan and investigated the role of initial neck dissection with node-positive oropharyngeal squamous cell carcinomas. They found that the recurrent or persistent regional disease for 109 patients was 36.7% at a median of 11 months after the first therapy (27). In another study, which was conducted in Spanish patients with squamous HNC, the researchers reported the overall incidence of locoregional tumor recurrence about 19.9%, which had a negative impact on survival time (17). Based on the results of the present study, 52.5% of the patients had at least one relapse at a median of 13 months after diagnosis, which was higher than some other researches. Also, despite the more percentage of death in the relapse group, the median of OS time in these patients was longer than non-relapse ones. The reason for this disparity can be explained as follow; according to the previous researches, the 5-year OS rate for patients with relapse in the primary stage was longer than the advanced stage (83% versus 48%) (28) Moreover, many studies reported that more than half of the cases were at an advanced stage (6, 14), while only about 39% of the patients of this study had this condition. According to the results of this study, most of the patients with primary stage had relapse at least once (about 60%), and some of them died after the experience of repeated recurrences. On the other hand, most of the patients, who died without any relapse, were in advanced stage and had no opportunity to relapse. Thus, despite occurring more number of death in patients with relapse, the median survival time in these patients was longer than others.
The findings of the present research indicated that older age and advanced stage of disease at diagnosis caused higher risk of death on HNC patients. These findings are consistent with results of most studies (6, 14). Moreover, patients treated with surgery + radiotherapy + chemotherapy at diagnosis had significantly higher risk of relapse compared to surgery. This may be the result of treatment type, which was used for different stages of the disease. The single-modality therapy (such as surgery or radiation alone) and combined therapy were allocated to patients with primary and advance stages, respectively (29). On the other hand, the advance stage was known as an important factor to raise the risk of relapse in HNC patients (8, 26, 30). With regard to this study, the stage of more than two-thirds of the patients (77%) with only surgery were primary and more than half of the patients (55%) treated with combined therapy (radiotherapy + chemotherapy, surgery + radiotherapy + chemotherapy) were advanced. Thus, the high rate of relapse in patients with surgery + radiotherapy + chemotherapy can be due to more percentage of patients with advanced stages in this group.
Since access to other patients in other hospital was not possible, this study just included patients, who were referred to Taleghani Hospital. So, an important limitation of this study was low generalizability of these results. Despite its limitation, this study could give a general description of the two survival endpoints (recurrent and death time) and examine the effect of several potential risk factors on both these rates. Another considerable result of the present study was measuring two significant dependencies between consecutive relapses and between occurrences of relapse and death that were obtained, using general joint frailty model. This model not only indicated dependencies between the mentioned events, but also led to obtaining unbiased estimates of the model parameters. The present study seems to be the first that investigated these both outcomes of HNC patients and factors that are influencing them. Thus, the results of this study help better understanding about the indicators on recurrence and death rate and may help policymakers, who plan preventive program, to early detection of patients and reducing these rates.
5.1. Conclusion
The results of this study estimated survival and recurrence rates and assessed the influence of prognostic factors on both survival endpoints. We concluded that more than half of the patients experienced relapse at least once. The percentage of patients, who deceased in relapse group, was more than non-relapse, but the median of OS time in them was longer than non-relapse patients. Furthermore, we found that age older than 50 years and advanced stage at diagnosis had significant effect to increase the risk of death. Although the rate of recurrence in HNC patients was most common, early detection can at least prevent the premature death of patients.
Acknowledgements
References
-
1.
Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371(9625):1695-709. https://doi.org/10.1016/s0140-6736(08)60728-x.
-
2.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-86. [PubMed ID: 25220842]. https://doi.org/10.1002/ijc.29210.
-
3.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [PubMed ID: 25651787]. https://doi.org/10.3322/caac.21262.
-
4.
Ragin CC, Modugno F, Gollin SM. The epidemiology and risk factors of head and neck cancer: a focus on human papillomavirus. J Dent Res. 2007;86(2):104-14. [PubMed ID: 17251508]. https://doi.org/10.1177/154405910708600202.
-
5.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clinic. 2015;65(1):5-29. https://doi.org/10.3322/caac.21254.
-
6.
Pruegsanusak K, Peeravut S, Leelamanit V, Sinkijcharoenchai W, Jongsatitpaiboon J, Phungrassami T, et al. Survival and prognostic factors of different sites of head and neck cancer: an analysis from Thailand. Asian Pac J Cancer Prev. 2012;13(3):885-90. [PubMed ID: 22631666].
-
7.
Cooper JS, Pajak TF, Forastiere AA, Jacobs J, Campbell BH, Saxman SB, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N Engl J Med. 2004;350(19):1937-44. [PubMed ID: 15128893]. https://doi.org/10.1056/NEJMoa032646.
-
8.
Ho AS, Kraus DH, Ganly I, Lee NY, Shah JP, Morris LG. Decision making in the management of recurrent head and neck cancer. Head Neck. 2014;36(1):144-51. [PubMed ID: 23471843]. https://doi.org/10.1002/hed.23227.
-
9.
Lancaster T, Intrator O. Panel data with survival: hospitalization of HIV-positive patients. J American Statistic Associat. 1998;93(441):46-53. https://doi.org/10.1080/01621459.1998.10474086.
-
10.
Liu L, Wolfe RA, Huang X. Shared frailty models for recurrent events and a terminal event. Biometrics. 2004;60(3):747-56. [PubMed ID: 15339298]. https://doi.org/10.1111/j.0006-341X.2004.00225.x.
-
11.
Huang X, Liu L. A joint frailty model for survival and gap times between recurrent events. Biometrics. 2007;63(2):389-97. [PubMed ID: 17688491]. https://doi.org/10.1111/j.1541-0420.2006.00719.x.
-
12.
Rondeau V, Mathoulin-Pelissier S, Jacqmin-Gadda H, Brouste V, Soubeyran P. Joint frailty models for recurring events and death using maximum penalized likelihood estimation: application on cancer events. Biostatistics. 2007;8(4):708-21. [PubMed ID: 17267392]. https://doi.org/10.1093/biostatistics/kxl043.
-
13.
Mazroui Y, Mathoulin-Pelissier S, Soubeyran P, Rondeau V. General joint frailty model for recurrent event data with a dependent terminal event: Application to follicular lymphoma data. Stat Med. 2012;31(11-12):1162-76. [PubMed ID: 22307954]. https://doi.org/10.1002/sim.4479.
-
14.
Tiwana MS, Wu J, Hay J, Wong F, Cheung W, Olson RA. 25 year survival outcomes for squamous cell carcinomas of the head and neck: population-based outcomes from a Canadian province. Oral Oncol. 2014;50(7):651-6. [PubMed ID: 24731736]. https://doi.org/10.1016/j.oraloncology.2014.03.009.
-
15.
Ricketts K, Williams M, Liu ZW, Gibson A. Automated estimation of disease recurrence in head and neck cancer using routine healthcare data. Comput Methods Programs Biomed. 2014;117(3):412-24. [PubMed ID: 25306243]. https://doi.org/10.1016/j.cmpb.2014.08.008.
-
16.
Amar A, Chedid HM, Rapoport A, Dedivitis RA, Cernea CR, Brandao LG. Update of assessment of survival in head and neck cancer after regional recurrence. J Oncol. 2012;2012.
-
17.
Alvarez Marcos CA, Llorente Pendás JL, Franco Gutierrez V, Fernández Espina H, Alonso Guervos M, Suárez Nieto C, et al. Tumour recurrence in squamous head and neck cancer. Acta Otorrinolaringol (English Edition). 2007;58(4):156-63. https://doi.org/10.1016/s2173-5735(07)70324-1.
-
18.
Masoudi S, Montazeri SA, Pourdanesh F, Biglarian A, Kazemi M, Rahgozar M. A new approach to survival analysis of head and neck squamous cell carcinoma. Archiv Iran Med (AIM). 2017;20(8).
-
19.
Kavousi A, Meshkani MR, Mohammadzadeh M. Spatial analysis of relative risk of lip cancer in Iran: a Bayesian approach. Environmetric. 2009;20(4):347-59. https://doi.org/10.1002/env.927.
-
20.
Rad M, Chamani G, Zarei M, Hashemipour M. Epidemiological aspects of head and neck cancers in a group of Iranian population. J Dentistr Shiraz Univ Med Sci. 2010;10:50-6.
-
21.
Larizadeh MH, Damghani MA, Shabani M. Epidemiological characteristics of head and neck cancers in southeast of iran. Iran J Cancer Prev. 2014;7(2):80-6. [PubMed ID: 25250154]. [PubMed Central ID: PMC4142945].
-
22.
Mirzaei M, Hosseini SA, Ghoncheh M, Soheilipour F, Soltani S, Soheilipour F, et al. Epidemiology and trend of head and neck cancers in Iran. Glob J Health Sci. 2015;8(1):189-93. [PubMed ID: 26234980]. [PubMed Central ID: PMC4803954]. https://doi.org/10.5539/gjhs.v8n1p189.
-
23.
Novin K, Ameri A, Faraji S, Torbati P, Mortazavi N. Head and neck squamous cell carcinoma in Iran: clinico-pathological and treatment-related factors influencing survival. Iran J Cancer Preven. 2015;8(5). https://doi.org/10.17795/ijcp-3842.
-
24.
World Health Organization. Head and neck cancer. Union for international cancer control. 2014 review of cancer medicines on the WHO list of essential medicines. 2014. Available from: http://www.who.int/selection_medicines/committees/expert/20/applications/cancer/en/#.
-
25.
Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50(7):670-5. [PubMed ID: 24735546]. https://doi.org/10.1016/j.oraloncology.2014.03.008.
-
26.
Bernier J, Domenge C, Ozsahin M, Matuszewska K, Lefebvre JL, Greiner RH, et al. Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-52. [PubMed ID: 15128894]. https://doi.org/10.1056/NEJMoa032641.
-
27.
Sakashita T, Homma A, Hayashi R, Kawabata K, Yoshino K, Iwae S, et al. The role of initial neck dissection for patients with node-positive oropharyngeal squamous cell carcinomas. Oral Oncol. 2014;50(7):657-61. [PubMed ID: 24726547]. https://doi.org/10.1016/j.oraloncology.2014.03.003.
-
28.
Goodwin WJ. Salvage surgery for patients with recurrent squamous cell carcinoma of the upper aerodigestive tract: when do the ends justify the means? Laryngoscope. 2000;110(3 Pt 2 Suppl 93):1-18. [PubMed ID: 10714711]. https://doi.org/10.1097/00005537-200003001-00001.
-
29.
Kiyota N, Tahara M, Fujii M. Adjuvant treatment for post-operative head and neck squamous cell carcinoma. Jpn J Clin Oncol. 2015;45(1):2-6. [PubMed ID: 25411434]. https://doi.org/10.1093/jjco/hyu195.
-
30.
Bernier J, Cooper JS, Pajak TF, van Glabbeke M, Bourhis J, Forastiere A, et al. Defining risk levels in locally advanced head and neck cancers: a comparative analysis of concurrent postoperative radiation plus chemotherapy trials of the EORTC (#22931) and RTOG (# 9501). Head Neck. 2005;27(10):843-50. [PubMed ID: 16161069]. https://doi.org/10.1002/hed.20279.