Abstract
Context:
Prior research over the past few decades has shown that the hypofractionated external beam radiation therapy could be a valuable method with an outstanding potential to treat the prostate cancer. The alpha-beta ratio (α/β) is an indication of the fractionation sensitivity of a particular cell type. Values of α/β ≥ 10 Gy are responsible for early-responding normal tissues and most tumors, while values of α/β < 5 Gy are for late-responding normal tissues. The α/β ratio could be raised by either a local tumor control increase or a decrease of normal tissue late effects through the application of an unconventional fractionation schedules.Evidence Acquisition:
This review article aimed to investigation the details of radiobiological and clinical trials which are reported to highlight the characteristics of hypofractionation for the prostate cancer treatment based on the investigation of clinical results from hypofractionated treatments.Results:
With the sufficient radiobiological and physiological research studies, it may be claimed that if α/β value for prostate cancer is less than the adjacent surrounding organs at risk and by applying fewer and larger fractions; a better therapeutic ratio can be achieved for the patient.Conclusions:
Radiobiological modeling can play a major role in the design of new protocols. Also, one should be aware that in modeling the hypofractionated treatment, normal tissue iso-effects are significantly important and some assumptions needed to be made regarding α/β values for the critical normal tissue complication endpoint.Keywords
Prostate Cancer Hypofractionation Linear Quadratic Model Normal Tissue Complication Probability (NTCP) Tumor Control Probability (TCP)
1. Context
Mid 1930’s Coutard et al. (1) reported that his radiation fractionated strategy had an amazing result regarding the cure of deep-seated tumors which had previously thought to be hopeless. The fractionated technique employed has been used in external beam radiotherapy ever since. Coutard suggested that a protracted schedule of teletherapy using multiple fractions regime over several weeks could be beneficial. However, other researchers believed that the fractionated method is a “primitive” method (2, 3). They reasoned that the distinction in response to radiation and normal tissue “will favor the tumor if the cancerocidal dose is not applied in the first treatment”. Additionally, they used the law of Bergonie and Tribondeau added to this discussion that a fractionated schedule will increase the probability of irradiating a cancer cell during a radiosensitive phase of the cell cycle.
In the last two decades, radiation biology is applied in radiation therapy and recommended as a shortened fractionation schedule in the treatment of prostate cancer. Technological developments such as intensity-modulated radiation therapy (IMRT) and improved target localization such as using nanoparticles as a targeted therapy have been combined with radiation biology to generate enthusiasm for hypofractionated regimens (4, 5).
Based on the global cancer statistics literature, one of the most common male cancer diagnosed after nonmelanoma skin cancer is prostate cancer (6). Hence, finding a right treatment regime accompanied with using new software for accurate treatment planning will help to cure the prostate cancer (7-9). Since in most cases the prostate cancer at diagnosis is organ confined (10), prostatectomy, external beam radiation therapy (EBRT) and definitive radiotherapy were introduced as conventional methods to be used for treatment (11).
2. Evidence Acquisition
After the second world war, since there were not enough available therapy machines the tendency was to use the hypofractionated regimens for the patients (12). There are a couple of valuable reports that the treatment of the patients has been done by the radical external-beam radiotherapy at Saint Thomas’s hospital in London. This report, which has been gathered by Collins et al. (13) reported 232 patients from 1964 to 1984. Patients were treated with 3-field, 4-field, or a double-rotation technique from a cobalt-60 machine or linac over 3 weeks with six 6 Gy fractions (2 fractions per week) for a total dose of 36 Gy. Two patients developed rectal strictures, and “a few patients” had recurrent rectal bleeding. They tolerated well, with very few late complications. The survival curves demonstrated that the hypofractionated regimen had good results in comparison to other methods of treatments.
Livesey et al. (14) reported more than 700 men treated with a hypofractionated regimen from 1995 to 1998 using 3.13 Gy fractions to 50 Gy at the Christie hospital. They used modern techniques such as the use of risk-group stratification, CT scan for the treatment planning, and defined a clinical tumor volume (CTV) based on the risk of seminal vesicle invasion. They were stratified into low, intermediate, and high-risk disease based on clinical stage, prostate specific antigen (PSA), and Gleason score. The freedom from biochemical recurrence at 5 years was 82%, 56%, and 39% for the low, intermediate, and high-risk groups, respectively.
Both studies demonstrated a low rate of morbidity. Neither of these fractionation regimens was explicitly designed to exploit the fractionation sensitivity of prostate cancer. In the recent years, most of the trials, which have been done explicitly, were designed assuming that α/β was low (14, 15). This review article aimed to investigation the details of radiobiological and clinical trials which are reported to highlight the characteristics of hypofractionation for the prostate cancer treatment based on the investigating of clinical results from hypofractionated treatments.
3. Results
3.1. Fractionation and Radiobiology Model
Hypofractionation and radiobiology models have common parameters. These parameters are important to be considered for predicting the sensitivity of prostate cancer cells accurately.
The modern radiobiology has been started by the creation of the linear-quadratic model (LQ) formalism for the mammalian cell killing, which has been caused by induced radiation. This method predicts that the survival rate of the cell depends on factors such as overall radiation dose, dose per fraction, and the overall treatment time. Moreover, the α/β could provide a prediction of the dose response of tumors and normal tissues to the fractionated irradiation (4, 14). The Equation 1 present this model (16, 17):
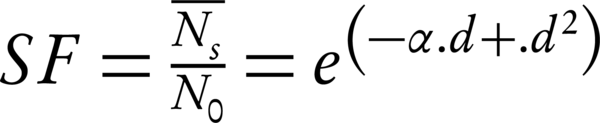
where N0 is the initial number of cells (clonogens), (N̄s) the mean number of surviving clonogens after a radiation dose d, SF the surviving fraction and α and β the cell-specific ‘single-hit’ and ‘double-hit’ coefficients, respectively. The formalism should have been averaged over the cell cycle for the asynchronous cell population cases (18).
By the implementing the model for the fractionated dose delivery (where n is fractions, and d is the dose), the surviving fraction at the end of the treatment course is given by:
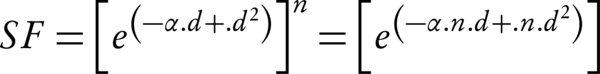
Note that for the Equation 2, it is assumed that the sublethal lesion repair has been completed in the interfraction interval. The Equation 3 could be rewritten according to the total dose D (= n × d):
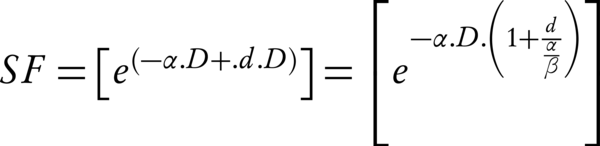
The term D [1 + d/(α/β)] is called the biologically effective dose (BED); if delivered in an infinite number of tiny fractions, a total dose equal to the BED is radiobiologically equivalent (achieves the same surviving fraction) to the regimen of interest (n is the number of fractions and d presents the size) (16). If the fraction size d tends to zero or (α/β) tends to infinity, then the product of the multiplication of [1 + d / (α/β)] and D, tends to unity. The Equation 3 can be written as SF = exp [-α.BED]. The BED could also represent the therapeutic ratio (TR) of the number of fractions. Hence, the BEDα/β = 3, could signify the late effect (normal tissue effect) and BEDα/β = 10, early effect (tumor effect), respectively (19). The maximum TR is attained at the smallest fraction sizes which are consistent with the steady decrease of BEDα/β = 3 (as the number of fractions increases).
3.2. Possible Cell-Killing Scenarios
Reviewing literature shows the linear-quadratic model overestimates cell killing at large fraction sizes (20-22). However, other clinical studies, which have been done by other research groups express that by utilizing an extreme hypofractionation, we could validate the LQ model (23-26). There is also some clinical research, which has been done presented by Song et al. (27) show that the LQ model underpredicts the level of reproductive cell death necessary to achieve the observed tumor control. Additionally, he states that there are some other mechanisms involved, such as indirect/necrotic cell death, which principally has been caused by the vascular damages. By looking at these diverse research results we could have conveyed that there is quite a confusion for the validation of LQ model and the clinical outcomes that have created controversy (4, 27).
3.3. Radical Alteration of Hypofractionation Criterion
One of the crucial factors in a radiotherapy treatment is the duration. If it is too fast (e.g. multiple fractions per day), the therapy dose does not allow for rapidly dividing cells such as mucosal cells to repopulate themselves, which have been killed by the radiation (5, 28, 29). A technique, which could be used for a hypofractionated regimen is to convert the dose received by the mucosal cells that could be equal to the tumor prescribed dose, or to a 2 Gy equivalent dose (EQD2-Gy), applying an α/β = 10 Gy value and afterward see if it is within the ‘safe’ limit for the total treatment time (30).
A high value of α/β (which is similar to 10 Gy) could characterize an effect of radiation, which is so-called ‘acute’ effects caused by radiation treatment. However, hypofractionation with a much lower α/β values has been demonstrated less problematic for ‘acute’ than for ‘late’ complications (30).
3.4. Contrast Between Hypofractionation and Standard Fractionation
Some comprehensive investigations, which have been done on the hypofractionation effect for the prostate carcinoma predicted α/β values ranges from 1.5 Gy to 8.5 Gy with 1 Gy interval according to the modern radiobiological model (30). Those research findings indicated an α/β value of 5 Gy for late complications for both rectum and bladder as the organs at risk (OARs). However, α/β of 3 Gy was predicted for the normal tissue for the late complications (31).
These studies adopted a variable, generalized equivalent uniform dose normalized at 2 Gy per fraction (gEUD2) to explain the radiobiological implication of the dose distribution. This model considered both sensitivity to fractionation (through the linear-quadratic [LQ] model) and volume effects (through generalized equivalent uniform dose [gEUD]). Equation 4 demonstrates the value of gEUD2 for the prostate tumor and for the OARs (both rectum and bladder).

Using a Poisson distribution model, Equations 5 calculate tumor control probability (TCP) and normal tissue complication probability (NTCP) values:
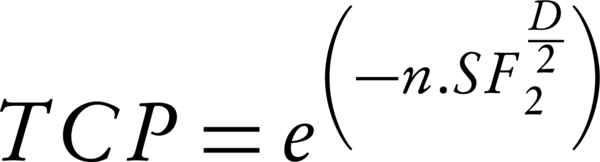
In Equation 5, n and SF2 present the number of clonogenic cells per tumor and the surviving fraction at 2 Gy, respectively. D is the calculated gEUD2 for tumor.
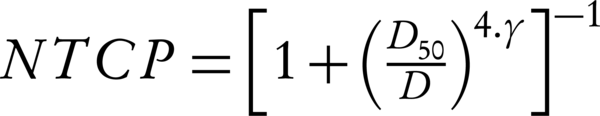
In Equation 6, D50 is the 50% response dose, D presents the calculated gEUD2 for OAR, and γ is the maximum normalized dose-response gradient. The values D50 and γ cited in the studies of Mavroidis et al. (32) the endpoints for such D50 values are smaller for bladder and necrosis and stenosis for rectum. The summary is shown in Table 1 (33).
Biologic Parameters for Bladder and Rectum Used in NTCP Calculation
| Organ at Risk | D50 (Gy) | γ |
|---|---|---|
| Bladder | 80 | 3 |
| Rectum | 75 | 2.5 |
Kallman et al. (34) introduced a plan ranking factor, P+. Equation 7 used NTCP and TCP to calculate P+. This combination of NTCP and TCP in P+ has been done in order to rank the treatment plans.
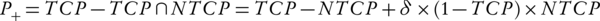
In this equation, δ signifies a fraction of the patients with statistically independent tumor and normal tissue responses. It should have a value less than 20% (32, 33).
Liao et al. (35) in a clinical study considered two sets of NTCP values, one for bladder and one for rectum to calculate P+ values. Since in clinical radiation therapy, late rectal toxicity plays a significant role (33). And applying NTCP for the rectum can be a great help for the decision-making process in the treatment planning.
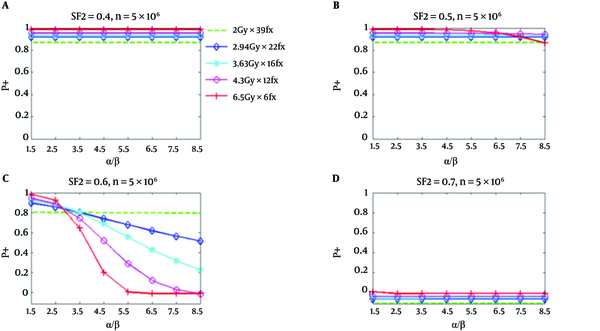
From Figure 1, it can be seen that all hypofractionated treatments have greater P+ values than the standard fractionation for relatively responsive tumor cells (SF2 = 0.4 and 0.5) (this happened when NTCP for the bladder is considered). However, for less responsive tumor cells (SF2 = 0.6), the P+ for all hypofractionation regimens at α/β values between 2.8 Gy and 3.5 Gy do not show any superiority to the standard fractionation Figures 1 and 3 illustrated P+ values for the bladder and rectum (34). It has been assumed that there are 5 × 106 clonogenic cells per tumor. The implemented radiobiologic model has demonstrated improved clinical results for hypofractionation with respect to the standard fractionation under the given conditions. This improvement is for both low α/β and high α/β values.
P+ Using NTCP of the Bladder as a Function of α/β for Five Regimens at SF2 of 0.4, 0.5, 0.6, and 0.7, Respectively; The number of clonogenic cells per tumor is about 5 × 106 (34).
The number of clonogenic per tumor cells assumed to be or 10 × 106, 5 × 106 (19, 35). For final investigation of the different α/β and SF2, 5 × 106 clonogenic cells per tumor has been selected. For the prostate tumor cancer, it is roughly about 0.5 (36). However, the uncertainty of SF2 could be notable (37). Since the uncertainty of SF2 could be indicative, its impact was studied for SF2 ranging from 0.3 to 0.9. Also, NTCP has been calculated according to P+ model.
Figure 2 shows similar P+ results for the rectum. However, P+ for hypofractionation treatments reduces faster and drops below standard fractionation at a lower value of α/β. In Figure 1, for SF2 = 0.4, and α/β of 8.5 Gy, all hypofractionation treatments are anticipated to show superiority with respect to a standard fractionation. For SF2 = 0.5, and the hypofractionation treatments of 4.7 Gy/fraction and 6.5 Gy/fraction, the P+ values are lower than the standard fractionation for α/β higher than 6.5 Gy and 4.7 Gy, correspondingly; however, at all α/β up to 8.5 Gy hypofractionation treatments continued to be superior. At α/β of 2.5 Gy for SF2 = 0.6, the P+ for all hypofractionation treatments shows inferiority respect to the standard fractionation. At high α/β, an improved result has been predicted for all hypofractionation, which has been predicted to produce by the notable decrease of predicted NTCP respect to TCP loss (34).
P+ Using Normal Tissue Complication Probability of Rectum as a Function of α/β for Five Regimens at SF2 of 0.4, 0.5, 0.6, and 0.7, Respectively; The number of clonogenic cells per tumor is about 5 × 106 (34).
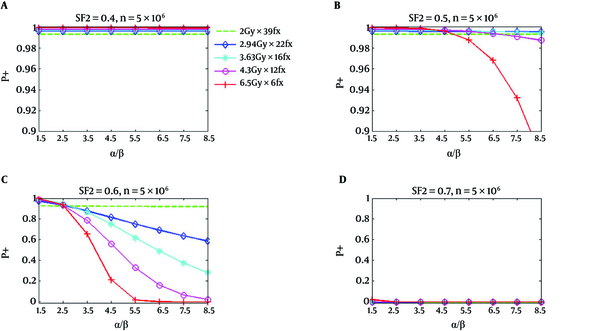
As it has been shown in part d of Figures 1 and 2, the calculated P+ values for the standard and hypofractionation treatments are smaller than zero for the radiation resistant tumor cells (SF2 = 0.7). This signifies that there is no reasonable obtainable complication-free tumor control possibility obtained with the fractionation and if a greater TCP is desirable, the dose should be increased.
Nahum et al. (19) suggested a mean α/β value of 8.3 Gy for prostate tumor cells in a couple of reported radiobiologic clonogenic assays. Additionally, in order to demonstrate the consistency of his results, he listed other in-vitro studies in his publication. Those studies presented relatively high α/β values ranging from 3.48 to 11 Gy (38, 39).
There are a number of studies and evidence showing that the value of α/β is relatively low for the prostate and breast cancers (18, 40, 41). These results have been shown in Figure 3 as promising outcomes for both breast and prostate tumors in the hypofractionated treatment protocols. However, for the prostate tumors case, still, there is some controversy about low α/β (18, 19, 42).
TCP for a Target Volume (Receiving a Homogenous Dose) Over a Range of Fraction Numbers (1 - 50) for Different Tumor α/β; All curves are for the same NTCP, i.e. ‘isotoxic’, here for rectal bleeding (4.3%) for which α/β = 3 Gy has been used. Open circles, α/β 10 Gy; triangles, α/β = 5 Gy; squares, α/β = 3 Gy; diamonds, α/β = 1.5 Gy (43).
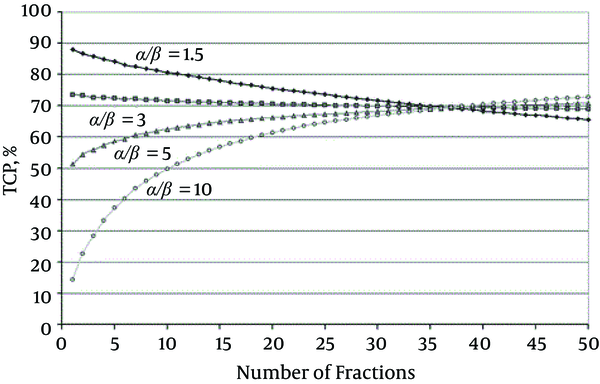
Fowler et al. (44) reported a series of fairly satisfied clinical results from the treatments of the early stage of non-small cell lung cancer (NSCLC) tumors, which have been done by external beam radiotherapy and conventional 1.8 - 2.2 Gy and excess fraction sizes. In other studies, Yarnold et al. and Miralbell et al. (41, 42) reported promising clinical results from prostate cancer treatment by the external beam radiotherapy with hypofractionated regimens for relatively for provides low α/β tumors. Both scenarios primarily follow the LQ model.
Tang et al. (45) used hypofractionation by external beam radiotherapy for prostate cancer beyond the daily 3 Gy per fraction schedules. There are other trials that used a five-fraction schedule delivering 35 Gy in five fractions (45, 46).
The α/β value for prostate cancer ranging from 1 to 4 were addressed, but they are based on clinical data sets for the patients with early and intermediate-risk prostate cancer (47-49). For more aggressive, poor-risk a relatively higher α/β value was estimated (50).
The critical dose-limiting organ at risk near the prostate with a low value of α/β such as the anterior rectal wall created a challenge for the dose-fractionation regimens for the prostate cancer treatment. While the α/β ratio for the rectal wall is 3.5, some studies reported it to be as high as 5 or 6. This means that normal tissue cannot be spared by the use of fractionation (35).
In 2008, Tang et al. (45) combined IMRT with image-guided radiotherapy (IGRT) and used fiducial gold seeds implanted before radiotherapy considering OARs such as both bladder and bowel at the time of radiation therapy and utilized a custom vacuum lock bag for the immobilization. They defined an action level based on the portal imaging to deliver IGRT using a tolerance of only 2 mm.
If increasing the biological dose to the prostate gland with applying hypofractionation is the best solution, developing a routine procedure with applying IGRT with IMRT would be the best approach for the treatment. Since a simple external beam would not be the solution, as a routine procedure simple external beam techniques, such as those in current practice, are unlikely to be adequate (51).
There is a clinical report, in which a high dose rate brachytherapy has been used as a boost after the external beam radiotherapy (52). The other groups reported monotherapy schedules of 36 Gy in four fractions and 31.5 Gy in three fractions (53). They exploited the low value of α/β ratio of prostate cancer by utilizing a few large doses per fraction in order to deliver a radical dose. That could be a great motivation for other therapy centers to encourage them to develop a high dose brachytherapy procedure. This satisfactory result comes from the conceptual physical fact that the brachytherapy method follows an inverse square law, which makes achieving a steep dose gradients adjacent to the critical OARs to be possible. Additionally, a 3 - 5 mm boundary around the prostate gland allows a microscopic spread and extension of the target volume into the base of the seminal vesicles. The brachytherapy method has the capability to eliminate the errors of the internal organ movement and set-up variability, which are the concerns in the external beam radiotherapy. Those parameters enable an accurate high-dose delivery to the CTV while protecting the critical normal tissues.
4. Conclusions
The reduction of the normal TCP without lowering the EQD2 to the tumor can be achieved by the hypofractionation treatment regimens for the prostate carcinoma. However, this is true if it is assumed that α/β for tumor is lower than OARs. An improved therapeutic ratio in hypofractionated regimens could have expected if the α/β ratio of the prostate cancer is lower than the α/β ratio of OARs such as rectum and bladder (14). Still, an optimal fractionation schedule for the prostate cancer treatment remains controversial.
The radiobiological models which have been adopted to perform the studies of TCP is a function of dose, α/β, SF2, and a number of clonogenic cells. The clinical outcomes confirm that all three parameters have a crucial role for the models (34).
In the clinical studies which have been performed by Ritter et al. (54) utilizing hypofractionation regimens for the prostate carcinoma with high α/β ratios up to 8.5 Gy with the different dose per fraction (Gy/fraction) such as 2.94, 3.63, and 4.3, superior clinical results were predicted over those of standard fractionation if the SF2 is 0.5 or less and number of clonogenic cells per tumor is less than 5 × 106.
Acknowledgements
References
-
1.
Coutard H. Roentgen therapy of epitheliomas of the tonsillar region, hypopharynx and larynx from 1920 to 1926. Am J Roentgenol. 1932;28:313-31.
-
2.
Weissberg JB, Prosnitz LR. Treatment of early breast cancer with primary radiation therapy: rationale, results, and techniques. Bull N Y Acad Med. 1982;58(2):203-13. [PubMed ID: 6956406].
-
3.
Shahbazi Gahrouei D. Radiobiological modeling in radiation oncology. J Radiobiol. 2014;1(1):17-8. https://doi.org/10.15171/jrb.2014.05.
-
4.
Fowler JF. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44(3):265-76. [PubMed ID: 16076699]. https://doi.org/10.1080/02841860410002824.
-
5.
Shahbazi-Gahrouei D, Abdolahi M, Zarkesh-Esfahani SH, Laurent S, Sermeus C, Gruettner C. Functionalized magnetic nanoparticles for the detection and quantitative analysis of cell surface antigen. Biomed Res Int. 2013;2013:349408. [PubMed ID: 23484112]. https://doi.org/10.1155/2013/349408.
-
6.
Shahbazi Gahrouei D, Gookizadeh A, Abdollahi M. Comparison of conventional radiotherapy techniques with different energies in treating prostate cancer, employing a designed pelvis phantom. J Med Sci. 2008;8(4):429-32. https://doi.org/10.3923/jms.2008.429.432.
-
7.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [PubMed ID: 21296855]. https://doi.org/10.3322/caac.20107.
-
8.
Shahbazi-Gahrouei D, Saeb M, Monadi S, Jabbari I. Clinical Implications of TiGRT Algorithm for External Audit in Radiation Oncology. Adv Biomed Res. 2017;6:117. [PubMed ID: 28989910]. https://doi.org/10.4103/abr.abr_268_16.
-
9.
Karbalaee M, Shahbazi-Gahrouei D, Tavakoli MB. An Approach in Radiation Therapy Treatment Planning: A Fast, GPU-Based Monte Carlo Method. J Med Signals Sens. 2017;7(2):108-13. [PubMed ID: 28553584].
-
10.
Rezaee V, Shahbazi Gahrouei D, Monadi S, Saeb M. Evaluation of error doses of treatment planning software using solid anthropomorphic phantom. J Isfahan Med Sch. 2016;34(393):908-13.
-
11.
Abdolahi M, Shahbazi-Gahrouei D, Laurent S, Sermeus C, Firozian F, Allen BJ, et al. Synthesis and in vitro evaluation of MR molecular imaging probes using J591 mAb-conjugated SPIONs for specific detection of prostate cancer. Contrast Media Mol Imaging. 2013;8(2):175-84. [PubMed ID: 23281290]. https://doi.org/10.1002/cmmi.1514.
-
12.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [PubMed ID: 25651787]. https://doi.org/10.3322/caac.21262.
-
13.
Collins CD, Lloyd-Davies RW, Swan AV. Radical external beam radiotherapy for localised carcinoma of the prostate using a hypofractionation technique. Clin Oncol (R Coll Radiol). 1991;3(3):127-32. [PubMed ID: 2069876].
-
14.
Livsey JE, Cowan RA, Wylie JP, Swindell R, Read G, Khoo VS, et al. Hypofractionated conformal radiotherapy in carcinoma of the prostate: five-year outcome analysis. Int J Radiat Oncol Biol Phys. 2003;57(5):1254-9. [PubMed ID: 14630259].
-
15.
Miles EF, Lee WR. Hypofractionation for prostate cancer: a critical review. Semin Radiat Oncol. 2008;18(1):41-7. [PubMed ID: 18082587]. https://doi.org/10.1016/j.semradonc.2007.09.006.
-
16.
Hoffmann AL, Nahum AE. Fractionation in normal tissues: the (alpha/beta)eff concept can account for dose heterogeneity and volume effects. Phys Med Biol. 2013;58(19):6897-914. [PubMed ID: 24029492]. https://doi.org/10.1088/0031-9155/58/19/6897.
-
17.
Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62(740):679-94. [PubMed ID: 2670032]. https://doi.org/10.1259/0007-1285-62-740-679.
-
18.
Chapman JD. Single hit mechanism of tumour cell killing by radiation. Int J Radiat Biol. 2003;79(2):71-81. [PubMed ID: 12569011].
-
19.
Nahum AE. The radiobiology of hypofractionation. Clin Oncol (R Coll Radiol). 2015;27(5):260-9. [PubMed ID: 25797579]. https://doi.org/10.1016/j.clon.2015.02.001.
-
20.
Nahum AE, Movsas B, Horwitz EM, Stobbe CC, Chapman JD. Incorporating clinical measurements of hypoxia into tumor local control modeling of prostate cancer: implications for the alpha/beta ratio. Int J Radiat Oncol Biol Phys. 2003;57(2):391-401. [PubMed ID: 12957250].
-
21.
Carlone M, Wilkins D, Raaphorst P. The modified linear-quadratic model of Guerrero and Li can be derived from a mechanistic basis and exhibits linear-quadratic-linear behaviour. Phys Med Biol. 2005;50(10):9-13. [PubMed ID: 15876677]. https://doi.org/10.1088/0031-9155/50/10/B01.
-
22.
Wang JZ, Huang Z, Lo SS, Yuh WT, Mayr NA. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Transl Med. 2010;2(39):39-48. [PubMed ID: 20610850]. https://doi.org/10.1126/scitranslmed.3000864.
-
23.
Sheu T, Molkentine J, Transtrum MK, Buchholz TA, Withers HR, Thames HD, et al. Use of the LQ model with large fraction sizes results in underestimation of isoeffect doses. Radiother Oncol. 2013;109(1):21-5. [PubMed ID: 24060173]. https://doi.org/10.1016/j.radonc.2013.08.027.
-
24.
Mehta N, King CR, Agazaryan N, Steinberg M, Hua A, Lee P. Stereotactic body radiation therapy and 3-dimensional conformal radiotherapy for stage I non-small cell lung cancer: A pooled analysis of biological equivalent dose and local control. Pract Radiat Oncol. 2012;2(4):288-95. [PubMed ID: 24674167]. https://doi.org/10.1016/j.prro.2011.10.004.
-
25.
Brown JM, Carlson DJ, Brenner DJ. The tumor radiobiology of SRS and SBRT: are more than the 5 Rs involved? Int J Radiat Oncol Biol Phys. 2014;88(2):254-62. [PubMed ID: 24411596]. https://doi.org/10.1016/j.ijrobp.2013.07.022.
-
26.
Baker C, Carver A, Nahum A. PO 0910, Local control prediction for NSCLC using a common LQbased TCP model for both SABR and 3D CRT fractionation. Radiother Oncol. 2015;115:471. https://doi.org/10.1016/s0167-8140(15)40902-8.
-
27.
Song CW, Cho LC, Yuan J, Dusenbery KE, Griffin RJ, Levitt SH. Radiobiology of stereotactic body radiation therapy/stereotactic radiosurgery and the linear-quadratic model. Int J Radiat Oncol Biol Phys. 2013;87(1):18-9. [PubMed ID: 23608235]. https://doi.org/10.1016/j.ijrobp.2013.03.013.
-
28.
Withers HR, Thames HD, Peters LJ. A new isoeffect curve for change in dose per fraction. Radiother Oncol. 1983;1(2):187-91. [PubMed ID: 6680223].
-
29.
Fenwick JD, Lawrence GP, Malik Z, Nahum AE, Mayles WP. Early mucosal reactions during and after head-and-neck radiotherapy: dependence of treatment tolerance on radiation dose and schedule duration. Int J Radiat Oncol Biol Phys. 2008;71(2):625-34. [PubMed ID: 18474317]. https://doi.org/10.1016/j.ijrobp.2008.02.008.
-
30.
Strigari L, Pedicini P, D'Andrea M, Pinnaro P, Marucci L, Giordano C, et al. A new model for predicting acute mucosal toxicity in head-and-neck cancer patients undergoing radiotherapy with altered schedules. Int J Radiat Oncol Biol Phys. 2012;83(5):697-702. [PubMed ID: 22578541]. https://doi.org/10.1016/j.ijrobp.2012.02.004.
-
31.
Mayles P, Nahum A, Rosenwald J. Handbook of radiotherapy physics, theory and practice. Florida, United States: CRC Press; 2007.
-
32.
Mavroidis P, Ferreira BC, Papanikotaou N, Svensson R, Kappas C, Lind BK, et al. Assessing the difference between planned and delivered intensity-modulated radiotherapy dose distributions based on radiobiological measures. Clin Oncol (R Coll Radiol). 2006;18(7):529-38. [PubMed ID: 16969983].
-
33.
Brenner DJ. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60(4):1013-5. [PubMed ID: 15519768]. https://doi.org/10.1016/j.ijrobp.2004.04.014.
-
34.
Kallman P, Agren A, Brahme A. Tumour and normal tissue responses to fractionated non-uniform dose delivery. Int J Radiat Biol. 1992;62(2):249-62. [PubMed ID: 1355519].
-
35.
Liao Y, Joiner M, Huang Y, Burmeister J. Hypofractionation: what does it mean for prostate cancer treatment? Int J Radiat Oncol Biol Phys. 2010;76(1):260-8. [PubMed ID: 19879698]. https://doi.org/10.1016/j.ijrobp.2009.06.043.
-
36.
Dasu A. Is the alpha/beta value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol (R Coll Radiol). 2007;19(5):289-301. [PubMed ID: 17517328]. https://doi.org/10.1016/j.clon.2007.02.007.
-
37.
Woynarowska BA, Roberts K, Woynarowski JM, MacDonald JR, Herman TS. Targeting apoptosis by hydroxymethylacylfulvene in combination with gamma radiation in prostate tumor cells. Radiat Res. 2000;154(4):429-38. [PubMed ID: 11023607].
-
38.
Bromfield GP, Meng A, Warde P, Bristow RG. Cell death in irradiated prostate epithelial cells: role of apoptotic and clonogenic cell kill. Prostate Cancer Prostatic Dis. 2003;6(1):73-85. [PubMed ID: 12664070]. https://doi.org/10.1038/sj.pcan.4500628.
-
39.
Leith JT, Quaranto L, Padfield G, Michelson S, Hercbergs A. Radiobiological studies of PC-3 and DU-145 human prostate cancer cells: x-ray sensitivity in vitro and hypoxic fractions of xenografted tumors in vivo. Int J Radiat Oncol Biol Phys. 1993;25(2):283-7. [PubMed ID: 8420876].
-
40.
Algan O, Stobbe CC, Helt AM, Hanks GE, Chapman JD. Radiation inactivation of human prostate cancer cells: the role of apoptosis. Radiat Res. 1996;146(3):267-75. [PubMed ID: 8752304].
-
41.
Yarnold J, Bentzen SM, Coles C, Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: myths and realities. Int J Radiat Oncol Biol Phys. 2011;79(1):1-9. [PubMed ID: 20950960]. https://doi.org/10.1016/j.ijrobp.2010.08.035.
-
42.
Miralbell R, Roberts SA, Zubizarreta E, Hendry JH. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5,969 patients in seven international institutional datasets: alpha/beta = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82(1):17-24. [PubMed ID: 21324610]. https://doi.org/10.1016/j.ijrobp.2010.10.075.
-
43.
Valdagni R, Italia C, Montanaro P, Lanceni A, Lattuada P, Magnani T, et al. Is the alpha-beta ratio of prostate cancer really low? A prospective, non-randomized trial comparing standard and hyperfractionated conformal radiation therapy. Radiother Oncol. 2005;75(1):74-82. [PubMed ID: 15878104]. https://doi.org/10.1016/j.radonc.2004.12.019.
-
44.
Fowler JF, Tome WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys. 2004;60(4):1241-56. [PubMed ID: 15519797]. https://doi.org/10.1016/j.ijrobp.2004.07.691.
-
45.
Tang CI, Loblaw DA, Cheung P, Holden L, Morton G, Basran PS, et al. Phase I/II study of a five-fraction hypofractionated accelerated radiotherapy treatment for low-risk localised prostate cancer: early results of pHART3. Clin Oncol (R Coll Radiol). 2008;20(10):729-37. [PubMed ID: 18838256]. https://doi.org/10.1016/j.clon.2008.08.006.
-
46.
Hoskin PJ, Dearnaley DP. Hypofractionation in clinical trials for prostate cancer. Clin Oncol. 2007;19(5):287-8. https://doi.org/10.1016/j.clon.2007.03.007.
-
47.
Khoo VS, Dearnaley DP. Question of dose, fractionation and technique: ingredients for testing hypofractionation in prostate cancer--the CHHiP trial. Clin Oncol (R Coll Radiol). 2008;20(1):12-4. [PubMed ID: 18036791]. https://doi.org/10.1016/j.clon.2007.10.008.
-
48.
Fowler J, Chappell R, Ritter M. Is alpha/beta for prostate tumors really low? Int J Radiat Oncol Biol Phys. 2001;50(4):1021-31. [PubMed ID: 11429230].
-
49.
Brenner DJ, Martinez AA, Edmundson GK, Mitchell C, Thames HD, Armour EP. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys. 2002;52(1):6-13. [PubMed ID: 11777617].
-
50.
Bentzen SM, Ritter MA. The alpha/beta ratio for prostate cancer: what is it, really? Radiother Oncol. 2005;76(1):1-3. [PubMed ID: 15990189]. https://doi.org/10.1016/j.radonc.2005.06.009.
-
51.
Williams SG, Taylor JM, Liu N, Tra Y, Duchesne GM, Kestin LL, et al. Use of individual fraction size data from 3756 patients to directly determine the alpha/beta ratio of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68(1):24-33. [PubMed ID: 17448868]. https://doi.org/10.1016/j.ijrobp.2006.12.036.
-
52.
Hoskin PJ. Hypofractionation in prostate cancer, How far can we go? Clin Oncol. 2008;20(10):727-8. https://doi.org/10.1016/j.clon.2008.09.002.
-
53.
Chin YS, Bullard J, Bryant L, Bownes P, Ostler P, Hoskin PJ. High dose rate iridium-192 brachytherapy as a component of radical radiotherapy for the treatment of localised prostate cancer. Clin Oncol (R Coll Radiol). 2006;18(6):474-9. [PubMed ID: 16909971].
-
54.
Ritter MA, Forman JD, Kupelian PA, Petereit DG, Lawton C, Walker W, et al. A phase I, II trial of dose per fraction escalation for prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69(3):174. https://doi.org/10.1016/j.ijrobp.2007.07.315.