Abstract
Introduction:
Malignant melanomas of the parotid gland are relatively uncommon and usually seen as metastases from cutaneous or mucous sites of the head and neck region. Some malignant melanomas may metastasize before they regress. Therefore, identifying the primary origin of metastatic melanoma is sometimes difficult. Furthermore, metastasis to the breast from an extramammary site is uncommon and challenging. It may present as a well-defined rounded mass that histopathologically mimics the various architecture and cellular phenotypes. In addition, the immunohistochemical stains of some metastatic melanomas are equivocal and challenging.Case Presentation:
We presented a case of parotid gland malignant melanoma in a 42-year-old woman with metastasis to the breast in a short interval. Biopsy of parotid and breast lesions showed loss of immune-reactivity for several melanoma markers and was initially considered as malignant peripheral nerve sheath tumor and primary breast tumor, respectively.Conclusions:
This case highlights the importance of obtaining past clinical history in surgical pathology cases to make a correct diagnosis. It also enhances our understanding regarding malignant melanoma as a mysterious tumor with various morphology and immunophenotype.Keywords
1. Introduction
Malignant melanomas of the parotid gland are uncommon and have been reported in sporadic cases. They mostly occur as metastases originating from cutaneous or mucous sites of the head and neck region. As there is a delay in diagnosis of this entity, the prognosis is poor and the pathogenesis is controversial (1, 2). Some malignant melanomas may metastasize and regress, so the metastasis and its primary source are not simultaneously identified.
The prevalence of primary malignant tumors of the salivary gland is 1 per 100000 people. Salivary gland tumors arise commonly from the parotid gland (80%), among which 25% are malignant. Adenoid cystic carcinoma, mucoepidermoid carcinoma, and acinar duct carcinoma are among the most common types of salivary gland cancers (3). About 25% of parotid tumors are metastatic, which mostly originate from the head and neck region. The main parotid gland metastatic tumors are squamous cell carcinoma and malignant melanoma (4).
Secondary tumors in the breast represent 1.3 to 2.7% of all malignant mammary tumors. The breast is a metastatic site for tumors such as leukemia, lung cancer, and melanoma (5). Metastatic melanoma to breast tissue may be difficult to recognize if the primary lesion is occult and the tumor lacks immunoreactivity for melanocytic markers.
2. Case Presentation
We reported a case of malignant melanoma in a 42-year-old woman, with a primary presentation of left cervical mass near the parotid gland. The initial biopsy of the lesion was diagnosed as a malignant peripheral nerve sheath tumor with rhabdomyoblastic differentiation based on the morphology of rhabdoid tumor cells, immunoreactivity of some tumor cells for S100, and negativity of melanocytic markers including Melan A and HMB45. Desmin immunostaining shows positivity in scattered rhabdoid tumor cells. A week later, the patient underwent surgery including resection of the mass, part of the parotid gland, the mandibular branch of the facial nerve, sternocleidomastoid muscle, and internal jugular vein and skin. Pectoralis flap and nerve graft reconstruction were, then, performed.
On gross examination, a well-defined mass, measuring 6 × 5.5 × 4 cm was identified. The mass was 0.8 cm far from the skin surface and no gross pathology was present on the skin.
Microscopic examination showed a neoplasm composed of the spindle to oval tumor cells with eccentrically-located nuclei, scant cytoplasm, and some prominent nucleoli. The tumor cells were arranged in solid sheets with alveolar patterns and infiltrative margins. Wide areas of necrosis and high mitoses rate were also observed. The skin was unremarkable. No evidence of lymphoid tissue at the periphery of the lesion was seen. A rim of parotid tissue around the tumor was identified.
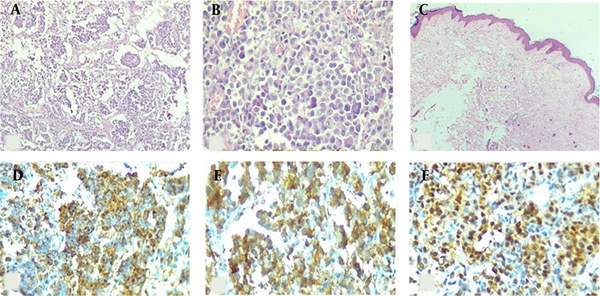
IHC study showed diffuse positive reactivity of tumor cells for Melan A, HMB45, SOX10, and S100 with scattered positivity for desmin (Figure 1A-F). A high proliferation index (60 - 70% nuclear positivity for Ki67) was also noted. Myogenin, Myo D1, P63, and CD57 exhibited no immunostaining. The diagnosis of “malignant melanoma” was made based on morphological and immunohistochemical findings.
Histological and immunohistochemical features of malignant melanoma of the parotid gland. (A) The tumoral cells were arranged in solid sheets and in alveolar pattern with infiltrative margins. (B) H&E ×400 section shows spindle to oval cells with eccentrically-located nuclei, scant cytoplasm and some with prominent nucleoli (400×). (C) The overlying skin was unremarkable (H&E ×100). (D-F) Immunoreactivity of tumoral cells for melanocytic markers (IHC ×100); (D) HMB45, (E) Melan A, (F) SOX10.
The patient underwent adjuvant treatment for melanoma, and follow-up radiologic investigations demonstrated a well-defined mass in the upper-outer-quadrant of the left breast (BIRADS: 4a). Core needle biopsy of the lesion showed a malignant spindle cell tumor with no immunoreactivity for melanocytic markers. CKAE1/AE3, P63, and CK 5/6 showed scattered positivity. Therefore, pathology report released as malignant spindle tumor with differential diagnosis of metaplastic carcinoma versus melanoma and final diagnosis deferred after the excision of the lesion.
One month later, the aforementioned mass was excised. The mass was well-delineated in macroscopic examination with morphological features of malignant spindle cell tumor.
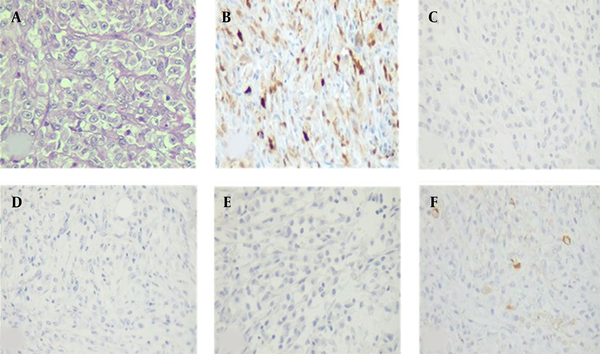
The tumor cells of resected breast lesion showed positive immune-reactivity for S100, while they were negative for HMB45, Melan-A, SOX10. CKAE1/AE3 was positive in some tumor cells (Figure 2A-E). PCR analysis for BRAF V600E mutation showed negative results in both specimens.
Histological and immunohistochemical features of metastatic malignant melanoma of the breast. (A) H&E section shows malignant spindle cell tumor with vesicular nuclei and prominent nucleoli (H&E ×400). (B) positive immunoreactivity of tumor cells for S100. (C-D) Negative immunoreactivity for melanocytic markers (IHC ×100); (C) HMB45, (D) Melan-A, (E) SOX10. (F) Occasional scattered positivity of tumoral cells for CKAE1/AE3.
Although immune-staining were not so supportive, considering the previous history of the patient, similarity of morphology with previous parotid tumor, and positive immune-reactivity of neoplastic cells for S100, metastatic malignant melanoma was concluded as a proper diagnosis. Regrettably, her condition started deteriorating and she succumbed to the disease within 6 months of diagnosis.
3. Discussion
Primary parotid malignant melanoma is extremely rare and comprises for approximately 0.68% of parotid malignant neoplasms. The majority of parotid melanoma tumors are metastatic lesions from skin or mucosal sites in head and neck regions. These original sites may include the scalp, mucosa membranes of the nose, paranasal sinuses, and throat. The 2 sources of metastasis include intra-lymphatic spread and direct melanoma extension from adjacent soft tissue (2).
The exact etiology of malignant melanoma of the parotid gland is unclear and many hypotheses have been proposed regarding the pathogenesis of the tumor. In 1997, Takeda identified melanocytes in parotid interlobular duct and hypothesized that primary parotid malignant melanoma was originated from these cells (6).
In 1993, Woodward et al. cited in Villarreal et al. proposed an extensive diagnostic criterion for primary parotid malignant melanoma. Some of these criteria were predominant intra-parotid mass, undistinguishable lymph node tissue, no evidence of ocular, dermal, nasopharyngeal, oral, esophageal, and a genital malignant melanoma or malignant melanoma in other sites. In other words, there is no evidence of previous malignant melanoma or progressive pigmented lesion (7). In our case, no evidence of pigmented or amelanotic lesion suggestive of the primary extra-parotid tumor was detected at the time of diagnosis. No regressed lesion was reported in the history of the patient. No residual lymphoid tissue was observed in the periphery or background of the tumor.
Since no primary source was identified for the metastasis and no residual lymphoid tissue surrounding the tumor was identified, considering our case as a primary parotid malignant melanoma based on the above criteria could be acceptable.
Primary or metastatic melanoma of the breast could occur in breast parenchyma or the overlying skin. breast metastatic lesions from extra-mammary origins are uncommon and account for 2% of all breast tumors (8).
The most common site of metastasis in the breast is its lateral aspect due to good vascularity and supplementary glandular tissue. The upper outer quadrant of the breast was involved in approximately 50% of cases, as the presented case. Metastatic breast tumors are frequently superficial and do not attach to the surrounding breast tissue, which is independent of skin involvement. The common presentation of these tumors is a firm and well-circumscribed masse. In approximately 10% of cases, breast involvement is bilateral, which indicates a widely disseminated disease with a poor prognosis (5).
Metastatic melanoma, as a breast tumor, can have multiple histological appearances and the diagnosis may be difficult. Furthermore, the immune profile of these tumors may have a large spectrum ranging from tumors without conventional melanocytic marker expression to tumors that express aberrant antigen of other cell lineages, including epithelial tissue, and smooth muscle (5, 9). In our case, immunoreactivity for melanocytic markers was not found in both biopsies of the parotid and breast lesions, which could be due to heterogeneity of neoplastic cells in different areas of the tumor. Furthermore, the loss of multiple melanocytic markers in metastatic lesions could be related to the high number of various mutations that contribute to intratumor and intratumor heterogeneity of melanoma (10). Because of the large size of the tumor specimen, it was technically difficult to stain the entire lesion and highlight the divergent immunoreactivity of tumor cells in different areas of the lesion. This heterogeneity may explain the discrepancy of the immunohistochemistry results in biopsy and resection specimens.
The findings of this study indicated that the challenge and difficulty of this entity, especially when the biopsy is not representative of the lesion, should not be underestimated. It is, therefore, wise to be cautious in the interpretation of the results, especially in small biopsy samples. Furthermore, as some metastatic malignant melanoma lose their melanocytic immunophenotype and become more challenging, the clinical history is important in the conclusion and accurate diagnosis. Moreover, it is recommended to use a wide battery of melanocytic markers when there is high level of suspicion to prevent misdiagnosing of uncommon immunophenotypes of melanoma.
In some cases, it may not be possible to observe the primary site and metastatic parotid malignant melanoma simultaneously. Therefore, evaluating previous photographs of patients may be helpful to detect possible regressed malignant melanomas.
The significant factors that affect patient improvement include early diagnosis, appropriate tumor resection by surgery, and comprehensive adjuvant.
3.1. Conclusions
This case report and literature review could improve our understanding regarding various presentations and immunophenotypes of malignant melanoma and enhance our ability in diagnosing similar cases.
References
-
1.
Kilickaya MM, Aynali G, Ceyhan AM, Ciris M. Metastatic Malignant Melanoma of Parotid Gland with a Regressed Primary Tumor. Case Rep Otolaryngol. 2016;2016. [PubMed ID: 27478668]. [PubMed Central ID: PMC4949337]. https://doi.org/10.1155/2016/5393404.
-
2.
Mohammed D, Tang R. Primary Malignant Melanoma of the Parotid Gland Combined 18F-FDGPET/CT And Immunochemical Diagnosis With Literature Review. J Dig Dis Diagn. 2017;1(2). https://doi.org/10.14302/issn.2574-4526.jddd-16-1322.
-
3.
Neto T, Nunes R, Amado I, Balhau R, Marques H, Sanz D, et al. Parotid metastasis of an amelanotic melanoma of the scalp: The great masquerader. Head Neck. 2016;38(4):91-4. [PubMed ID: 26348327]. https://doi.org/10.1002/hed.24222.
-
4.
Mesa M, Quesada JL, Pinas J. Metastasis of amelanotic melanoma of unknown origin in the parotid gland. Br J Oral Maxillofac Surg. 2009;47(7):569-71. [PubMed ID: 19167137]. https://doi.org/10.1016/j.bjoms.2008.10.011.
-
5.
Al Samaraee A, Khout H, Barakat T, Fasih T. Breast metastasis from a melanoma. Ochsner Journal. 2012;12(2):149-51.
-
6.
Takeda Y. Melanocytes in the human parotid gland. Pathol Int. 1997;47(8):581-3. [PubMed ID: 9293542]. https://doi.org/10.1111/j.1440-1827.1997.tb04545.x.
-
7.
Villarreal IM, Arellano B, Sáenz J, Martin L, Castello JR, García-Berrocal JR. Primary Malignant Parotid Melanoma: Searching for a Rare Entity. Int J Otorhinolaryngol Head Neck Surg. 2014;3(5):228-32. https://doi.org/10.4236/ijohns.2014.35042.
-
8.
Saad Abdalla Al-Zawi A, Osayi K, Eades J. Breast Metastasis from a Malignant Melanoma- A Case Report. Int J Radiol. Radiat Ther. 2017;3(3). https://doi.org/10.15406/ijrrt.2017.03.00062.
-
9.
Aisner DL, Maker A, Rosenberg SA, Berman DM. Loss of S100 antigenicity in metastatic melanoma. Hum Pathol. 2005;36(9):1016-9. [PubMed ID: 16153466]. [PubMed Central ID: PMC2656365]. https://doi.org/10.1016/j.humpath.2005.07.010.
-
10.
Grzywa TM, Paskal W, Wlodarski PK. Intratumor and Intertumor Heterogeneity in Melanoma. Transl Oncol. 2017;10(6):956-75. [PubMed ID: 29078205]. [PubMed Central ID: PMC5671412]. https://doi.org/10.1016/j.tranon.2017.09.007.