Abstract
Background:
As one of the most prevalent cancers in men, prostate cancer is a condition with multiple causes. Viral infections have been identified as one of the major sources of elevated incidence of prostate cancer.Objectives:
The purpose of this research was to assess the association of the risk of prostate cancer and its aggressiveness with seropositivity of herpes simplex virus 2 (HSV-2) and/or human herpesvirus 8 (HHV-8).Methods:
Totally, 103 men with prostate cancer as cases and 81 healthy individuals as controls were included in this case-control analysis and provided a serum sample. The specific IgG antibodies against HSV-2 and HHV-8 were screened by enzyme-linked immunosorbent assay (ELISA). To determine the association between HSV-2, HHV-8, prostate-specific antigen (PSA) level, and demographic variables with incidence of prostate cancer, univariate and multivariate logistic regression models were applied.Results:
The results of the univariate logistic regression model showed a statistically significant association between HSV-2 and HHV-8 seropositivity, PSA level, age, and smoking with prostate cancer incidence (P ≤ 0.20). The multivariate logistic regression model results after adjusting for the potential confounding variables showed a significant statistical association between the mean of PSA level [adjusted odds ratio (OR): 3.44; 95% CI: 2.15 - 5.51; P < 0.001) and incidence of prostate cancer. Moreover, the results of univariate and multivariate logistic regression model showed a significant statistical association between age [adjusted OR: 0.88; 95% confidence interval (CI): 0.81 - 0.95; P = 0.001] and HSV-2 and also significant statistical association was found between PSA (adjusted OR: 1.02; 95% CI: 1.005 - 1.03; P = 0.006) and HHV-8.Conclusions:
Although the seroprevalence of HSV-2 and HHV-8 was higher in patients with prostate cancer than in the control group, it cannot be concluded that there is a significant association between the seropositivity of these viruses and prostate cancer incidence. However, the findings showed a significant statistical association between age and seropositivity of HSV-2 and also a significant statistical association between PSA levels and seropositivity of HHV-8.Keywords
Prostate Cancer Herpes Simplex Virus Type 2 Human Herpesvirus 8 Sexually Transmitted Infections Enzyme-Linked Immunosorbent Assay Seroprevalence
1. Background
Prostate cancer is the second leading malignancy and the 6th major cause of cancer-related death among males worldwide (1). A previous study reported prostate cancer as the second most prevalent cancer among urological malignancies in Iranian males (2).
Prostate tumor is a multifactorial disease and several genetic and environmental risk factors have been associated with its development and progression, such as aging, ethnicity, and genetic predisposition (3). Seroepidemiologic research has proposed a correlation between sexually transmitted infections (STIs) and the possibility of prostate cancer. Infectious agents such as herpes simplex virus type 2 (HSV‑2) and human herpesvirus type 8 (HHV‑8) have been assumed to contribute to prostate tumorigenesis (4-6).
Herpes virus is one of the viral etiologies that has been associated with the pathogenesis of cancer and plays a significant role in the carcinogenesis via induction of deoxyribonucleic acid (DNA) synthesis as well as inhibition of cell apoptosis (7). On the other hand, energy is essential for any function, and one of the new hypotheses is the effect of energy depletion in human cells on the development of cancer cells. Mitochondria are involved in cellular metabolic processes and also play a major role against viruses. Many viral proteins also react with mitochondrial proteins and regulate cellular responses. Therefore, one of the new aspects of carcinogenesis is the effect of viruses including HSV-2 and HHV-8 on cellular mitochondria (8, 9).
The possible association of HHV 8 and HSV 2 in prostate cancer has been reported in some previous studies (10, 11). However, some other studies reported contradictory results and failed to find such a relationship (6, 12, 13). Therefore, the correlation between HSV 2 and/or HHV 8 infection and prostate cancer remains controversial.
2. Objectives
The current case-control study aimed at evaluating the potential association between the STIs and the risk of prostate cancer and its aggressiveness by studying the seropositivity of HSV-2 and/or HHV-8, using serological methods.
3. Methods
3.1. Study Design
In the current case-control study, between 2017 and 2019, 184 samples comprising 103 histologically confirmed prostate cancer patients (as cases), who had undergone one or more treatment programs for prostate cancer (including radical prostatectomy, hormone-suppression therapy, radiation therapy), and 81 age-matched healthy controls were enrolled. All participants (both cases and controls) completed a self-administered questionnaire for relevant clinical characteristics and demographic data and provided a serum sample for serological analysis. Individuals with a history of any other malignancies and/or cancer metastasized to the prostate from another origin as well as the ones with the history of organ transplantation and human immunodeficiency virus (HIV) were excluded. All participants have signed informed consent and the work was accepted by the institutional review board (approval ID: IR.TUMS.VCR.REC.1397.1028). In the patient group (cases), prostate-specific antigen (PSA) levels at the time of diagnosis were considered, and in the control group (controls), their PSA levels were measured when individuals entered the study. The differentiation level of the prostate tumor was evaluated by a pathologist, using the Gleason score (GS) system (well-differentiated, GS < 7; moderately differentiated, GS = 7; poorly differentiated, GS > 7) (14).
3.2. Serology
The specific IgG antibodies against HSV-2 were detected, using a recognized sandwich third-generation enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's guidelines (DIAPRO Diagnostic; Bioprobes Milano Italy) (15). Moreover, the presence of antibodies specific to HHV-8 was screened by ELISA (16). Briefly, the microtiter plates were coated with the diluted ORF65 protein, which is extremely immunogenic in humans. All wells were blocked with 5% no-fat dry milk in PBS (2 hours at room temperature). Diluted serums samples were also blocked with 5% no-fat dry milk in PBS. Subsequently, a 2-step ELISA was performed for the detection of IgG, using horseradish peroxidase (HRP)–conjugated goat antibody to mouse IgG and gamma-chain-specific monoclonal antibodies specific for γ-chain, which were diluted to a concentration of 1: 2000 and 1: 800, respectively. Then, the mixture was incubated with the substrate (1 hour/room temperature). Finally, the absorption measurement was performed (415 nm). All tests were performed in duplicate and the laboratory technicians were blinded to the cases and controls.
3.3. Statistical Analysis
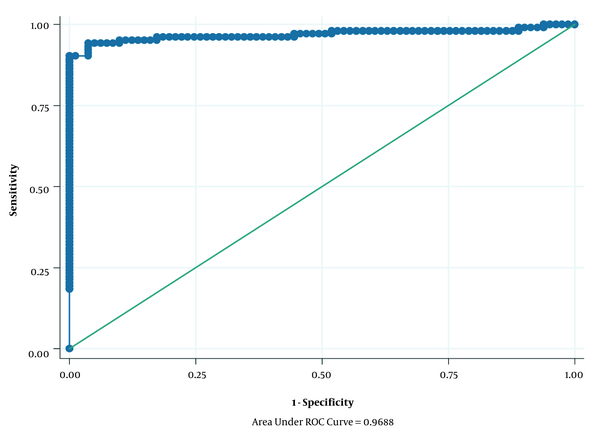
The relevant data were entered into the STATA14 for analysis. In descriptive analyzes, mean and standard deviation (SD) were used for quantitative variables, and number and relative frequency were used for qualitative variables. Moreover, to determine the association between HSV-2, HHV-8, PSA level, and demographic variables with incidence of prostate cancer, univariate and multivariate logistic regression models were applied; then, the crude and adjusted OR with 95% confidence interval (CI) were calculated. Additionally, the area under a ROC curve was used for the assessment discriminative ability of the multivariable logistic regression analysis (Figure 1). Also, the univariate and multivariate logistic regression models were used to determine the association of HSV-2 and HHV-8 with Gleason score, PSA, and age in case subjects. Finally, P < 0.05 was considered as a statistically significant level.
The area under the curve for variables entered in the multivariable logistic regression model.
4. Results
A total of 103 cases (patients with prostate cancer) and 81 healthy controls were recruited for the present study. The mean age ± SD for cases and controls was 65.70 ± 9.92 and 61.96 ± 8.53 years, respectively (Table 1). Additionally, Table 1 shows the association of HSV-2, HHV-8, PSA, and demographic variables with the incidence of prostate cancer. The results of the univariate logistic regression model showed a statistically significant association between HSV-2, HHV-8, PSA, age, and smoking with the incidence of prostate cancer (P ≤ 0.20). After the univariate logistic regression model, to eliminate potential confounding variables, we introduced all the significant variables in univariate logistic regression analysis (P ≤ 0.20) simultaneously into a multivariate logistic regression model. An independent variable should be examined in the univariate model. Then, only variables that seem to be closely associated with the studied outcome (P < 0.2) are imported into the final multivariate model (17). The results of the multivariate logistic regression model after adjusting for the confounding variables showed a significant statistical association between mean PSA level [adjusted odds ratio (OR): 3.44; 95% CI: 2.15 - 5.51; P < 0.001] and prostate cancer incidence; this means that for every one-unit increase in the mean PSA level, the odds of incidence of prostate cancer increase by 3.44 times. However, other variables did not show a statistically significant association with the incidence of prostate cancer (P > 0.05) (Table 1). Figure 1 shows the area under the ROC curve for variables entered into the multivariate logistic regression model, which was 0.9688.
The Association Between HSV2, HHV8, PSA, and Demographic Variables with Prostate Cancer by Univariate & Multivariate Logistic Regression Model a
| Variables | Case | Control | Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value |
|---|---|---|---|---|---|---|
| HSV-2 | 0.122 | |||||
| Seronegative | 89 (86.4) | 75 (92.6) | Reference | 0.187 | Reference | |
| Seropositive | 14 (13.6) | 6 (7.4) | 1.97 (0.72 - 5.37) | 3.91 (0.6922.03) | ||
| HHV-8 | 0.789 | |||||
| Seronegative | 92 (89.3) | 79 (97.5) | Reference | 0.048 | Reference | |
| Seropositive | 11 (10.7) | 2 (2.5) | 4.72 (1.01 - 21.94) | 0.63 (0.02 - 18.51) | ||
| Smoking | 0.494 | |||||
| Non-smoker | 44 (42.7) | 55 (67.9) | Reference | 0.001 | Reference | |
| Smoker | 59 (57.3) | 26 (32.1) | 2.83 (1.54 - 5.21) | 1.50 (0.47 - 4.83) | ||
| PSA | 25.35 ± 32.43 | 3.41 ± 1.28 | 3.40 (2.19 -5.28) | < 0.001 | 3.44 (2.15 - 5.51) | < 0.001 |
| Age (y) | 65.70 ± 9.92 | 61.96 ± 8.53 | 1.04 (1.01 - 1.07) | 0.009 | 0.93 (0.00 - 6.24) | 0.082 |
Then, the univariate and multivariate logistic regression model was used to examine the association of HSV-2 and/or HHV-8 seropositivity with Gleason score, PSA level, and age. Finally, the results of the multivariate logistic regression model showed a significant statistical association between age (adjusted OR: 0.88; 95% CI: 0.81 - 0.95; P = 0.001) and HSV-2 seropositivity; this means that for every one-unit increase of the age, the odds of seropositivity for HSV-2 decrease by 12% (intra-group analysis was performed in cases) (Table 2).
The Association Between HSV-2 with Gleason Score, PSA, and Age by Univariate & Multivariate Logistic Regression Model a
| Variables | HSV2 | Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
|---|---|---|---|---|---|---|
| Seronegative | Seropositive | |||||
| Gleason score | 7 ± 1.18 | 6.5 ± 1.65 | 0.71 (0.44 - 1.15) | 0.168 | 0.86 (0.52 - 1.43) | 0.573 |
| PSA | 15.36 ± 25.61 | 18.46 ± 34.15 | 0.99 (0.98 - 1.01) | 0.951 | - | - |
| Age (y) | 64.97 ± 9.26 | 56.55 ± 8.11 | 0.87 (0.81 - 0.94) | < 0.001 | 0.88 (0.81 - 0.95) | 0.001 |
In addition, a significant statistical association was found between PSA levels (adjusted OR: 1.02; 95% CI: 1.005 - 1.03; P = 0.006) and HHV-8 seropositivity; this means that for every one-unit increase in the mean of PSA level, the odds of seropositivity for HHV8 increase by 1.02 times (intra-group analysis was performed in cases) (Table 3).
The Association Between HHV-8 with Gleason Score, PSA, and Age Cancer by Univariate & Multivariate Logistic Regression Model a
| Variables | HHV8 | Crude OR (95% CI) | P-Value | Adjusted OR (95% CI) | P-Value | |
|---|---|---|---|---|---|---|
| Seronegative | Seropositive | |||||
| Gleason Score | 6.91 ± 1.25 | 7.10 ± 1.37 | 1.12 (0.68 - 1.83) | 0.656 | - | - |
| PSA | 13.34 ± 23.11 | 46.64 ± 45.68 | 1.02 (1.005 - 1.03) | 0.006 | 1.02 (1.005 - 1.03) | 0.006 |
| Age (y) | 63.97 ± 9.38 | 65.15 ± 11.16 | 1.01 (0.95 - 1.08) | 0.668 | - | - |
5. Discussion
HSV-2 is one of the sexually transmitted viruses, which is typically most frequent in people with high-risk behaviors (18). The worldwide incidence of HSV-2 infection in populations aged 15 to 49 years in 2012 was roughly 11% (19). In general, the seroprevalence of HSV-2 varies in different demographic groups from 6% in the healthy population to 40% in patients with STIs (19). The frequency of HSV-2 has been documented to be very high in some regions of Africa and South America and low in Asia (7). There is limited data on the prevalence of HSV-2 in Asia and, specifically, Iran (20). Our findings showed that the total seroprevalence of HSV-2 in the statistical population of the study was 10.9%, which is less than the incidence reported in some of the earlier studies in Iran (21, 22). A meta-analysis in Iran reported the incidence of HSV-2 to be 42.04% (20). Moreover, a previous study in Iran showed the prevalence of HSV-2 as 23.3% in a sample of asymptomatic Iranian students (21). However, the prevalence of HSV-2 IgG antibody was previously reported to be 5.8% in an analysis of pregnant women residing in northwestern Iran (22).
Furthermore, the seroprevalence of HHV-8 differs in various geographical regions and races in different countries (23). The highest incidence of the virus is in sub-Saharan Africa with 36 to 60% (24). The majority of the available studies reported the seroprevalence of the virus among high-risk populations, including the men who have sex with men (MSMs) or HIV-positives; however, the incidence of the virus in the healthy population has not been well documented (25-27). The HHV-8 seroprevalence in healthy populations was reported to be 1.3 to 4.4% in Southeast Asia and the Caribbean (28) and 7.3% in the United States (29). It was 1.7% in renal transplant recipients in Saudi Arabia (30). The seroprevalence of HHV-8 was 7.1% in the statistical population of our research; however, the seroprevalence of the control group was 2.5%. One of Iran's infrequent tumors is Kaposi's sarcoma (31). The prevalence of HHV-8 is estimated to be 2% in the normal Iranian community, but this figure rises to 45.7% in HIV patients (32). The prevalence of HHV-8 was reported to be about 10% in hemodialysis patients (33) and 5.5% in HIV-positive patients (34). In addition, no positive HHV-8 DNA cases were observed in the two groups of a previous study in Iran (healthy and HIV-patient group) and reported a very low seroprevalence for the virus. The authors claimed that Iran is probably one of the regions with low HHV-8 prevalence (35).
Herpes viruses play a central role in carcinogenesis by suppressing cellular apoptosis and activating the DNA synthesis (11). The role of HSV-2 and HHV-8 viruses in prostate cancer has been a controversial subject in studies. These differences could be due to the varying prevalence of prostate cancer and HSV-2 and HHV-8 viruses, both of which are dependent on geographical region and age (7). Another explanation for the disparity of results on the role of these viruses in prostate cancer is the use of various approaches in detecting the virus. Due to the lack of healthy prostate tissue samples that can be used as the control for the study of the relationship between viral infections and prostate cancer, serological methods may be preferable to others (12). Moreover, the herpes virus serological tests are less complicated than tissue tests and are also more popular in investigations (7). Moreover, there is no reliable and adequate data on the comparability or consistency of the results from serological testing with tissue analysis in diagnosing the herpes virus infections (7). Several studies have reported a strong association between serological results of HHV-8 positives and an increased risk of prostate cancer (10, 36). A previous study showed that HHV-8 expresses IL-6, a human-like viral interleukin that effectively induces the proliferation of prostate cancer cells (37). Moreover, in a case-control study on serum level of inflammatory cytokine in prostate cancer patients and controls, it was found that the cytokine profile in men with prostate cancer was different from that of the healthy men. The researchers reported a T-helper 2 response with an increase in IL-13 levels as well as a decrease in the T-helper 1 response, and the level of IL-12P70 was present in both prostate cancer patients and HHV-8 serologically positive patients. Hence, the authors of the study regarded these findings as supportive of their theory on the presence of an interaction between chronic HHV-8 infection and increased risk of prostate cancer (38). Previous research on men living in Trinidad and Tobago and the United States showed increased HHV-8 seropositivity in prostate cancer patients (36). In another study on Tobago men, it was shown that a latent HHV-8 infection leads to inflammation and macrophage infiltration in the prostate. Notably, Tobago is one of the areas with the highest rates of prostate cancer mortality (39).
The results of a meta-analysis of 11 trials, consisting of a total of 2 999 cases and 3 875 controls, indicated a significant association between HSV-2 infection and an increased risk of prostate cancer in the North and South American populations; however, such a relationship was not observer for HHV-8 infection (7). While the geographical region factor was considered in the meta-analysis before the research, no reports from Asia and Africa were included in the study, which, in turn, is one of the drawbacks of this review for comparative purposes (7). The study reported the lack of adequate information from Asia and Africa as inhibitors of extensive and reliable meta-analyses investigating the relationship of these viruses and the risk of prostate cancer (7).
Our findings showed that 13.6% of the cases and 7.4% of the control group were seropositive for HSV-2, but there was no statistically significant difference in the prevalence of HSV-2 between the two groups (P = 0.181). Although there was no correlation between HHV-8 and HSV-2 seropositivity with prostate cancer based on the findings of the logistic regression model of multivariate analysis after adjusting the confounding variables in our study, these results are consistent with some earlier studies; namely, some studies reject the presence of a relationship between such viruses infection and prostate cancer (4, 12, 13, 40). For example, during a seroepidemiological analysis in Finnish men, over a 24-year follow-up, no significant association was observed between HHV-8 and HSV-2 infections and an increased risk of prostate cancer (12). Moreover, a protective impact of HHV-8 chemokines was proposed on prostate cancer in an article, establishing an inverse association between virus infection and prostate cancer. However, the authors claimed the Mediterranean population as an intervening variable (6).
Furthermore, according to the findings shown in Tables 2 and 3, no significant association was observed between the seropositivity of HHV-8 and HSV-2 with the Gleason score of prostate tumors.
Based on the findings of the multivariate logistic regression model after adjusting the potential confounders in the present research, a statistically significant association was observed between the mean level of PSA and the occurrence of prostate cancer. These findings indicate a 3.44-fold rise in the incidence of prostate cancer per unit of mean PSA levels.
The results of the univariate and multivariate logistic regression model have also demonstrated a significant statistical association between PSA levels and HHV-8 seropositivity. These results are consistent with the results of the previous case-cohort report, in which the increased PSA levels in prostate cancer caused by HHV-8 infection were identified as a potential source for their previously reported significant relationship between the virus infection and prostate cancer (4).
Increasing age is an accepted factor in developing prostate cancer (41). The mean age of the case and control groups in the present study was 65.70 and 61.96 years, respectively. Despite the different age ranges of patients in the groups, no significant association was observed between age and prostate cancer according to the results of the multivariate logistic regression model. The previous studies have described age as a risk factor for HSV-2 infection (20). Furthermore, the prevalence of HSV-2 has been documented to increase with age, from a very low percentage in below 12 to 80% in high ages (42), with a maximum infection occurring around the age of 40 (43); however, it should be noted that many aspects such as sexual activity have a major role in this process (42).
HHV-8 also becomes more prevalent with age (13). However, the results of our analysis revealed no significant relationship between HHV-8 seropositivity and the patients' age. Nevertheless, the results of the multivariate logistic regression model showed a significant statistical association between age and HSV-2 seropositivity, as opposed to previous research, in which greater seropositivity was reported in older ages (42). The older age of our research sample could explain this finding because the population studied in the case group were men with prostate cancer, who are usually old.
5.1. Conclusions
Although the seroprevalence of oncogenic and sexually transmitted viruses was higher in patients with prostate cancer than in the control group, it cannot be concluded that there is a significant association between the seropositivity of these viruses and prostate cancer and the grade of the tumor. However, the results of our research showed a significant relationship between the age and seropositivity of HSV-2 and also a significant statistical association between PSA levels and seropositivity of HHV-8. Nonetheless, multicenter experiments on several groups of people of varying ages and races, as well as the concurrent use of various virus detection and assessment approaches are essential to endorse or rule out the correlation of such viruses with prostate cancer. In addition, aside from the carcinogenic characteristics of these viruses, chronic infections, and estimates of their role in increasing the prevalence of cancer, the screening and surveillance of the prevalence of these viruses is another important concern for the implementation of instructional, prevention, and treatment initiatives, particularly in communities at higher risk of infection with these viruses.
Acknowledgements
References
-
1.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69-90. [PubMed ID: 21296855]. https://doi.org/10.3322/caac.20107.
-
2.
Akbari ME, Hosseini SJ, Rezaee A, Hosseini MM, Rezaee I, Sheikhvatan M. Incidence of genitourinary cancers in the Islamic Republic of Iran: A survey in 2005. Asian Pac J Cancer Prev. 2008;9(4):549-52. [PubMed ID: 19256736].
-
3.
Perdana NR, Mochtar CA, Umbas R, Hamid AR. The risk factors of prostate cancer and its prevention: A literature review. Acta Med Indones. 2016;48(3):228-38. [PubMed ID: 27840359].
-
4.
McDonald AC, Jenkins FJ, Bunker CH, Wilson JW, Patrick AL, Weissfeld JL. A case-cohort study of human herpesvirus 8 seropositivity and incident prostate cancer in Tobago. Infect Agent Cancer. 2011;6:25. [PubMed ID: 22151996]. [PubMed Central ID: PMC3248833]. https://doi.org/10.1186/1750-9378-6-25.
-
5.
Hrbacek J, Urban M, Hamsikova E, Tachezy R, Eis V, Brabec M, et al. Serum antibodies against genitourinary infectious agents in prostate cancer and benign prostate hyperplasia patients: A case-control study. BMC Cancer. 2011;11:53. [PubMed ID: 21291519]. [PubMed Central ID: PMC3039631]. https://doi.org/10.1186/1471-2407-11-53.
-
6.
Sutcliffe S, Giovannucci E, Gaydos CA, Viscidi RP, Jenkins FJ, Zenilman JM, et al. Plasma antibodies against Chlamydia trachomatis, human papillomavirus, and human herpesvirus type 8 in relation to prostate cancer: A prospective study. Cancer Epidemiol Biomarkers Prev. 2007;16(8):1573-80. [PubMed ID: 17684131]. [PubMed Central ID: PMC3012386]. https://doi.org/10.1158/1055-9965.EPI-07-0134.
-
7.
Ge X, Wang X, Shen P. Herpes simplex virus type 2 or human herpesvirus 8 infection and prostate cancer risk: A meta-analysis. Biomed Rep. 2013;1(3):433-9. [PubMed ID: 24648964]. [PubMed Central ID: PMC3917040]. https://doi.org/10.3892/br.2013.82.
-
8.
Ohta A, Nishiyama Y. Mitochondria and viruses. Mitochondrion. 2011;11(1):1-12. [PubMed ID: 20813204]. [PubMed Central ID: PMC7105242]. https://doi.org/10.1016/j.mito.2010.08.006.
-
9.
Akbari H, Taghizadeh-Hesary F, Heike Y, Bahadori M. Cell energy: A new hypothesis in decoding cancer evolution. Arch Iran Med. 2019;22(12):733-5. [PubMed ID: 31823627].
-
10.
Dennis LK, Coughlin JA, McKinnon BC, Wells TS, Gaydos CA, Hamsikova E, et al. Sexually transmitted infections and prostate cancer among men in the U.S. military. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2665-71. [PubMed ID: 19755645]. https://doi.org/10.1158/1055-9965.EPI-08-1167.
-
11.
Ewald PW. An evolutionary perspective on parasitism as a cause of cancer. Adv Parasitol. 2009;68:21-43. [PubMed ID: 19289189]. https://doi.org/10.1016/S0065-308X(08)00602-7.
-
12.
Korodi Z, Wang X, Tedeschi R, Knekt P, Dillner J. No serological evidence of association between prostate cancer and infection with herpes simplex virus type 2 or human herpesvirus type 8: A nested case-control study. J Infect Dis. 2005;191(12):2008-11. [PubMed ID: 15897985]. https://doi.org/10.1086/430354.
-
13.
Jenkins FJ, Hayes RB, Jackson A, Pizza G, Mbisa G, Whitby D, et al. Human herpesvirus 8 seroprevalence among prostate cancer case patients and control subjects. J Infect Dis. 2007;196(2):208-11. [PubMed ID: 17570107]. https://doi.org/10.1086/518790.
-
14.
Epstein JI, Allsbrook WJ, Amin MB, Egevad LL, Isup Grading Committee. The 2005 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma. Am J Surg Pathol. 2005;29(9):1228-42. [PubMed ID: 16096414]. https://doi.org/10.1097/01.pas.0000173646.99337.b1.
-
15.
Vestergaard BF, Grauballe PC. ELISA for herpes simplex virus (HSV) type-specific antibodies in human sera using HSV type 1 and type 2 polyspecific antigens blocked with type-heterologous rabbit antibodies. Acta Pathol Microbiol Scand B. 1979;87(4):261-3. [PubMed ID: 227226]. https://doi.org/10.1111/j.1699-0463.1979.tb02436.x.
-
16.
Tedeschi R, Dillner J, De Paoli P. Laboratory diagnosis of human herpesvirus 8 infection in humans. Eur J Clin Microbiol Infect Dis. 2002;21(12):831-44. [PubMed ID: 12525917]. https://doi.org/10.1007/s10096-002-0836-8.
-
17.
Jewell NP. Statistics for epidemiology. Florida, USA: CRC Press; 2003.
-
18.
Bohl DD, Katz KA, Bernstein K, Wong E, Raymond HF, Klausner JD, et al. Prevalence and correlates of herpes simplex virus type-2 infection among men who have sex with men, san francisco, 2008. Sex Transm Dis. 2011;38(7):617-21. [PubMed ID: 21278625]. https://doi.org/10.1097/OLQ.0b013e31820a8c10.
-
19.
Looker KJ, Magaret AS, Turner KM, Vickerman P, Gottlieb SL, Newman LM. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS One. 2015;10(1). e114989. [PubMed ID: 25608026]. [PubMed Central ID: PMC4301914]. https://doi.org/10.1371/journal.pone.0114989.
-
20.
Malary M, Abedi G, Hamzehgardeshi Z, Afshari M, Moosazadeh M. The prevalence of Herpes Simplex Virus type 1 and 2 infection in Iran: A meta-analysis. Int J Reprod Biomed. 2016;14(10):615-24. [PubMed ID: 27921084]. [PubMed Central ID: PMC5124323].
-
21.
Tayyebi D, Tabatabaee M, Rahsaz M. Seroepidemiology of infection with Herpes Simplex Virus type 2 (HSV2) among asymptomatic students attending Islamic Azad University of Kazeroun, southwest of Iran. Retrovirology. 2010;7(Suppl 1). https://doi.org/10.1186/1742-4690-7-s1-p88.
-
22.
Rostamzadeh Z, Gharehaghaji F, Hashemi M, Barzeghari R, Shermohammadi Z. Herpes simplex virus type 2: Seroprevalence in pregnant women. Iran J Neonatol. 2012;3(Supplement):7. https://doi.org/10.22038/IJN.2012.1145.
-
23.
Tornesello ML, Biryahwaho B, Downing R, Hatzakis A, Alessi E, Cusini M, et al. Human herpesvirus type 8 variants circulating in Europe, Africa and North America in classic, endemic and epidemic Kaposi's sarcoma lesions during pre-AIDS and AIDS era. Virology. 2010;398(2):280-9. [PubMed ID: 20079510]. https://doi.org/10.1016/j.virol.2009.12.005.
-
24.
Tumwine LK, Lukande R, Zhao W, Ayers L. Human herpes virus 8 (HHV-8)-associated lymphoproliferative disorders in Kampala, Uganda. J Microbiol Bio Scie. 2017;5.
-
25.
Liu Z, Fang Q, Zuo J, Wang J, Chen Y, Minhas V, et al. High seroprevalence of human herpesvirus 8 and herpes simplex virus 2 infections in men who have sex with men in Shanghai, China. J Med Virol. 2017;89(5):887-94. [PubMed ID: 27769110]. https://doi.org/10.1002/jmv.24718.
-
26.
Nzivo MM, Lwembe RM, Odari EO, Kang'ethe JM, Budambula NLM. Prevalence and risk factors of human herpes virus type 8 (HHV-8), Human immunodeficiency virus-1 (HIV-1), and syphilis among female sex workers in Malindi, Kenya. Interdiscip Perspect Infect Dis. 2019;2019:5345161. [PubMed ID: 31320897]. [PubMed Central ID: PMC6610739]. https://doi.org/10.1155/2019/5345161.
-
27.
Engels EA, Atkinson JO, Graubard BI, McQuillan GM, Gamache C, Mbisa G, et al. Risk factors for human herpesvirus 8 infection among adults in the United States and evidence for sexual transmission. J Infect Dis. 2007;196(2):199-207. [PubMed ID: 17570106]. https://doi.org/10.1086/518791.
-
28.
de Sanjose S, Mbisa G, Perez-Alvarez S, Benavente Y, Sukvirach S, Hieu NT, et al. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J Infect Dis. 2009;199(10):1449-56. [PubMed ID: 19351262]. https://doi.org/10.1086/598523.
-
29.
Minhas V, Wood C. Epidemiology and transmission of Kaposi's sarcoma-associated herpesvirus. Viruses. 2014;6(11):4178-94. [PubMed ID: 25375883]. [PubMed Central ID: PMC4246215]. https://doi.org/10.3390/v6114178.
-
30.
Alzahrani AJ, El-Harith el HA, Milzer J, Obeid OE, Stuhrmann M, Al-Dayel A, et al. Increased seroprevalence of human herpes virus-8 in renal transplant recipients in Saudi Arabia. Nephrol Dial Transplant. 2005;20(11):2532-6. [PubMed ID: 16115855]. https://doi.org/10.1093/ndt/gfi058.
-
31.
Jalilvand S, Shoja Z, Mokhtari-Azad T, Nategh R, Gharehbaghian A. Seroprevalence of Human herpesvirus 8 (HHV-8) and incidence of Kaposi's sarcoma in Iran. Infect Agent Cancer. 2011;6:5. [PubMed ID: 21527020]. [PubMed Central ID: PMC3108908]. https://doi.org/10.1186/1750-9378-6-5.
-
32.
Kakavand-Ghalehnoei R, Shoja Z, Najafi A, Mollahoseini MH, Shahmahmoodi S, Marashi SM, et al. Prevalence of human herpesvirus-8 among HIV-infected patients, intravenous drug users and the general population in Iran. Sex Health. 2016;13(3):295-8. [PubMed ID: 26886227]. https://doi.org/10.1071/SH15192.
-
33.
Shokri S, Mahmoudvand S, Makvandi M, Shahani T, Samadi A, Shahbazian H. Prevalence of human herpesvirus 8 infection in patients undergoing hemodialysis using nested-PCR. J Acute Dis. 2019;8(6):250.
-
34.
Hesamizadeh K, Keyvani H, Bokharaei-Salim F, Monavari SH, Esghaei M, Jahanbakhsh Sefidi F. Molecular epidemiology of Kaposi's Sarcoma-associated herpes virus, and risk factors in HIV-infected patients in Tehran, 2014. Iran Red Crescent Med J. 2016;18(11). e32603. [PubMed ID: 28191343]. [PubMed Central ID: PMC5292624]. https://doi.org/10.5812/ircmj.32603.
-
35.
Ramezani A, Saboori E, Azadmanesh K, Mohraz M, Kazemimanesh M, Karami A, et al. No evidence of human herpesvirus 8 among Iranian Patients infected with HIV. Iran J Public Health. 2016;45(7):935-40. [PubMed ID: 27517001]. [PubMed Central ID: PMC4980349].
-
36.
Hoffman LJ, Bunker CH, Pellett PE, Trump DL, Patrick AL, Dollard SC, et al. Elevated seroprevalence of human herpesvirus 8 among men with prostate cancer. J Infect Dis. 2004;189(1):15-20. [PubMed ID: 14702148]. https://doi.org/10.1086/380568.
-
37.
Mygatt JG, Singhal A, Sukumar G, Dalgard CL, Kaleeba JA. Oncogenic herpesvirus HHV-8 promotes androgen-independent prostate cancer growth. Cancer Res. 2013;73(18):5695-708. [PubMed ID: 24005834]. https://doi.org/10.1158/0008-5472.CAN-12-4196.
-
38.
Henning JD, Bonachea LA, Bunker CH, Patrick AL, Jenkins FJ. Human herpesvirus 8 infection contributes to a T helper 2 immune response in men from Tobago with prostate cancer. Int J Urol. 2017;24(1):64-8. [PubMed ID: 27734534]. https://doi.org/10.1111/iju.13243.
-
39.
Henning JD, Bunker CH, Patrick AL, Jenkins FJ. Human herpesvirus 8 establishes a latent infection in prostates of Tobago men resulting in increased macrophage infiltration. Prostate. 2016;76(8):735-43. [PubMed ID: 26848067]. https://doi.org/10.1002/pros.23163.
-
40.
Huang WY, Hayes R, Pfeiffer R, Viscidi RP, Lee FK, Wang YF, et al. Sexually transmissible infections and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17(9):2374-81. [PubMed ID: 18768506]. [PubMed Central ID: PMC2559953]. https://doi.org/10.1158/1055-9965.EPI-08-0173.
-
41.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87-108. [PubMed ID: 25651787]. https://doi.org/10.3322/caac.21262.
-
42.
Smith JS, Robinson NJ. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J Infect Dis. 2002;186(Suppl 1):S3-28. [PubMed ID: 12353183]. https://doi.org/10.1086/343739.
-
43.
Cusini M, Ghislanzoni M. The importance of diagnosing genital herpes. J Antimicrob Chemother. 2001;47 Suppl T1:9-16. [PubMed ID: 11160031]. https://doi.org/10.1093/jac/47.suppl_1.9.