Abstract
Context:
Glioblastoma is the most invasive brain tumor with a poor prognosis and rapid progression. The standard therapy (surgical resection, adjuvant chemotherapy, and radiotherapy) ensures survival only up to 18 months. In this article, we focus on innovative types of radiotherapy, various combinations of temozolomide with novel substances, and methods of their administration and vector delivery to tumor cells.Evidence Acquisition:
For a detailed study of the various options for chemotherapy and radiotherapy, Elsevier, NCBI MedLine, Scopus, Google Scholar, Embase, Web of Science, The Cochrane Library, EMBASE, Global Health, CyberLeninka, and RSCI databases were analyzed.Results:
The most available method is oral or intravenous administration of temozolomide. More efficient is the combined chemotherapy of temozolomide with innovative drugs and substances such as lomustine, histone deacetylase inhibitors, and chloroquine, as well as olaparib. These combinations improve patient survival and are effective in the treatment of resistant tumors. Compared to standard fractionated radiotherapy (60 Gy, 30 fractions, 6 weeks), hypofractionated is more effective for elderly patients due to lack of toxicity; brachytherapy reduces the risk of glioblastoma recurrence, while radiosurgery with bevacizumab is more effective against recurrent or inoperable tumors. Currently, the most effective treatment is considered to be the intranasal administration of anti-Ephrin A3 (anti-EPHA3)-modified containing temozolomide butyl ester-loaded (TBE-loaded) poly lactide-co-glycolide nanoparticles (P-NPs) coated with N-trimethylated chitosan (TMC) to overcome nasociliary clearance.Conclusions:
New radiotherapeutic methods significantly increase the survival rates of glioblastoma patients. With some improvement, it may lead to the elimination of all tumor cells leaving the healthy alive. New chemotherapeutic drugs show impressive results with adjuvant temozolomide. Anti-EPHA3-modified TBE-loaded P-NPs coated with TMC have high absorption specificity and kill glioblastoma cells effectively. A new “step forward” may become a medicine of the future, which reduces the specific accumulation of nanoparticles in the lungs, but simultaneously does not affect specific absorption by tumor cells.Keywords
EPHA3 Antibody Glioblastoma Multiforme (GBM) Nose-to-brain Delivery Temozolomide Brachytherapy Gamma Knife Radiosurgery External-beam Radiotherapy Lomustine Suberoylanilide hydroxamic acid (SAHA) Olaparib
1. Context
1.1. Fatal Neoplasms: The Ghosts of Unknown Etiology
Glioblastomas are currently the most common aggressive brain tumors with a poorly predicted prognosis. Their nature is still not clear. There are various hypotheses and one of them is that viruses cause glioblastoma. In any case, the reliable cause of the glioblastoma occurrence is not known to mankind, but methods for treating glioblastomas have already been discovered. Depending on the treatment method, different survival rates are achieved after the initial diagnosis of glioblastoma. At the moment, the average maximum survival duration reaches 18 to 19 months. But, the unique capabilities of individuals and auxiliary conditions, for example, the concomitant presence of cytomegalovirus antigens during the entire period of homeostatic proliferation of T-lymphocytes after radiation therapy, temozolomide, and corticosteroids (which cause lymphopenia) can increase survival up to 7 years and more (1, 2).
In this article, we will review various new radiological and chemotherapeutic treatment methods for glioblastoma (including vector drug delivery).
2. Evidence Acquisition
To study new methods of chemotherapy and radiotherapy in the treatment of glioblastoma multiforme, articles from the Elsevier, NCBI MedLine, Scopus, Google Scholar, Embase, Web of Science, The Cochrane Library, EMBASE, Global Health, CyberLeninka, and RSCI databases were analyzed. While searching articles, the following keywords were used: “Glioblastoma multiforme”, “EPHA3 antibody”, “Nose-to-brain delivery”, “Gamma knife radiosurgery", “Temozolomide”, “Lomustine”, “External-beam radiotherapy”, “Suberoylanilide hydroxamic acid (SAHA)”, “Olaparib”, and “Brachytherapy”.
The assessment process of the English and Russian sources was carried out in several stages; the titles, abstracts, and full-text articles were viewed. Moreover, if needed, an additional search for the sources indicated in the selected articles was performed.
Articles that included original studies that provided preliminary results of studies or duplicated the results of previous studies were excluded. The main focus of this article was on the glioblastoma multiforme treatment used worldwide from 2005 to 2020.
3. Results
3.1. In What Way Can Radiotherapeutic Features Fight Glioblastoma?
Radiotherapy of glioblastoma is based on the subjecting tumor cell to ionizing radiation. Ionizing radiation can affect the DNA of glioblastoma cells directly (damaging the structure of hereditary material directly by radiation) or indirectly (radiolysis of water in the cytoplasm is accompanied by releasing reactive oxygen species, which damage the DNA). Ionizing radiation has a much stronger influence on actively dividing cells during mitosis due to the compactification of their DNA and the inactivity of repair enzymes during division.
The standard pattern of radiotherapy for glioblastoma has become outdated.
External beam radiotherapy (EBRT) was used as an independent methodic of treating glioblastoma until 2005. In 2005, R. Stupp et al. (3) published an article providing the data that EBRT is more effective in combination with temozolomide (TMZ) compared with radiotherapy alone (overall survival rates increased from 12.1 till 14.6 months). Today, the standard of glioblastoma treatment includes surgical resection of the tumor and fractionated EBRT combined with chemotherapy (TMZ) sometime after the operation (4). Based on this, the main prognostic factors for radiotherapy are the patient’s age, the Karnofsky performance status, totally absorbed radiation dose, and hypermethylation of MGMT (O(6)-methylguanine DNA methyltransferase) (for adjuvant TMZ therapy) (5).
The standard course of EBRT includes a total absorbed dose of 60 Gy, divided into 30 fractions of 1 fraction per day, 5 days a week for 6 weeks. Reducing the total dose does not show significant results and an increase in overall survival time. Increased doses are connected with high risks of adverse effects (6).
3.2. Hypofractionated EBRT: New Hope for elderly or Weakened Patients?
Hypofractioning is an EBRT regimen that consists of using small amounts of large (more than 2 Gy) fractions of radiation. Due to the long-time intervals between fractions, adverse effects are rarely observed, that is why it is the most effective therapy for elderly or weakened patients.
According to literature evidence (7, 8), the usage of hypofractionated radiotherapy (HFRT) as an independent methodic of treatment is not effective, but another thing is its combination with adjuvant TMZ therapy. HFRT with intensity modulation provides many advantages to form radiation beams and change their intensity following the size and shape of the tumor. According to many data (7, 8), the average overall survival rates of elderly patients who administered this therapy are 9 to 20 months, while standard therapy provides 6 to 8 months.
These results are encouraging. But there is a need for further researches to find the most fractionating regimen.
3.3. Brachytherapy; a “Heavy Artillery” Against Tumor Growth and Recurrence
Brachytherapy (BT) of glioblastoma is performing by introducing capsules (a.k.a. “grains”) with titanium shell, containing radioactive isotopes I-125 or Ir-192, which produce radiation with different intensity into the tumor’s bed. The decay of I-125 by electronic capture leads to its transformation into Te-125, which emits gamma-rays inducing therapeutic effects of capsules. Ir-192 decays simultaneously with releasing beta- and gamma-rays and transforming into Pt-192.
Due to the high intensity of radiation, iridium is used in high-dose-rate brachytherapy (HDR-BT). It is necessary to remove its grains from the body after some time. I-125 is also used in low-dose-rate brachytherapy (LDR-BT). Such grains, if necessary, can be left in the patient’s body for a whole life without adverse effects.
The combination of surgical resection of the tumor and LDR-BT, followed by adjuvant HFRT 4 weeks later, was proposed by Chen et al. (9), led to high risks of adverse effects but demonstrated high values of patient’s survival (overall survival, 28.5 months, progression-free survival 13.2 months).
Waters et al. (10) and Welsh et al. (11) studied the use of the standard treatment with the addition of HDR-BT between surgery and EBRT. There was an increase in both progression-free survival and overall survival rates by 3 months compared to the standard treatment.
According to Kickingereder et al. (12) and Chatzikonstantinou et al. (13), the use of brachytherapy for inoperable patients can significantly increase their overall survival rate compared to supportive treatment.
The advantage of brachytherapy is its local effect and reduced distance from the radiation source to the glioblastoma due to the delivery of radioactive “grains” directly to the tumor bed. It provides a possibility to significantly decrease the frequency of tumor recurrence at adequate radiation doses (with standard therapy, recurrence appears in > 80% of cases, with brachytherapy from 18% to 80%) (14).
According to Schwartz et al. (15) and Chatzikonstantinou et al. (16), LDR-BT for patients with small-size recurrent glioblastoma can increase their time-to-treatment-fail up to 6 months, overall survival rates up to 9 months and survival rates from initial diagnosis up to 29 months, and HDR-BT as a component of complex treatment up to 4.5, 9, and 20 months, respectively.
3.4. Radiosurgery; a Modern and Effective Method for Patients with Recurrent Glioblastoma
Radiosurgery with high accuracy influences the tumor bed with large doses of ionizing radiation in one or more (up to 5) fractions.
It is effective for recurrent glioblastoma treatment. Some authors (7, 17) report that radiosurgery is more useful during the tumor progression rather than initial stages and increases patients’ survival rates, which is relevant for inoperable patients.
According to scientists of the University of Pittsburgh (18), the average survival rate of such patients after radiosurgery is approximately 9 months, and from an initial diagnosis of 18 months. Execution of chemotherapy for patients before tumor recurrence and after radiosurgery increase in radiation dose and tumor resection after radiosurgery provides the increase in survival.
Unfortunately, radiosurgery focuses radiation only on the tumor site, which is visible on MR-images with gadolinium contrast. Due to its high invasiveness, glioblastoma cells can spread beyond the tumor bed without an appearance on the MR-images, and it can lead to tumor recurrence. It makes radiosurgery inadvisable to use at the initial stages of treatment due to the inability of decreasing the risk of tumor recurrence (7).
It is interesting to combine radiosurgery with bevacizumab therapy (a medicament of monoclonal antibodies to vascular endothelial growth factor). Oxygen diffuses from the capillaries at a distance of 100 - 150 microns; so, far tumor cells necrotize because of excessive hypoxia. Bevacizumab increases this effect, which leads to the inhibition of tumor cell proliferation. Radiosurgery complements its action, causing the death of still-living cells. Many researchers (18-21) note a sharp increase in recurrent glioblastoma patients’ survival rates with reduced risks of adverse effects with such therapies.
Despite the advantages of radiosurgery, it is not widely used in practice today because of complicated equipment, lack of specialists, high energy costs for the procedure, and other factors.
3.5. Adverse Effects, or a Little About the Sad
Not only tumor cells, but also rapidly dividing red bone marrow cells are highly sensitive to radiotherapy. So, most of the adverse effects are observed from the circulatory system: leukopenia, lymphopenia, neutropenia, and thrombocytopenia. If glioblastoma of the brain stem is irradiated, there can be observed nausea and constipation (22).
If radiation doses are inadequately high, dangerous disorders of the nervous system might be observed: memory impairment, radionecrosis, brain hemorrhages (they are extremely rarely observed), etc. (14).
The importance of side by side application of radiotherapy with chemotherapy has already been discussed and now we will focus on the latter one.
3.6. There Is no Room for Solo Players: The Combination of TMZ with Innovative Chemotherapy as One of the Most Effective Methods for the Treatment of Glioblastoma Multiforme
TMZ is a compound from the triazene class (chemical formula: RN = N-NR1R2), an alkylating chemotherapeutic antitumor drug approved for the treatment of patients with glioblastoma multiforme (21). After oral administration, it is rather quickly and completely absorbed, the percentage of drug binding to plasma proteins is insignificant; therefore, the simultaneous administration of other drugs ensures their minimal interaction with TMZ (22). TMZ is a lipophilic molecule that can penetrate the blood-brain barrier (BBB) and, hence, has antitumor activity in the central nervous system (23). Compared to other chemotherapeutic drugs, the toxicity of TMZ is low; however, the use of this drug can lead to significant side effects (such as thrombocytopenia, neutropenia, lymphopenia, and significant myelosuppression when combined with radiotherapy) (24).
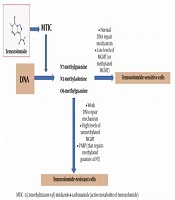
The most common position of DNA methylation caused by the active metabolite of TMZ 5-(3-methyltriazen-1-yl) imidazole-4-carboxamide (MTIC) is N7 of guanine followed by methylation at position N3 of adenine and position O6 of guanine (O6-MeG). In normal cells, direct repair of O6-MeG with the MGMT effectively removes methyl adduct and restores guanine (25). DNA damage caused by TMZ can be eliminated with MGMT; therefore, a decrease in MGMT activity can enhance the effect of the drug (26). It was shown that alkylation products are not detected in cells with a weak DNA repair mechanism and, hence, they are resistant to TMZ, even if MGMT is absent in them (27). Thus, TMZ is most cytotoxic in cells with low levels of MGMT and normal DNA repair mechanisms (Figure 1).
TMZ and its anti-glioblastoma activity in sensitive and resistant cells
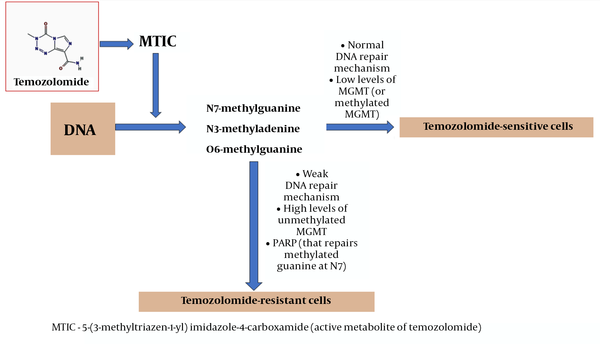
Poly (ADP-ribose) polymerase (PARP) can restore methylated guanine at position N7. It is known that methylation at this region of the nucleotide does not have a significant cytotoxic effect; however, PARP inactivation contributes to the treatment of cancer, which justifies the synergistic effect of TMZ with PARP inhibitors (28, 29).
If the MGMT promoter in glioblastomas is methylated, MGMT is not capable of transcription and usually, such tumors can be treated with TMZ, unlike tumors with unmethylated MGMT (28, 29). Specific mutations in tumors are the main factors of resistance and growth (30). Unlike genetic mutations, epigenetic changes such as promoter methylation or histone acetylation status are reversible and can be targeted with drugs (31).
It was shown that combined chemotherapy with lomustine (this drug promotes the formation of interchain bonds in the DNA molecule and leads to carbamoylation of amino acids, which ultimately leads to a change in transcriptional, translational, and post-transcriptional processes) and TMZ increased overall survival in the group of patients with methylated MGMT glioblastoma (32).
TMZ in combination with histone deacetylase (HDAC) inhibitors (for example, suberoylanilide hydroxamic acid, which is a specific inhibitor of HDAC 1, 2, 3, and 6, is currently approved for the treatment of cutaneous T-cell lymphoma) and chloroquine (chemosensitizing agent) have been tested for various types of cancer, and clinical trials have been conducted to treat glioblastoma. Chloroquine blocks the late stages of the protective cancer cells' reaction-autophagy and promotes apoptosis of the tumor (33, 34). Histone deacetylase inhibitors promote histone acetylation, which leads to changes in chromatin dynamics; moreover, they favor transcription factor acetylation, which affects gene expression (35). It has been shown that HDAC inhibitors suppress the cell population resistant to treatment with alkylating drugs and eliminate resistance to these drugs (36).
Preclinical trials of olaparib (an inhibitor of poly [ADP-ribose] polymerases, PARP) have shown that PARP inhibitors can be considered as a promising class of radiosensitizers (provide a more effective response to radiotherapy) (37, 38). The combination of TMZ/olaparib/radiotherapy can be used in the treatment of partially resected or non-resected glioblastomas and can help to improve survival rates, with virtually no effect on healthy tissues and neurocognitive functions (39).
Thus, the combined use of TMZ with different substances can be helpful in the treatment for glioblastoma; however, additional clinical research to confirm the effectiveness on big groups of patients has to be done. Currently, vector drug delivery is gaining momentum and is a true perspective method of glioblastoma treatment. The most recent technologies of this method are discussed in the following section.
3.7. Nanotechnologies and Vector Drug Delivery
Significant progress has been made in recent years in surgery, radiotherapy, and chemotherapy; however, even the most active chemotherapeutic treatment can only slightly improve overall survival.
3.8. What Surprises Does Temozolomide Bring with Vector Delivery?
TMZ is considered the most effective drug for treating glioblastomas; it is most often administered orally or intravenously (40). However, there are several problems associated with its use:
1) Short plasma half-life;
2) The high toxicity associated with the limitation of the drug dose usage (hematological toxicity, acute cardiomyopathy, ulceration of the oral cavity, and myelosuppression) (41);
3) The use of TMZ in combination with radiation therapy led only to moderate improvements (42).
3.9. The Successful trio of TMZ/Nanoparticles/Polylactide-co-Glycolide
However, science does not stand still, and some studies are currently being carried out aimed to overcome these obstacles in the treatment of glioblastomas; for instance, the compositions of microspheres, various implants, and combinations of TMZ with several system components for targeted drug delivery to tumor cells are being actively studied (43).
The most commonly used and promising for the treatment of glioblastoma are considered nanoparticle-based vector delivery systems (44-46).
As a rule, polylactide-co-glycolide (PLGA), is (A) biodegradable, (B) biocompatible, and (C) universal.
Nevertheless, an obstacle arises with TMZ: its poor solubility in aqueous and organic solvents, which leads to significant difficulties in encapsulation of TMZ in PLGA-based nanoparticles (P-NPs) (47). However, a way out of this situation was found by Wang (48): it was proposed to add to TMZ molecule 4 - 10 carbon chain; so, TMZ esters were successfully synthesized with activity comparable to unmodified TMZ.
However, in addition to the difficulties associated with the chemical and physical properties of the drug, there are several problems associated with its delivery to tumor cells:
1) The penetration of the drug through the BBB,
2) Vector delivery of the drug to the lesion site of the brain (49).
It has been a long trip: technology for the delivery of chemotherapy to glioblastoma cells through the nose to brain pathway.
In this article, we focus on the technology of drug delivery to glioblastoma cells through the nose to brain pathway.
This method has several advantages (50-52):
1) If intranasal administration, direct drug delivery from the nasal mucosa to the brain via the olfactory and trigeminal nerve pathways is ensured;
2) Bypassing the BBB, it is possible to avoid systemic side effects and primary metabolization of the drug, which prevents its enzymatic/chemical degradation;
3) In comparison with intravenous administration, this method is safer due to its faster action and greater antitumor activity, which allows reducing the dose and frequency of drug administration; this helps to increase patient survival. However, when administrating the drug, it is necessary to take into account the features of nasal mucociliary clearance, which significantly affect the absorption of the drug (53).
An interesting feature of this method is the use of an auxiliary adhesive polymer applied to the nasal mucosa, which increases the retention time of the drug in the nasal cavity (53). Chitosan (an amino sugar derived from chitin) is most often used for this, but it is insoluble and does not have adhesive properties at a neutral pH (54). But, the solution was found to overcome these obstacles: N-trimethylated chitosan (TMC), obtained by reductive methylation of chitosan, has good adhesion and solubility even at neutral pH (55). In the study of du Plessis et al., it was found that TMC has stronger adhesion to the nasal mucosa compared to unmethylated chitosan, and TMC also reduces mucociliary clearance.
To enhance the directed action of the drug on glioblastoma, a complementary interaction of the drug ligand and the overexpressed receptor in tumor cells are used (56, 57). One of these receptors is the type A3 ephrin receptor (ephrin type-A receptor 3; EPH receptor A3; EPHA3). This membrane-bound receptor is overexpressed in stroma and vasculature in gliomas, but almost not expressed normally (58). Anti-EPHA3-recombinant non-fucosylated (fucose-free) IgG1k (human f-allotype) is a monoclonal antibody that can specifically interact with EPHA3 tyrosine kinase receptor (59, 60). In recent studies (50) human bronchial epithelial cells (16HBE), C6 cells, and glioma tissue were used to confirm specific expression of EPHA3 by glioblastoma cells. The presence of EPHA3 was determined by solid-phase immunoenzyme analysis (IEA). To count the percentage of EPHA3 expression by these cells, the following formula was used: EPHA3 (%) = C (EPHA3)/C (total) × 100%. Levels of EPHA3 expression in glioma tissues and C6-cells were 4.06 ± 0.2% and 2.49 ± 0.15%, respectively, why there was almost no expression of EPHA3 in 16HBE-cells. Thus, EPHA3 expression by glioblastoma cells was confirmed for the first time. It was determined that in preclinical models, anti-EPHA3 antibody showed significant efficacy and slight toxicity (61). Currently, the medicine of anti-EPHA3 antibody (KB004) has entered phase I of clinical trials (50, 59, 62). The experiment to establish cytotoxicity was performed for 6 hours because of the transport of nanoparticles with airflow during respiration. The results showed no significant difference in cell viability between the administrations of PLGA-nps filled or unfilled with TMZ butyl ether; TMC/PLGA-NPs and anti-EPHA3-TMC/PLGA-NPs. It indicates the safety of delivering temozolomide butyl ester-loaded (TBE) nanoparticles to the brain through the nasal mucosa and the possibility of its performing using anti-EPHA3-TMC/PLGA-NPs as a vector. Then, studies for estimation of cytotoxicity of nanoparticles with TBE were conducted: C6-cell line was cultivated with different amounts of different nanoparticles with TBE; in this connection, the viability of tumor cells depended on the concentration of the chemotherapy drug. The following conclusion was made from this experiment: anti-EPHA3-modified nanoparticles directly interacted with C6-cells due to binding anti-EPHA3 antibody to the receptor, which increased absorption of nanoparticles by tumor cells. For instance, cell viability with anti-EPHA3-T/P-TBE-NPs was 25.76% at 60 mg/mL TBE, while cell viability with T/P-TBE-NPs and P-TBE-NPs was 42.40% and 43.15%, respectively. Moreover, it was found that cytotoxicity, associated with delivering the drug to target cells, was negligible in the PLGA concentration range from 0.23 to 2.35 mg/mL (50). Also, T/P-NPs killed tumor cells more effectively than uncoated nanoparticles; it can be explained by more effective attaching of positively charged T/P-NPs to negatively charged cells compared to uncoated nanoparticles (63).
This evidence proves that anti-EPHA3-antibodies is suitable for boosting vector delivery of chemotherapy drugs following the “nose-brain” pathway for glioblastoma treatment.
Thus, anti-EPHA3-modified TBE-loaded P-NPs, coated with TMC, proved their high effectiveness. The results of the cytotoxicity analysis for C6-cells and subsequent experiments on specific cellular absorption of nanoparticles showed that the modification of anti-EPHA3 antibodies can enhance the exactness of their effect when delivering the drug to glioblastoma. The distribution of fluorescence in rats with glioma confirmed the utility of this drug for treating glioblastoma. These results indicate that anti-EPHA3-T/P-NPs could potentially be used as a system of drug-delivering along the “nose-brain” pathway for specific vector therapy of glioblastoma.
4. Discussion
4.1. Will There be Light at the End of the Tunnel?
4.1.1. Outstanding Questions Box
1) How to evaluate the effectiveness of HFRT with bevacizumab against glioblastoma?
2) Are there new radioisotopes with lower penetrating possibilities for use in brachytherapy? The grains implantation of such radioisotopes would be able to suppress tumor growth without damaging the underlying tissue.
3) Are there any contrasting substances that could manifest cell masses that migrate outside the tumor and can cause its recurrence?
4) What are the future ways of delivering drugs modifying radiotherapeutic susceptibility to healthy nerve tissue? This would allow increasing the dose of radiation against tumor cells without increasing the risk of developing radionecrosis in healthy tissue?
5) What kind of additional screening tests based on specific biomarkers will help to predict the effectiveness of PARP inhibitors in the future?
6) How to realize genomic signatures that provide an approximation of the functional status of the DNA repair machinery?
7) What is the way to integrating targeted DNA sequencing to find mutations to provide epigenome analysis?
8) Are there any other molecular targets in managing glioblastoma?
9) What is the mechanism of transport of nanoparticles through these nerve pathways and what is the effect on them? Do nanoparticles affect eyesight and smell? Is there too much adsorption on the surface of the neurons of the tract?
10) What can be done to reduce the accumulation of nanoparticles in the lungs? Should it be introduced some kind of aerosol to reduce adhesion to the surface of lung cells?
11) Is it possible to additionally introduce some drugs to help remove already accumulated nanoparticles in the lungs from the tissues but so that the accumulation in the tumor remains?
12) Is it possible to modify nanoparticles to supplement their activity with properties to suppress the anti-drug activity of Til-cells? It may be worthwhile to supplement their design with an additional surface (so there is a greater chance of interaction and less steric obstacles) conformationally small (not to disrupt the transport and structure of the nanoparticles) agent for suppressing the anti-drug activity of Til-cells.
4.2. Concluding Remarks
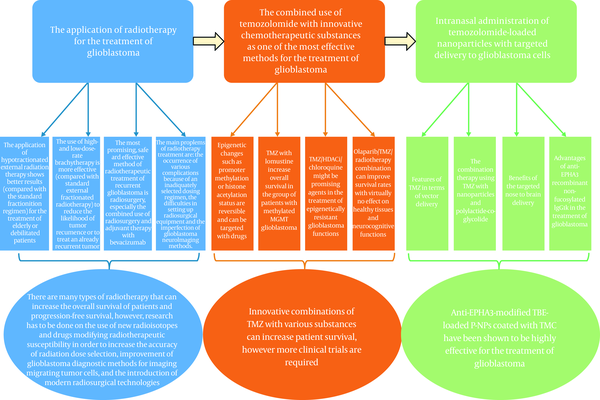
Glioblastomas are deadly; they are progressing very quickly and aggressively. Recent years show significant progress, which has been made in radiotherapy and chemotherapy and has helped to improve overall survival, on average, for several months. New radiotherapeutic methods that were listed above are encouraging for increasing survival rates of glioblastoma patients. With some improvement, such as drugs modifying radiotherapeutic susceptibility and development of glioblastoma diagnostic methods for imaging migrating tumor cells, radiotherapy may lead to the elimination of all tumor cells leaving the healthy alive. Temozolomide and its various combinations with other drugs are one of the most effective methods of glioblastoma and gliomas treatment. Impressing results were shown when using anti-EPHA3-modified TBE-loaded P-NPs, coated with TMC, that have high absorption specificity and enough levels of cytotoxicity to kill glioblastoma cells. But, it does not mean that we need to stop where we are now. We need to continue developing new, less toxic, and more effective drugs. Probably, one of the new “step forwards” may become a development of new medicine, which reduces the specific accumulation of anti-EPHA3-modified TBE-loaded P-NPs, coated with TMC, in lungs, but simultaneously does not affect specific absorption by tumor cells and does not pass the blood-brain barrier and blood-brain tumor barrier. Also, the authors find it interesting to improve nanoparticles to suppress the anti-drug properties of TIL-cells (tumor-infiltrating lymphocytes) (Figure 2).
the summary of the most crucial points of the article.
References
-
1.
Lamano JB, Quaggin-Smith JA, Horbinski CM, Tate MC, Grimm SA, Kumthekar PU, et al. Long-term glioblastoma survival following recovery from cytomegalovirus colitis: A case report. J Clin Neurosci. 2019;64:18-21. [PubMed ID: 30948314]. https://doi.org/10.1016/j.jocn.2019.03.051.
-
2.
Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, et al. Long-term Survival in Glioblastoma with Cytomegalovirus pp65-Targeted Vaccination. Clin Cancer Res. 2017;23(8):1898-909. [PubMed ID: 28411277]. [PubMed Central ID: PMC5559300]. https://doi.org/10.1158/1078-0432.CCR-16-2057.
-
3.
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987-96. [PubMed ID: 15758009]. https://doi.org/10.1056/NEJMoa043330.
-
4.
Batash R, Asna N, Schaffer P, Francis N, Schaffer M. Glioblastoma Multiforme, Diagnosis and Treatment; Recent Literature Review. Curr Med Chem. 2017;24(27):3002-9. [PubMed ID: 28521700]. https://doi.org/10.2174/0929867324666170516123206.
-
5.
Alifieris C, Trafalis DT. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol Ther. 2015;152:63-82. [PubMed ID: 25944528]. https://doi.org/10.1016/j.pharmthera.2015.05.005.
-
6.
Barani IJ, Larson DA. Radiation therapy of glioblastoma. Cancer Treat Res. 2015;163:49-73. [PubMed ID: 25468225]. https://doi.org/10.1007/978-3-319-12048-5_4.
-
7.
Shah JL, Li G, Shaffer JL, Azoulay MI, Gibbs IC, Nagpal S, et al. Stereotactic Radiosurgery and Hypofractionated Radiotherapy for Glioblastoma. Neurosurgery. 2018;82(1):24-34. [PubMed ID: 28605463]. https://doi.org/10.1093/neuros/nyx115.
-
8.
Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol. 2012;85(1017):e770-81. [PubMed ID: 22919020]. [PubMed Central ID: PMC3487099]. https://doi.org/10.1259/bjr/83827377.
-
9.
Chen AM, Chang S, Pouliot J, Sneed PK, Prados MD, Lamborn KR, et al. Phase I trial of gross total resection, permanent iodine-125 brachytherapy, and hyperfractionated radiotherapy for newly diagnosed glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2007;69(3):825-30. [PubMed ID: 17512132]. https://doi.org/10.1016/j.ijrobp.2007.03.061.
-
10.
Waters JD, Rose B, Gonda DD, Scanderbeg DJ, Russell M, Alksne JF, et al. Immediate post-operative brachytherapy prior to irradiation and temozolomide for newly diagnosed glioblastoma. J Neurooncol. 2013;113(3):467-77. [PubMed ID: 23673513]. https://doi.org/10.1007/s11060-013-1139-x.
-
11.
Welsh J, Sanan A, Gabayan AJ, Green SB, Lustig R, Burri S, et al. GliaSite brachytherapy boost as part of initial treatment of glioblastoma multiforme: a retrospective multi-institutional pilot study. Int J Radiat Oncol Biol Phys. 2007;68(1):159-65. [PubMed ID: 17331666]. https://doi.org/10.1016/j.ijrobp.2006.11.053.
-
12.
Kickingereder P, Hamisch C, Suchorska B, Galldiks N, Visser-Vandewalle V, Goldbrunner R, et al. Low-dose rate stereotactic iodine-125 brachytherapy for the treatment of inoperable primary and recurrent glioblastoma: single-center experience with 201 cases. J Neurooncol. 2014;120(3):615-23. [PubMed ID: 25151509]. https://doi.org/10.1007/s11060-014-1595-y.
-
13.
Chatzikonstantinou G, Ulrich P, Archavlis E, Zamboglou N, Strouthos I, Zoga E, et al. Interstitial high-dose-rate brachytherapy in the primary treatment of inoperable glioblastoma multiforme. J Contemp Brachytherapy. 2019;11(3):215-20. [PubMed ID: 31435428]. [PubMed Central ID: PMC6701379]. https://doi.org/10.5114/jcb.2019.85722.
-
14.
Barbarite E, Sick JT, Berchmans E, Bregy A, Shah AH, Elsayyad N, et al. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg Rev. 2017;40(2):195-211. [PubMed ID: 27180560]. https://doi.org/10.1007/s10143-016-0727-6.
-
15.
Schwartz C, Romagna A, Thon N, Niyazi M, Watson J, Belka C, et al. Outcome and toxicity profile of salvage low-dose-rate iodine-125 stereotactic brachytherapy in recurrent high-grade gliomas. Acta Neurochir (Wien). 2015;157(10):1757-64. discussion 1764. [PubMed ID: 26298594]. https://doi.org/10.1007/s00701-015-2550-1.
-
16.
Chatzikonstantinou G, Zamboglou N, Archavlis E, Strouthos I, Zoga E, Milickovic N, et al. CT-guided interstitial HDR-brachytherapy for recurrent glioblastoma multiforme: a 20-year single-institute experience. Strahlenther Onkol. 2018;194(12):1171-9. [PubMed ID: 30203110]. https://doi.org/10.1007/s00066-018-1358-3.
-
17.
Villavicencio AT, Burneikiene S, Romanelli P, Fariselli L, McNeely L, Lipani JD, et al. Survival following stereotactic radiosurgery for newly diagnosed and recurrent glioblastoma multiforme: a multicenter experience. Neurosurg Rev. 2009;32(4):417-24. [PubMed ID: 19633875]. https://doi.org/10.1007/s10143-009-0212-6.
-
18.
Niranjan A, Monaco EI, Kano H, Flickinger JC, Lunsford LD. Stereotactic Radiosurgery in the Multimodality Management of Residual or Recurrent Glioblastoma Multiforme. Prog Neurol Surg. 2018;31:48-61. [PubMed ID: 29393176]. https://doi.org/10.1159/000466998.
-
19.
Abbassy M, Missios S, Barnett GH, Brewer C, Peereboom DM, Ahluwalia M, et al. Phase I Trial of Radiosurgery Dose Escalation Plus Bevacizumab in Patients With Recurrent/Progressive Glioblastoma. Neurosurgery. 2018;83(3):385-92. [PubMed ID: 28973311]. https://doi.org/10.1093/neuros/nyx369.
-
20.
Morris SL, Zhu P, Rao M, Martir M, Zhu JJ, Hsu S, et al. Gamma Knife Stereotactic Radiosurgery in Combination with Bevacizumab for Recurrent Glioblastoma. World Neurosurg. 2019;127:e523-33. [PubMed ID: 30954746]. https://doi.org/10.1016/j.wneu.2019.03.193.
-
21.
Cuneo KC, Vredenburgh JJ, Sampson JH, Reardon DA, Desjardins A, Peters KB, et al. Safety and efficacy of stereotactic radiosurgery and adjuvant bevacizumab in patients with recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2012;82(5):2018-24. [PubMed ID: 21489708]. [PubMed Central ID: PMC3690566]. https://doi.org/10.1016/j.ijrobp.2010.12.074.
-
22.
Feng E, Sui C, Wang T, Sun G. Temozolomide with or without Radiotherapy in Patients with Newly Diagnosed Glioblastoma Multiforme: A Meta-Analysis. Eur Neurol. 2017;77(3-4):201-10. [PubMed ID: 28192785]. https://doi.org/10.1159/000455842.
-
23.
Schreck KC, Grossman SA. Role of Temozolomide in the Treatment of Cancers Involving the Central Nervous System. Oncology (Williston Park). 2018;32(11):555-60. 569. [PubMed ID: 30474103].
-
24.
Thomas RP, Recht L, Nagpal S. Advances in the management of glioblastoma: the role of temozolomide and MGMT testing. Clin Pharmacol. 2013;5:1-9. [PubMed ID: 23293540]. [PubMed Central ID: PMC3534290]. https://doi.org/10.2147/CPAA.S26586.
-
25.
Portnow J, Badie B, Chen M, Liu A, Blanchard S, Synold TW. The neuropharmacokinetics of temozolomide in patients with resectable brain tumors: potential implications for the current approach to chemoradiation. Clin Cancer Res. 2009;15(22):7092-8. [PubMed ID: 19861433]. [PubMed Central ID: PMC2908372]. https://doi.org/10.1158/1078-0432.CCR-09-1349.
-
26.
Diehl A, Yarchoan M, Hopkins A, Jaffee E, Grossman SA. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget. 2017;8(69):114268-80. [PubMed ID: 29371985]. [PubMed Central ID: PMC5768402]. https://doi.org/10.18632/oncotarget.23217.
-
27.
Thomas A, Tanaka M, Trepel J, Reinhold WC, Rajapakse VN, Pommier Y. Temozolomide in the Era of Precision Medicine. Cancer Res. 2017;77(4):823-6. [PubMed ID: 28159862]. [PubMed Central ID: PMC5313339]. https://doi.org/10.1158/0008-5472.CAN-16-2983.
-
28.
Christmann M, Verbeek B, Roos WP, Kaina B. O(6)-Methylguanine-DNA methyltransferase (MGMT) in normal tissues and tumors: enzyme activity, promoter methylation and immunohistochemistry. Biochim Biophys Acta. 2011;1816(2):179-90. [PubMed ID: 21745538]. https://doi.org/10.1016/j.bbcan.2011.06.002.
-
29.
Karran P, Hampson R. Genomic instability and tolerance to alkylating agents. Cancer Surv. 1996;28:69-85. [PubMed ID: 8977029].
-
30.
Nagel ZD, Margulies CM, Chaim IA, McRee SK, Mazzucato P, Ahmad A, et al. Multiplexed DNA repair assays for multiple lesions and multiple doses via transcription inhibition and transcriptional mutagenesis. Proc Natl Acad Sci U S A. 2014;111(18):E1823-32. [PubMed ID: 24757057]. [PubMed Central ID: PMC4020053]. https://doi.org/10.1073/pnas.1401182111.
-
31.
Higuchi F, Nagashima H, Ning J, Koerner MVA, Wakimoto H, Cahill DP. Restoration of Temozolomide Sensitivity by PARP Inhibitors in Mismatch Repair Deficient Glioblastoma is Independent of Base Excision Repair. Clin Cancer Res. 2020;26(7):1690-9. [PubMed ID: 31900275]. [PubMed Central ID: PMC7192178]. https://doi.org/10.1158/1078-0432.CCR-19-2000.
-
32.
Gatenby RA, Brown J. Mutations, evolution and the central role of a self-defined fitness function in the initiation and progression of cancer. Biochim Biophys Acta Rev Cancer. 2017;1867(2):162-6. [PubMed ID: 28341421]. [PubMed Central ID: PMC5441954]. https://doi.org/10.1016/j.bbcan.2017.03.005.
-
33.
Nadeem Abbas M, Kausar S, Wang F, Zhao Y, Cui H. Advances in Targeting the Epidermal Growth Factor Receptor Pathway by Synthetic Products and Its Regulation by Epigenetic Modulators As a Therapy for Glioblastoma. Cells. 2019;8(4). [PubMed ID: 31013819]. [PubMed Central ID: PMC6523687]. https://doi.org/10.3390/cells8040350.
-
34.
Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, open-label, phase 3 trial. Lancet. 2019;393(10172):678-88. [PubMed ID: 30782343]. https://doi.org/10.1016/S0140-6736(18)31791-4.
-
35.
Lohitesh K, Saini H, Srivastava A, Mukherjee S, Roy A, Chowdhury R. Autophagy inhibition potentiates SAHAmediated apoptosis in glioblastoma cells by accumulation of damaged mitochondria. Oncol Rep. 2018;39(6):2787-96. [PubMed ID: 29658588]. https://doi.org/10.3892/or.2018.6373.
-
36.
Hori YS, Hosoda R, Akiyama Y, Sebori R, Wanibuchi M, Mikami T, et al. Chloroquine potentiates temozolomide cytotoxicity by inhibiting mitochondrial autophagy in glioma cells. J Neurooncol. 2015;122(1):11-20. [PubMed ID: 25528635]. https://doi.org/10.1007/s11060-014-1686-9.
-
37.
Li JY, Horwitz S, Moskowitz A, Myskowski PL, Pulitzer M, Querfeld C. Management of cutaneous T cell lymphoma: new and emerging targets and treatment options. Cancer Manag Res. 2012;4:75-89. [PubMed ID: 22457602]. [PubMed Central ID: PMC3308634]. https://doi.org/10.2147/CMAR.S9660.
-
38.
Goncalves RM, Agnes JP, Delgobo M, de Souza PO, Thome MP, Heimfarth L, et al. Late autophagy inhibitor chloroquine improves efficacy of the histone deacetylase inhibitor SAHA and temozolomide in gliomas. Biochem Pharmacol. 2019;163:440-50. [PubMed ID: 30878553]. https://doi.org/10.1016/j.bcp.2019.03.015.
-
39.
Lesueur P, Lequesne J, Grellard JM, Dugue A, Coquan E, Brachet PE, et al. Phase I/IIa study of concomitant radiotherapy with olaparib and temozolomide in unresectable or partially resectable glioblastoma: OLA-TMZ-RTE-01 trial protocol. BMC Cancer. 2019;19(1):198. [PubMed ID: 30832617]. [PubMed Central ID: PMC6399862]. https://doi.org/10.1186/s12885-019-5413-y.
-
40.
Sharma S, Italiya K, Mittal A, Chitkara D. New strategies for cancer management: how can temozolomide carrier modifications improve its delivery? Ther Deliv. 2017;8(7):475-7. [PubMed ID: 28633588]. https://doi.org/10.4155/tde-2017-0016.
-
41.
Trinh VA, Patel SP, Hwu WJ. The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf. 2009;8(4):493-9. [PubMed ID: 19435405]. https://doi.org/10.1517/14740330902918281.
-
42.
Chakravarti A, Erkkinen MG, Nestler U, Stupp R, Mehta M, Aldape K, et al. Temozolomide-mediated radiation enhancement in glioblastoma: a report on underlying mechanisms. Clin Cancer Res. 2006;12(15):4738-46. [PubMed ID: 16899625]. https://doi.org/10.1158/1078-0432.CCR-06-0596.
-
43.
Song S, Mao G, Du J, Zhu X. Novel RGD containing, temozolomide-loading nanostructured lipid carriers for glioblastoma multiforme chemotherapy. Drug Deliv. 2016;23(4):1404-8. [PubMed ID: 26203687]. https://doi.org/10.3109/10717544.2015.1064186.
-
44.
Stoyanov GS, Dzhenkov D, Ghenev P, Iliev B, Enchev Y, Tonchev AB. Cell biology of glioblastoma multiforme: from basic science to diagnosis and treatment. Med Oncol. 2018;35(3):27. [PubMed ID: 29387965]. https://doi.org/10.1007/s12032-018-1083-x.
-
45.
Jain KK. A Critical Overview of Targeted Therapies for Glioblastoma. Front Oncol. 2018;8:419. [PubMed ID: 30374421]. [PubMed Central ID: PMC6196260]. https://doi.org/10.3389/fonc.2018.00419.
-
46.
Ramalho MJ, Sevin E, Gosselet F, Lima J, Coelho MAN, Loureiro JA, et al. Receptor-mediated PLGA nanoparticles for glioblastoma multiforme treatment. Int J Pharm. 2018;545(1-2):84-92. [PubMed ID: 29715532]. https://doi.org/10.1016/j.ijpharm.2018.04.062.
-
47.
Lee CY, Ooi IH. Preparation of Temozolomide-Loaded Nanoparticles for Glioblastoma Multiforme Targeting-Ideal Versus Reality. Pharmaceuticals (Basel). 2016;9(3). [PubMed ID: 27618068]. [PubMed Central ID: PMC5039507]. https://doi.org/10.3390/ph9030054.
-
48.
Wang Y. Pharmaceutical composition comprising temozolomide ester. Google Patents; 2009.
-
49.
Gao H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B. 2016;6(4):268-86. [PubMed ID: 27471668]. [PubMed Central ID: PMC4951594]. https://doi.org/10.1016/j.apsb.2016.05.013.
-
50.
Chu L, Wang A, Ni L, Yan X, Song Y, Zhao M, et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug Deliv. 2018;25(1):1634-41. [PubMed ID: 30176744]. [PubMed Central ID: PMC6127843]. https://doi.org/10.1080/10717544.2018.1494226.
-
51.
Chung K, Ullah I, Kim N, Lim J, Shin J, Lee SC, et al. Intranasal delivery of cancer-targeting doxorubicin-loaded PLGA nanoparticles arrests glioblastoma growth. J Drug Target. 2020;28(6):617-26. [PubMed ID: 31852284]. https://doi.org/10.1080/1061186X.2019.1706095.
-
52.
Khan AR, Liu M, Khan MW, Zhai G. Progress in brain targeting drug delivery system by nasal route. J Control Release. 2017;268:364-89. [PubMed ID: 28887135]. https://doi.org/10.1016/j.jconrel.2017.09.001.
-
53.
Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3(1):42-62. [PubMed ID: 23316447]. [PubMed Central ID: PMC3539067]. https://doi.org/10.1007/s13346-012-0108-9.
-
54.
Gartziandia O, Herran E, Pedraz JL, Carro E, Igartua M, Hernandez RM. Chitosan coated nanostructured lipid carriers for brain delivery of proteins by intranasal administration. Colloids Surf B Biointerfaces. 2015;134:304-13. [PubMed ID: 26209963]. https://doi.org/10.1016/j.colsurfb.2015.06.054.
-
55.
Hagenaars N, Mania M, de Jong P, Que I, Nieuwland R, Slutter B, et al. Role of trimethylated chitosan (TMC) in nasal residence time, local distribution and toxicity of an intranasal influenza vaccine. J Control Release. 2010;144(1):17-24. [PubMed ID: 20100528]. https://doi.org/10.1016/j.jconrel.2010.01.027.
-
56.
Belhadj Z, Ying M, Cao X, Hu X, Zhan C, Wei X, et al. Design of Y-shaped targeting material for liposome-based multifunctional glioblastoma-targeted drug delivery. J Control Release. 2017;255:132-41. [PubMed ID: 28390902]. https://doi.org/10.1016/j.jconrel.2017.04.006.
-
57.
Fan K, Jia X, Zhou M, Wang K, Conde J, He J, et al. Ferritin Nanocarrier Traverses the Blood Brain Barrier and Kills Glioma. ACS Nano. 2018;12(5):4105-15. [PubMed ID: 29608290]. https://doi.org/10.1021/acsnano.7b06969.
-
58.
Janes PW, Slape CI, Farnsworth RH, Atapattu L, Scott AM, Vail ME. EphA3 biology and cancer. Growth Factors. 2014;32(6):176-89. [PubMed ID: 25391995]. https://doi.org/10.3109/08977194.2014.982276.
-
59.
Swords RT, Greenberg PL, Wei AH, Durrant S, Advani AS, Hertzberg MS, et al. KB004, a first in class monoclonal antibody targeting the receptor tyrosine kinase EphA3, in patients with advanced hematologic malignancies: Results from a phase 1 study. Leuk Res. 2016;50:123-31. [PubMed ID: 27736729]. https://doi.org/10.1016/j.leukres.2016.09.012.
-
60.
Offenhauser C, Al-Ejeh F, Puttick S, Ensbey KS, Bruce ZC, Jamieson PR, et al. EphA3 Pay-Loaded Antibody Therapeutics for the Treatment of Glioblastoma. Cancers (Basel). 2018;10(12). [PubMed ID: 30562956]. [PubMed Central ID: PMC6316644]. https://doi.org/10.3390/cancers10120519.
-
61.
Vail ME, Murone C, Tan A, Hii L, Abebe D, Janes PW, et al. Targeting EphA3 inhibits cancer growth by disrupting the tumor stromal microenvironment. Cancer Res. 2014;74(16):4470-81. [PubMed ID: 25125683]. https://doi.org/10.1158/0008-5472.CAN-14-0218.
-
62.
Bi C, Wang A, Chu Y, Liu S, Mu H, Liu W, et al. Intranasal delivery of rotigotine to the brain with lactoferrin-modified PEG-PLGA nanoparticles for Parkinson's disease treatment. Int J Nanomedicine. 2016;11:6547-59. [PubMed ID: 27994458]. [PubMed Central ID: PMC5153272]. https://doi.org/10.2147/IJN.S120939.
-
63.
Meng Q, Wang A, Hua H, Jiang Y, Wang Y, Mu H, et al. Intranasal delivery of Huperzine A to the brain using lactoferrin-conjugated N-trimethylated chitosan surface-modified PLGA nanoparticles for treatment of Alzheimer's disease. Int J Nanomedicine. 2018;13:705-18. [PubMed ID: 29440896]. [PubMed Central ID: PMC5798568]. https://doi.org/10.2147/IJN.S151474.