Abstract
Background:
There is increasing awareness that patients with cancer desire information as well as strategies to support their capacity to actively participate in informed decision-making. This study will evaluate outcomes of using a question prompt list (QPL) on shared decision making (SDM), decision-making self-efficacy, and preferences for participation among Iranian women with breast cancer, who are referred to a Tehran Comprehensive Cancer Center.Methods:
This research will utilize a randomized controlled trial. The research population is patients with breast cancer, who are referred to the Oncology Radiotherapy Unit, Imam Khomeini Hospital, Tehran following tumor resection. After completing baseline surveys (demographics and health survey, decision self-efficacy scale, and control preferences scale), participants will be randomized into either a control or a treatment group based on block design. The treatment group will receive routine care along with the QPL that provides information on decision-making relative to treatment options (chemotherapy, radiotherapy, or both treatments) following meeting their oncologist. They will be trained to use the QPL which they will use to ask questions about their treatment choices when meeting with their physician or through computer-mediated modalities such as WhatsApp or other social messengers. These patients will have the opportunity to think about the treatment options and will be referred for medical treatment following their decision. The control group will receive routine care (physician discussion and receipt of treatment information). Following decision-making regarding treatment, the questionnaires will be administered (9-item SDM questionnaire, decision self-efficacy scale, and control preferences scale). Data will be analyzed using SPSS 16.Discussion:
The current study will provide experimental evidence for the preliminary efficacy or lack of an intervention that has the potential to improve shared decision-making outcomes, a better understanding of personal preferences related to decision-making and self-efficacy in medical decision-making for Iranian patients with breast cancer.Keywords
Shared-Decision Making Self-Efficacy Preferences Participation Question Prompt List Cancer Iran
1. Background
Breast cancer is a common and highly prevalent disease with a high incidence globally. It includes 1.7 million new cases per year and 25% of all types of cancers (1). In Iran, cancers, including breast, are the third cause of death following coronary heart disease and accidents. In Iran, the incidence rate of cancer is 98 - 100 per 100,000 population (2).
There is increasing awareness that patients with cancer desire information as well as strategies to support their capacity to actively participate in informed decision-making (3). With shared decision making, the physician provides the patient with information about the disease, potential complications and risks, treatment options, and advantages and disadvantages of different alternatives. A mutual decision is then agreed upon based on the patient’s preferences and priorities. Shared decision-making thus provides a mechanism for using evidence to evaluate treatment options, while also taking into account the beliefs and desires of patients (4). Although many patients want detailed information, their actual involvement in decision making is considerably variable (5). For example, Khammarnia et al. (6) reported that 52% of patients with cancer (n = 374) in their study were passive in treatment decision making involvement.
Self-efficacy, a personal belief, and confidence that one can successfully perform the behaviors required to produce expected outcomes are essential in order to be actively engaged in one’s life. Higher self-efficacy may facilitate the active involvement of patients in treatment decision making which has been associated with better coping and enhanced well-being (7, 8). Psycho-educational interventions to bolster self-efficacy are a way to help patients make informed decisions that are based on both benefits and challenges of possible treatment options (9). While most patients with cancer express a desire for full information about their illness and treatment, they are uncertain about what relevant questions they should discuss with their health care team. Further, clinicians may not be certain relative to the type and degree of information the patient desires, especially given treatment discussion may contribute to patient distress (10). Research suggests that information needs regarding treatment decisions are individualized and vary among patients (11).
A question prompt list (QPL) is an innovative method that consists of a structured list of patient questions that can potentially be asked from health providers including both physicians and nurses. The QPL is designed to assist patients in order to obtain information that is suited to personal needs at their own pace (11). For example, the “three questions to ask your doctor” is a personal QPL that has been introduced as an initiative in the British health system (12). Communication aids such as QPL were developed to help patients identify their concerns and to address questions they have about their diagnosis and treatment while encouraging them to seek information and appropriate answers (10). In Australia, QPLs have been developed, in particular, for use in cancer care (12).
The QPL may potentially improve patient participation in decision-making about breast cancer treatment (13). The QPL is user-friendly and requires limited financial and human resources, and can be implemented in a busy care environment (10). The QPL purpose is to support patients who need information related to their diagnosis and treatment. The QPL provides a platform for patients to express concerns, while also potentially improving therapeutic alliances between the patient and physician that enhances the patients participation in their care, including as treatment decision-making (14). Thus, QPL is potentially an important tool that may facilitate information exchange between the health team and their patients.
Although there has been increasing interest in the use of QPL for improvement of clinical outcomes, there are gaps in the knowledge relative to its efficacy in improving patients’ capacity to make informed decisions (12). Previous studies have evaluated the effectiveness of QPL on outcomes such as anxiety levels (15), appropriate numbers of questions to query the healthcare team (16), patients’ self-confidence in asking questions (17), and preferences to participate in informed decision-making (18). However, studies remain limited, and in particular, very few clinical trials with this context have been conducted (19). QPL has been introduced as a method to facilitate information exchange between health providers and their patients. Given the socio-cultural climate of Iranian healthcare, patients with cancer may not ask questions about their treatment options during their short appointments. Thus using QPL might be particularly useful. Therefore, the aim of the proposed study is to determine if the QPL may impact shared decision making, self-efficacy in treatment decision-making, and preferences to participate in decision-making among patients with breast cancer who have completed surgery.
2. Methods
2.1. Study Setting
A randomized controlled trial will be conducted using parallel groups. The research population will consist of patients with breast cancer, who were referred to the Oncology Radiotherapy Unit, Imam Khomeini Hospital following tumor resection. This hospital is a Comprehensive Cancer Center located in Tehran, Iran.
2.2. Eligibility Criteria
Inclusion criteria consist of patients with breast cancer who are referred for adjuvant/neoadjuvant treatment following tumor resection, able to read and write Persian, and age 18 years or older. Exclusion criteria include a history of psychiatric illness that would prevent them from participating in treatment decision-making.
2.3. Intervention
The QPL is a list of selected questions that includes important information that patients desire relative to their health condition before participating in care plans. In advance of the proposed study, we interviewed patients who had been selected by purposeful sampling, to determine what their questions were about their respective breast cancer treatment. We found that many patients were not aware of what treatment they needed and/or when treatment would commence. Several patients were concerned about potential treatment side effects, including from chemotherapy, and whether the associated side effects would disrupt daily work and other duties. Many patients did not know how they would adapt to treatment-related changes. Some patients were not familiar with what radiotherapy is and its potential complications. Further, some were anxious and unable to analyze the information that their physicians provided to participate in decision-making, even though they had previously undergone surgery. Some patients did not know what questions to ask in order to make an informed decision for starting a particular treatment or even to withdraw from treatment. Given these issues identified that were associated with the Iranian women’s making decisions about breast cancer treatment, we asked the healthcare team to identify potential questions that could be added to the QPL to enable patients to participate in shared decision-making. Existing evidence and other QPLs were considered for developing the QPLs (chemotherapy-related QPL and radiotherapy-related QPL) to be used in the proposed study (18, 20-22).
2.4. Outcomes
2.4.1. Primary Outcome
The primary outcome of this study will be shared decision making.
2.4.2. Secondary Outcome
The secondary outcomes will include (1) preferences to participate in decision-making and (2) decision-making self-efficacy.
2.5. Sampling
The sample size was estimated using Openepi software and a previous study of Aminaie et al. (4) by evaluating the differences between two means. Using this method, the sample size is estimated to require 22 individuals in each group, based on the standard deviation of two groups (5.84), significant level (95%), statistical power (80%), and alteration in scores (5) as an evaluation of the intervention effectiveness. To account for the possibility of 10% attrition over the course of the study, 25 individuals will be assigned to each group.
2.6. Allocation Process
Allocation will include three stages of random sequence generation, allocation concealment, and implementation of the random allocation process. The production of a random allocation sequence will be carried out by the block method using software (23) with a fixed block size equaling 4. Allocation concealment will be carried out using sealed opaque envelopes in order to have a non-predictable sampling. These two stages will be carried out by RN, rather than the researcher performing the sampling.
2.7. Blinding
This study will be single-blinded.
2.8. Data Collection
Participants who were referred for adjuvant/ neoadjuvant treatment (following tumor resection) will be evaluated based on the aforementioned inclusion criteria. If eligible patients are interested and willing to participate, written informed consent will be elicited. Before random allocation, pre-test survey data will be collected. Participants in both groups will receive routine care via physician consultation about appropriate treatments (chemotherapy, radiotherapy, or a combination of these two treatments). For patients in the treatment group, the QPL will be provided. Depending on what is appropriate for their care based on their post-surgical profile, these patients will receive a QPL with specific information about chemotherapy, radiotherapy, or both.
Patients will be explicitly informed that they can use the QPL to develop questions that they can bring to their appointment, or request answers via phone call or through computer-mediated options such as WhatsApp application. Patients will be informed that they are free to ask questions that are not present in the QPL and are free to reflect and voice their concerns and questions until they are comfortable with a treatment decision.
Following treatment decision-making, post-testing will be conducted with all the study participants and will include the shared decision-making questionnaire, the decision self-efficacy scale, and the control preferences scale (Figure 1). Given participants may vary in the time needed to decide treatment, the duration of time will be recorded and time effects will be evaluated in the statistical analysis.
Study flow diagram. CPS = Control Preferences Scale, SDM-Q-9 = The 9-item Shared Decision-making Questionnaire
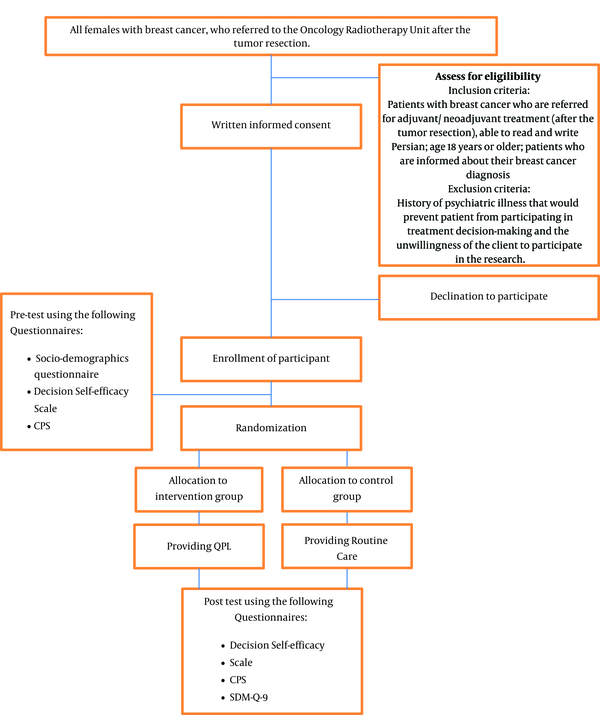
Confounding factors will be minimized by the use of randomization. However, we will control for potential confounding factors by making necessary adjustments using an appropriate regression model.
2.9. Measures
2.9.1. Demographic and Health Information Questionnaire
Demographic information will be collected including age, marital status, number of children, level of education, occupation, insurance, and income. Health information will include breast screening tests before current illness (monthly self-examination, an annual examination by a physician or midwife, mammography, and sonography), surgical background, underlying disease background, tumor resection time, co-morbid health conditions, and sources of health information.
2.9.2. The 9-Item Shared Decision-Making Questionnaire
The 9-Item Shared Decision-Making questionnaire (SDM-Q-9) will be used to evaluate the patients’ perceptions of their participation in decision-making. This questionnaire is a standard tool consisting of nine questions. The patients respond to questions on a 6-point Likert scale (completely disagree = 0 to completely agree = 5). The score range is from 0 to 45. In this study, questionnaire scores will be divided into three groups to compute the participation of patients in clinical decision-making. Scores between 0 to 15 are considered to be low participation (physician or health team-centered), 16 to 30 as average or equal participation (shared), and 31 to 45 as high participation in the decision-making (patient-centered) (4). This questionnaire will be translated using translation-back translation. Its validity and reliability will be evaluated to coordinate its Persian version with the original version. The validity and reliability of this questionnaire have been measured in the study of Kriston et al. (24) (Cronbach’s α = 0.938).
2.9.3. Control Preferences Scale
Developed by Degner et al. (25), The Control Preferences scale (CPS) is a self-report scale consisting of five options that evaluate the preference of patients to participate in their decision-making, ordered from the patient's preference to “independence in decision-making” to “complete authority of the physician in decision-making”. The patients will be asked to choose an option based on their role, which they prefer in the decision-making process. Then, this action will be repeated once more. If the first option does not demonstrate consistency with the second option in terms of the score (e.g., A after C), the test will be repeated. Based on the preference, A and B play an active role, C shared role, and D and E are inactive. In general, six scores will be achieved: (1) Active-active; (2) active-shared; (3) shared-active; (4) shared-inactive; (5) inactive-shared; and (6) inactive-inactive. These scores will be reported as three groups: active (active-active and active-shared); shared (shared-active and shared-inactive), and inactive (inactive-shared and inactive-inactive) (4). This questionnaire will be translated using the standard method of the translation-back translation. Its validity and reliability will be evaluated in accordance with its’ ability to coordinate its Persian version with the original version. The validity and reliability of this scale have been measured in the study of De Las Cuevas et al. (26) (Cronbach’s α = 0.72).
2.9.4. Decision Self-Efficacy Scale
This scale includes 11 Likert items with response scales running from zero (not confident) to four (very confident). Total scores will be divided into 11 and multiplied into 25. The scores will be arranged from zero (not confident) to a hundred (very confident) (27). This questionnaire will also be translated based on the standard method of translation-back translation. Its validity and reliability will be evaluated to coordinate its Persian version with the original version. The validity and reliability of this scale have been measured in the study of Bunn et al. (Cronbach’s α = 0.84) (28).
2.10. Statistical Analysis
Descriptive and analytical statistics will be used to analyze data. The mean (standard deviation) will be used to describe the quantitative variables and the frequency (percentage) for the categorical variables. Independent samples t-test will be used to evaluate the between-groups comparison of the mean outcome variables scores. ANCOVA will be conducted to adjust pre-test scores in comparing the post-test scores of outcome variables. Data analysis will be performed using SPSS software. A P value of less than 0.05 will be considered statistically significant. The intention to treat (ITT) approach will be used if more than 30% of individuals are not adherent to the QPL intervention.
2.11. Ethics Principles and Publication Plan
This clinical trial study protocol has been approved by the ethics committee of Tehran University of Medical Science and registered in the Iranian Registry of Clinical Trials with the number of IRCT20190626044032N1. Written informed consent form will be obtained. Eligible patients will receive information that their treatment will not be affected if they choose not to participate. Further, they will receive information that their anonymity will be assured and that all study materials are assigned a code as opposed to personal identifiers.
Findings will be presented at related conferences and papers will be generated for journals to inform both science and clinical practice. The published data will include outcomes useful for scientists and practitioners interested in enhancing patient care, and also researchers motivated towards conducting systematic and meta-analytical reviews on global data. This research will be published according to the CONSORT checklist.
3. Discussion
Patients with cancer need to be aware of their potential treatment options at all stages of their illness trajectory. Based on the findings in previous studies, patients may face challenges in making personal decisions about treatment during a highly stressful time (29). However, time is an important factor in cancer treatment, given the potential for disease advancement. Decreased quality of life that result from various cancer treatments may outweigh other advantages (30). In such potentially complex patient care situations, strategies for increasing the active participation of patients are needed. The QPL is an innovative strategy that may improve the capacity for patient participation in their care by encouraging patients to ask relevant questions and by facilitating patient communication with the healthcare team. The current study will provide experimental evidence for the preliminary efficacy or lack thereof of an intervention that carries the potential to improve shared decision-making outcomes, a better understanding of personal preferences related to decision-making and self-efficacy in medical decision-making for Iranian patients with breast cancer. If effectiveness is confirmed, QPL may be eventually added to the list of tools available to potentially enhance shared decision-making status in Iranian women with breast cancer.
Acknowledgements
References
-
1.
Ghoncheh M, Pournamdar Z, Salehiniya H. Incidence and mortality and epidemiology of breast cancer in the world. Asian Pac J Cancer Prev. 2016;17(S3):43-6. [PubMed ID: 27165206]. https://doi.org/10.7314/apjcp.2016.17.s3.43.
-
2.
Vakili M, Pirdehghan A, Adimi M, Sadeghian M, Akhondi M. Epidemiology and trend of cancer in Yazd, a central province of Iran, 2005-2009. J Res Health Sci. 2014;14(3):210-3. [PubMed ID: 25209908].
-
3.
Masters S, Gordon J, Whitehead C, Davies O, Giles LC, Ratcliffe J. Coaching older adults and carers to have their preferences heard (COACH): A randomised controlled trial in an intermediate care setting (study protocol). Australas Med J. 2012;5(8):444-54. [PubMed ID: 23024719]. [PubMed Central ID: PMC3442189]. https://doi.org/10.4066/AMJ.2012.1366.
-
4.
Aminaie N, Lehto RH, Negarandeh R. Iranian women's decision making: Preferred roles, experienced involvement, and decisional conflict when undergoing surgery for early-stage breast cancer. Clin J Oncol Nurs. 2019;23(5):529-36. [PubMed ID: 31538966]. https://doi.org/10.1188/19.CJON.529-536.
-
5.
Sinding C, Hudak P, Wiernikowski J, Aronson J, Miller P, Gould J, et al. "I like to be an informed person but..." negotiating responsibility for treatment decisions in cancer care. Soc Sci Med. 2010;71(6):1094-101. [PubMed ID: 20633970]. https://doi.org/10.1016/j.socscimed.2010.06.005.
-
6.
Khammarnia M, Eskandari M, Alinezhade Ranjbar A, Ansari-Moghadam A, Peyvand M. Participation rate of cancer patients in treatment decisions: A cross sectional study. J Health Literacy. 2017;2(3):156-63. https://doi.org/10.29252/jhl.2.3.156.
-
7.
Foster C, Breckons M, Cotterell P, Barbosa D, Calman L, Corner J, et al. Cancer survivors' self-efficacy to self-manage in the year following primary treatment. J Cancer Surviv. 2015;9(1):11-9. [PubMed ID: 25028218]. [PubMed Central ID: PMC4341005]. https://doi.org/10.1007/s11764-014-0384-0.
-
8.
Ng YK, Shah NM, Loong LS, Pee LT, Hidzir SAM, Chong WW. Attitudes toward concordance and self-efficacy in decision making: A cross-sectional study on pharmacist-patient consultations. Patient Prefer Adherence. 2018;12:615-24. [PubMed ID: 29731609]. [PubMed Central ID: PMC5923248]. https://doi.org/10.2147/PPA.S159113.
-
9.
Pedram Razi S, Satiyarvand A, Tabari F, Kazemnejad A, Tizh Maghz Z, Navidhamidi M. The effect of education and telephone counseling on the self-efficacy of women with breast cancer undergoing radiotherapy. Iran J Nurs Res. 2018;13(1):64-72.
-
10.
Dimoska A, Tattersall MH, Butow PN, Shepherd H, Kinnersley P. Can a "prompt list" empower cancer patients to ask relevant questions? Cancer. 2008;113(2):225-37. [PubMed ID: 18484592]. https://doi.org/10.1002/cncr.23543.
-
11.
Clayton J, Butow P, Tattersall M, Chye R, Noel M, Davis JM, et al. Asking questions can help: Development and preliminary evaluation of a question prompt list for palliative care patients. Br J Cancer. 2003;89(11):2069-77. [PubMed ID: 14647140]. [PubMed Central ID: PMC2376858]. https://doi.org/10.1038/sj.bjc.6601380.
-
12.
Sansoni J, Grootemaat P, Duncan C, Samsa P, Eagar K. A systematic literature review on question prompt lists in health care: Final report. Cent Heal Serv Dev Univ Wollongong. 2014.
-
13.
Sleath B, Carpenter DM, Davis SA, Watson CH, Lee C, Loughlin CE, et al. Acceptance of a pre-visit intervention to engage teens in pediatric asthma visits. Patient Educ Couns. 2017;100(11):2005-11. [PubMed ID: 28550963]. [PubMed Central ID: PMC5600669]. https://doi.org/10.1016/j.pec.2017.05.013.
-
14.
Sansoni JE, Grootemaat P, Duncan C. Question prompt lists in health consultations: A review. Patient Educ Couns. 2015. [PubMed ID: 26104993]. https://doi.org/10.1016/j.pec.2015.05.015.
-
15.
Lim L, Chow P, Wong CY, Chung A, Chan YH, Wong WK, et al. Doctor-patient communication, knowledge, and question prompt lists in reducing preoperative anxiety: A randomized control study. Asian J Surg. 2011;34(4):175-80. [PubMed ID: 22464834]. https://doi.org/10.1016/j.asjsur.2011.11.002.
-
16.
Shirai Y, Fujimori M, Ogawa A, Yamada Y, Nishiwaki Y, Ohtsu A, et al. Patients' perception of the usefulness of a question prompt sheet for advanced cancer patients when deciding the initial treatment: A randomized, controlled trial. Psychooncology. 2012;21(7):706-13. [PubMed ID: 21425387]. https://doi.org/10.1002/pon.1955.
-
17.
Kidd J, Marteau TM, Robinson S, Ukoumunne OC, Tydeman C. Promoting patient participation in consultations: A randomised controlled trial to evaluate the effectiveness of three patient-focused interventions. Patient Educ Couns. 2004;52(1):107-12. [PubMed ID: 14729297]. https://doi.org/10.1016/s0738-3991(03)00018-1.
-
18.
Brown RF, Butow PN, Dunn SM, Tattersall MH. Promoting patient participation and shortening cancer consultations: A randomised trial. Br J Cancer. 2001;85(9):1273-9. [PubMed ID: 11720460]. [PubMed Central ID: PMC2375236]. https://doi.org/10.1054/bjoc.2001.2073.
-
19.
Amundsen A, Bergvik S, Butow P, Tattersall MHN, Sorlie T, Nordoy T. Supporting doctor-patient communication: Providing a question prompt list and audio recording of the consultation as communication aids to outpatients in a cancer clinic. Patient Educ Couns. 2018;101(9):1594-600. [PubMed ID: 29703493]. https://doi.org/10.1016/j.pec.2018.04.011.
-
20.
McJannett M, Butow P, Tattersall MH, Thompson JF. Asking questions can help: development of a question prompt list for cancer patients seeing a surgeon. Eur J Cancer Prev. 2003;12(5):397-405. [PubMed ID: 14512805]. https://doi.org/10.1097/00008469-200310000-00009.
-
21.
Yeh JC, Cheng MJ, Chung CH, Smith TJ. Using a question prompt list as a communication aid in advanced cancer care. J Oncol Pract. 2014;10(3):e137-41. [PubMed ID: 24594680]. [PubMed Central ID: PMC4888050]. https://doi.org/10.1200/JOP.2013.001295.
-
22.
McLawhorn VC, Vess J, Dumas BP. Integrating a question prompt list on an inpatient oncology unit to increase prognostic awareness. Clin J Oncol Nurs. 2016;20(4):385-90. [PubMed ID: 27441510]. https://doi.org/10.1188/16.CJON.385-390.
-
23.
Research randomizer. 2019. Available from: https://www.randomizer.org.
-
24.
Kriston L, Scholl I, Holzel L, Simon D, Loh A, Harter M. The 9-item Shared Decision Making questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Educ Couns. 2010;80(1):94-9. [PubMed ID: 19879711]. https://doi.org/10.1016/j.pec.2009.09.034.
-
25.
Degner LF, Sloan JA, Venkatesh P. The Control Preferences scale. Can J Nurs Res. 1997;29(3):21-43. [PubMed ID: 9505581].
-
26.
De Las Cuevas C, Penate W. Validity of the Control Preferences scale in patients with emotional disorders. Patient Prefer Adherence. 2016;10:2351-6. [PubMed ID: 27895470]. [PubMed Central ID: PMC5118017]. https://doi.org/10.2147/PPA.S122377.
-
27.
User manual for decision self-efficacy-patient decision aids. 2019. Available from: https://decisionaid.ohri.ca/docs/develop/User_Manuals/UM_Decision_SelfEfficacy.pdf.
-
28.
Bunn H, O'Connor A. Validation of client decision-making instruments in the context of psychiatry. Can J Nurs Res. 1996;28(3):13-27. [PubMed ID: 8997937].
-
29.
Sankar SD, Dhanapal B, Shankar G, Krishnaraj B, Karra S, Natesan V. Desire for information and preference for participation in treatment decisions in patients with cancer presenting to the department of general surgery in a tertiary care hospital in india. J Glob Oncol. 2018;4:1-10. [PubMed ID: 30241243]. [PubMed Central ID: PMC6223434]. https://doi.org/10.1200/JGO.17.00144.
-
30.
Henselmans I, Smets EMA, de Haes J, Dijkgraaf MGW, de Vos FY, van Laarhoven HWM. A randomized controlled trial of a skills training for oncologists and a communication aid for patients to stimulate shared decision making about palliative systemic treatment (CHOICE): Study protocol. BMC Cancer. 2018;18(1):55. [PubMed ID: 29310605]. [PubMed Central ID: PMC5759304]. https://doi.org/10.1186/s12885-017-3838-8.

