Abstract
Background:
The role of human papillomavirus (HPV) in prostate cancer is unclear. The aim of this study was to investigate the relationship between HPV and prostate cancer.Methods:
In this case - control study, 133 paraffin embedded and formalin fixed prostate tissues were collected from the archive of pathology laboratory, Tohid Hospital, Sanandaj, Iran. A total of 58 tissues with malignant tumors (cases) and 75 tissues with benign prostatic hyperplasia (controls) were selected. Sections with thickness of 7 μm to 10 μm were prepared by sterile microtome blade. Sections were deparaffinized, deoxyribonucleic acid (DNA) was extracted, and stored at - 20 °C. To detect HPV infection, PCR test was conducted on all samples, using HPV general primers. Also, to detect high - risk HPV genotypes, PCR test was performed, using genotype specific primers. Genotypes of HPV positive samples were confirmed by sequencing.Results:
In the polymerase chain reaction (PCR) test using HPV general primers, 3 (2.3%) of the 133 samples were positive. Using genotype specific primers, HPV - 18 was positive in 2 (3.4%) of 58 cases and 1 (1.3%) of the 75 controls. The difference of HPV infection between 2 groups was not significant (P = 0.41). Other high - risk genotypes, HPV - 16, HPV - 31, and HPV - 33 were not found in both groups.Conclusions:
The findings of this study do not support the role of HPV in prostate cancer. So, there may be other factors involved in carcinogenesis of the prostate cancer in our population. It is necessary to confirm this result by different detection methods and in other populations.Keywords
Prostate Cancer Human Papilloma Virus (HPV) Carcinoma Benign Prostatic Hyperplasia (BPH)
1. Background
Prostate cancer is the most common malignancy in men worldwide. The incidence rates of prostate cancer have increased in Asian countries during the last decades (1). Therefore, the epidemiological and biological study of the etiology of prostate cancer is essential. Multiple etiologies have been hypothesized for the cause of prostate cancer, including genetic defects, infectious agents, and associated inflammation (2).
Human papillomavirus (HPV) infection is reported as a risk factor of prostate cancer. HPV is a member of viral family,papillomaviridae, containing double stranded and circular deoxyribonucleic acid (DNA). Oncogenesis of HPV in human tissue has been proven. Its oncogenicity is due to persistent infection with the expression of 2 viral early proteins, E6 and E7, inhibiting cellular tumor suppressor proteins such as Rb, and p53 (3).
HPV is commonly transmitted by sexual activity. High-risk or more carcinogenic types, such as HPV - 16 and 18 have been proved to be a cause of cervical cancer in women (4,5). Also, studies have suggested a possible link between HPV infection and other cancers in women, such as vulva, vagina, and breast cancers (5).
In addition, HPV infection has also been shown to be associated with the risk of cancers in anogenital and urinary sites of men such as anus, penis, and bladder. Several epidemiologic and molecular methods detected HPV in malignant, benign, or normal prostate tissues (5).
The association of HPV infections with prostate cancer was reported by a meta-analysis of 46 tissue - based studies, including 4919 prostate cancer cases worldwide. The overall prevalence of HPV infection was 18.93% in prostate cancer cases, and most of which were high - risk HPV types. The prevalence varied by region, PCR primers used, publication period, and Gleason score (5).
Pooled data of epidemiological studies in Iran suggested that the prevalence of HPV was 10.4% in prostate cancers and 3.8% in benign prostatic hyperplasia (BPH), respectively. HPV - 16 and 18 genotypes were almost equal in prostate cancers and BPHs (6).
In a study, immunohistochemical (IHC) staining was performed in 30 prostate adenocarcinoma and 90 BPH tissue blocks in Isfahan, Iran. HPV infection was positive in 10% (3/30) of cases with adenocarcinoma and 1.1% (1/90) of BPH (3).
The prevalence of high - risk HPV types in prostate cancer has not been reported in Kurdistan province until now. Furthermore, the role of HPV in prostate cancer is unclear and controversial. Therefore, the aim of this study was to investigate the relationship between human papillomavirus and prostate cancer.
2. Methods
2.1. Study Population and Specimens
In this case - control study, we found only 58 formalin fixed and paraffin embedded (FFPE) prostate samples with malignant tumors (cases); also, we selected 75 samples with benign prostatic hyperplasia (controls). Therefore, samples of FFPE prostate tissue blocks were collected from archive of pathology laboratory, Tohid Hospital, Sanandaj, Iran, during 2010 to 2012. Prostate tissues had been taken due to prostate problems from patients referring to Sanandaj hospitals. Adequate tumor tissues without necrosis and hemorrhage were the inclusion criteria of the samples to this study. Sections with thickness of 7 μm to 10 μm were prepared by sterile microtome blade. This study was approved by Ethics Committee of Kurdistan University of Medical Sciences (ethic code: MUK.REC.1393.124).
2.2. Tissue Deparaffinization
The sections were deparaffinized by xylol, using a kit (GenetBio, Korea), according to manufacturer’s instruction. Briefly, prostate tissue sections were thrown into 1ml xylol, incubated at 50 ºC to 60 ºC for 15 minutes. Then, supernatant was separated by centrifugation at 13000 rpm, repeated 3 times, and washed in 100%, 90%, 70%, 50% ethanol for 5 minutes.
2.3. DNA Extraction
DNA was extracted, using PrimePrepTMgenomic DNA extraction kit (GenetBio, Korea) from formalin fixed and deparaffinated prostate tissue sections and stored at - 20 °C until PCR testing. To verify the integrity of extracted DNA, a PCR test for betaglobin gene was performed on all samples.
2.4. Molecular Detection of HPV and Beta - globin
For detection of betaglobin and HPV, PCR tests were done in a total volume of 25 μL reaction mixture, including 10 μL of PCR 2 × master mix (SinaClon, Iran), 1 μL forward and reverse primers each, 5 μL DNA template, and 8 μL distilled water.
PCR amplification program for betaglobin was as: Initial denaturation at 94 ºC for 5 min; followed by 30 cycles of denaturation at 94 ºC 40 s, annealing at 58 ºC 30 s, extension at 72 ºC, 50 s, and final extension at 72 ºC for 5 min.
To detect HPV infection, PCR test was conducted on all samples, using HPV general primers (GP5+ and GP6+) for L1 region of HPV genome. Also, to detect high-risk HPV genotypes, PCR tests were performed, using genotype specific primers for E7 gene of HPV. The name and sequences of primers as well as the length of PCR products are shown in Table 1.
Name and Sequences of Primers Used in PCR Tests, the Target Gene, and the Length of PCR Products in Base Pairs (Bp)
| Primer Name | Primer Sequence | Target | Length of PCR Product in Base Pairs (bp) | Reference |
|---|---|---|---|---|
| PCO3 | ACACAACTGTGTTCACTAGC | Human Betaglobin gene | 110 bp | (7) |
| PCO4 | CAACTTCATCCACGTTCACC | |||
| GP5+ | TTTGTTACTGTGGTAGATACTAC | HPV General (L1 gene) | 150 bp | (8) |
| GP6+ | GAAAAATAAACTGTAAATCATATTC | |||
| F - 16 | TTATGAGCAATTAAATGACAGCTCAG | HPV - 16 (E7 gene) | 215 bp | (9) |
| R - 16 | TGAGAACAGATGGGGCACACAAT | |||
| F - 18 | GACCTTCTATGTCACGAGCAATTA | HPV - 18 (E7 gene) | 236 bp | |
| R - 18 | TGCACACCACGGACACACAAAG | |||
| F - 31 | AGCAATTACCCGACAGCTCAGAT | HPV - 31(E7 gene) | 210 bp | |
| R - 31 | GTAGAACAGTTGGGGCACACGA | |||
| F - 33 | ACTGACCTAYACTGCTATGAGCAA | HPV - 33 (E6 and E7 genes) | 229 bp | |
| R - 33 | TGTGCACAGSTAGGGCACACAAT |
PCR amplification program for HPV infection using general primers was as: Initial denaturation 94 ºC for 5 min; followed by 30 cycles of denaturation at 94 ºC 30 s, annealing at 50 ºC 30 s, extension at 72 ºC 40 s, and final extension at 72 ºC for 5 min.
PCR amplification program for HPV using genotype specific primers was as: Initial denaturation 94 ºC for 5 min; followed by 30 cycles of denaturation at 94 ºC 30 s, annealing at 58 ºC 30 s, extension at 72 ºC 40 s, and final extension at 72 ºC for 5 min.
Genomic DNA of HeLa cell line containing HPV - 18 sequence (NCBI Code: C552) was purchased from national cell bank, Pasteur institute of Iran. In addition, the HPV genotype specific DNAs (HPV - 16, 18, 31 and 33) were prepared from the research institute of virology, Messiah Daneshvari Hospital, Tehran, Iran. These DNAs were used as positive control in PCR tests.
2.5. Sequencing
Genotypes of HPV positive samples were confirmed by sequencing. The results of sequencing were compared with the sequences stored in Gene Bank database, using BLAST software.
2.6. Statistical Analysis
The data were entered into the SPSS software version 19 and were analyzed by Chi - square and Fisher statistical tests. In all steps, P values less than 0.05 were considered statistically significant.
3. Results
The age range in case group was 22 to 90 years (mean: 71.62) and in the control group 51 to 91 years (mean: 71.7), respectively. The difference of the age between 2 groups was not significant (P = 0.6). Using the Gleason system, 39.7% of the study population had grade < 5, 25.4% grade 5 - 6, 14.26% grade 7, and 20.64% high grade prostate adenocarcinoma.
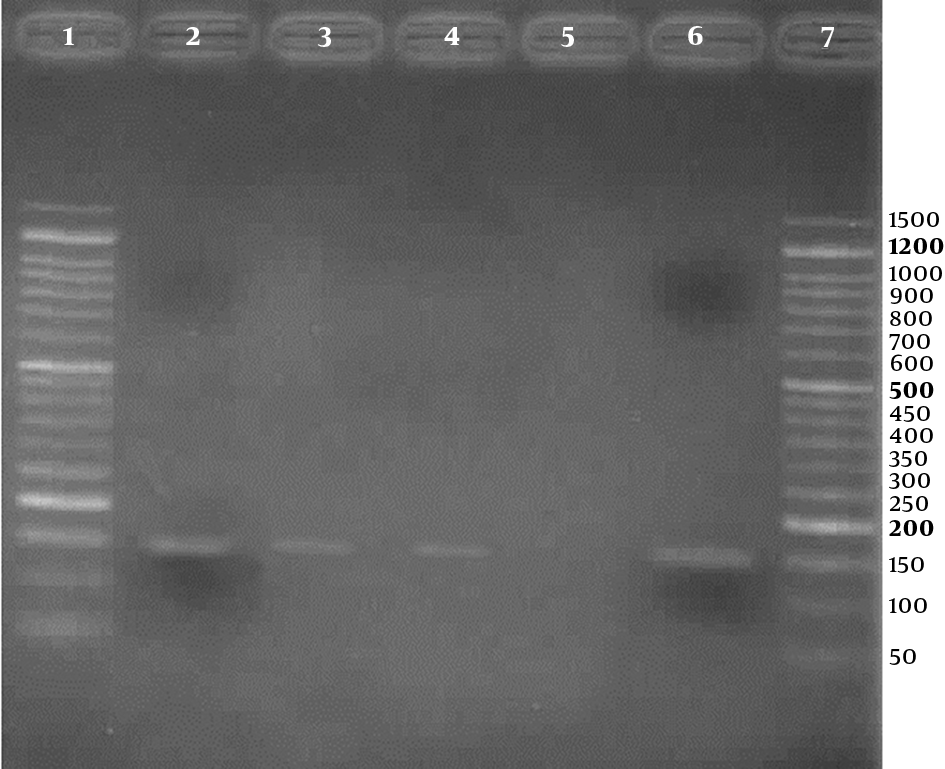
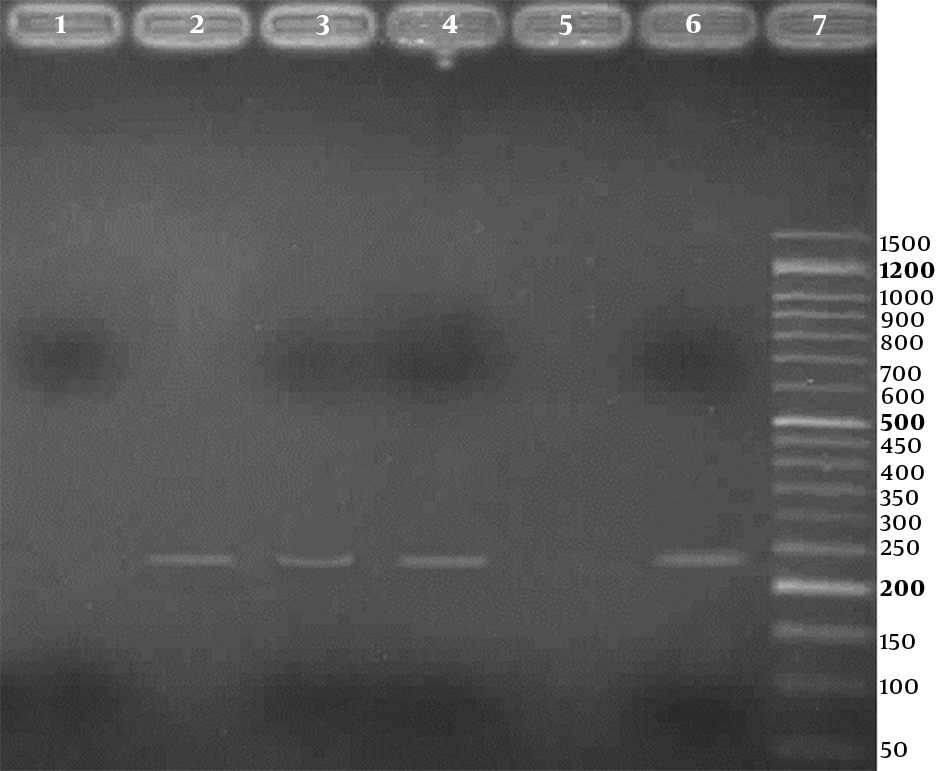
Three (2.3%) of the 133 samples were positive for HPV in the PCR test, using HPV general primers, 2 in case group, and 1 in control group, respectively. Also, using genotype specific primers, HPV - 18 was positive in 2 (3.4%) of 58 cases and 1 (1.3%) of 75 controls. For detection of human papillomavirus infection, representative agarose gel (2%) electrophoresis of PCR products are shown in Figures 1 and 2. DNA sequencing confirmed the HPV - 18 genotype in positive samples. The difference of HPV infection was not significant between 2 groups (P = 0.41). Other high - risk genotypes such as HPV - 16, HPV - 31, and HPV - 33 were not found in both groups.
Agarose gel (2%) electrophoresis of PCR products for detection of Human papillomavirus, using HPV general primers; Lanes 1 and 7: 50 bp DNA Ladder (CinnaClon, Iran); Lanes 2 to 4: three positive results (150 bp bands); Lane 5: negative control; Lane 6: positive control
Agarose gel (2%) electrophoresis of PCR products for detection of high risk human papillomavirus, using type specific primers; Lane 1: a negative result; Lanes 2 to 4: three positive results (236 bp bands); Lane 5: negative control; Lane 6: positive control; Line 7: 50 bp DNA Ladder (CinnaClon, Iran)
4. Discussion
In the present study, 3 (2.3%) of the 133 samples were positive for HPV, using HPV general primers. Also, HPV - 18 was positive in 2 (3.4%) of 58 cases and 1 (1.3%) of 75 controls, using genotype specific primers. The difference of HPV infection was not significant between 2 groups. Other high - risk genotypes, HPV - 16, HPV - 31, and HPV - 33 were not found in both groups.
The identification of human papillomavirus, as the cause of prostate cancer, is very important because it would facilitate the management of prostate cancer such as treatment and prevention. HPV vaccine is developed for the prevention of cervical cancer in some countries. If HPV infection is associated with the risk of prostate cancer, it would be necessary for the use of HPV vaccine in the prevention of prostate cancer in addition to its use in the prevention of cervical cancer.
A systematic study including 61 studies were conducted on different cancers in Iranian populations. The prevalence of HPV has been 77.5% in cervical cancers and 32.4% in head and neck cancers. Also, HPV has been detected in 23.1%, 22.2%, 10.4%, 30.9%, 14%, and 25.2% of esophagus, lung, prostate, urinary tract, breast, and skin cancers, respectively. HPV16 and 18 have been the most frequent in all cancers (6).
In the present study, the majority of histological type of prostate cancer was adenocarcinoma. That is compatible with the result of a meta - analysis (5). In another study, during 6 years (2003 - 2008), a total of 16071 cases of prostate cancer were recorded in Iran; most (95.2 %) were adenocarcinoma (10).
The results of the current study showed that 3 (2.3%) of the total 133 prostate samples were positive for HPV. Also, high - risk HPV - 18 was positive in 2 (3.4%) of 58 cases and 1 (1.3%) of the 75 controls. The difference of HPV infection was not significant between 2 groups.
Three meta - analyses have reported the association between HPV infection and the risk of prostate cancer, but the results were not consistent (1,11,12).
In a meta - analysis, the estimated summary odds ratios of the total 30 articles indicated that HPV - 16 infection significantly increased the risk of prostate cancer (1). In the United Kingdom, 100 prostate DNA samples were tested for HPVs by nucleic acid detection method. Only one sample was positive for HPV - 16 (13). But, we did not find HPV - 16 in our population.
In a study, 90 FFPE blocks with the diagnosis of BPH and 30 cases with adenocarcinoma were tested, using Immunohistochemical (IHC) staining to detect HPV infection in Isfahan, Iran. HPV infection was positive in 10% (3/30) of cases and 1.1% (1/90) of BPHs (3).
In two studies using FFPE blocks of prostate tissues, HPV was detected by MY09/MY11 and GP5+/GP6+ primers in a nested PCR. The first study included 29 prostate cancers and 167 BPH samples in Tehran, Iran. HPV DNA was found in 17.2% of prostate cancers and 4.8% of BPH samples and the difference was not significant (2). The second study included 104 primary prostate adenocarcinomas and 104 control tissues of BPH in Iran. HPV - DNA was found in 13 of 104 prostate cancers and 8 of 104 BPH samples. High - risk HPVs were detected in 10 of 13 prostate cancers and 5 of 8 BPH samples. Low - risk HPVs were detected in 3 of 13 prostate cancers and 3 of 8 BPH specimens. There was no significant difference between prostate cancer and BPH specimens regarding the HPV - DNA (14). In the present study, the difference of HPV infection was not significant between 2 groups, although we did not achieve PCR test to detect low - risk HPVs.
In a study, HPV - 18 and EBV were detected in approximately equal proportion in normal, benign, and prostate cancer specimens. HPV associated koilocytosis were identified in 24% of prostate cancers. Experimental evidence has demonstrated that HPV and EBV can collaborate in the proliferation of cultured cervical cells (15). Thus, other viruses may be the etiology of prostate cancer. But, we could not report koilocytosis in prostate tissues and did not intend to detect EBV. Also, in comparison to the prevalence of HPV - 18 in prostate tissue reported by mentioned studies in Iranian population; the result of the present study is lower.
A study indicated that the human apurinic/apyrimidinic endonuclease 1 (ApE1) 1349T > G polymorphism is associated with the increased risk of prostate cancer in northern Iran (16). Thus, genetic alteration of cell may be an etiology of prostate cancer.
PCR method is important in the detection of HPV infections in prostate cancer (5). Studies carried out on cancer tissues showed that PCR test using type - specific primer was more sensitive in the detection of HPV DNA, in comparison with the low detection rate of PCR test that uses HPV general primers. Because, HPV type - specific primers are designed to amplify shorter sequences of HPV DNA for example viral E gene, but HPV general primers are designed for long viral L1 gene (5). We used general and type specific primers. However, due to the instability of DNA in long - time maintenance and in extraction from FFPE tissue blocks, there is the possibility of PCR detection failure.
However, due to aging the population and changing the lifestyles, more studies are needed on the association of viruses with prostate carcinogenesis (10). We previously did not detect xenotropic murine leukemia virus - related virus (XMRV) in samples either from prostate cancer or benign prostate hyperplasia (17).
In conclusion, the aim of this study was to investigate the relationship between HPV and prostate cancer. But, the findings of the current study do not support the role of HPV in prostate cancer. So, there may be other factors involved in the oncogenesis of the prostate cancer in our population or we may have detection failure. Thus, it is necessary to confirm this result by further investigations by different detection methods in other populations.
Acknowledgements
References
-
1.
Bae JM. Human papillomavirus 16 infection as a potential risk factor for prostate cancer: an adaptive meta-analysis. Epidemiol Health. 2015;37. e2015005. [PubMed ID: 25687950]. https://doi.org/10.4178/epih/e2015005.
-
2.
Ghasemian E, Monavari SH, Irajian GR, Jalali Nodoshan MR, Roudsari RV, Yahyapour Y. Evaluation of human papillomavirus infections in prostatic disease: a cross-sectional study in Iran. Asian Pac J Cancer Prev. 2013;14(5):3305-8. [PubMed ID: 23803120].
-
3.
Mokhtari M, Taghizadeh F, Hani M. Is prostatic adenocarcinoma in a relationship with Human Papilloma Virus in Isfahan -Iran. J Res Med Sci. 2013;18(8):707-10. [PubMed ID: 24379849].
-
4.
Abreu AL, Souza RP, Gimenes F, Consolaro ME. A review of methods for detect human Papillomavirus infection. Virol J. 2012;9:262. [PubMed ID: 23131123]. https://doi.org/10.1186/1743-422X-9-262.
-
5.
Yang L, Xie S, Feng X, Chen Y, Zheng T, Dai M, et al. Worldwide Prevalence of Human Papillomavirus and Relative Risk of Prostate Cancer: A Meta-analysis. Sci Rep. 2015;5:14667. [PubMed ID: 26441160]. https://doi.org/10.1038/srep14667.
-
6.
Jalilvand S, Shoja Z, Hamkar R. Human papillomavirus burden in different cancers in Iran: a systematic assessment. Asian Pac J Cancer Prev. 2014;15(17):7029-35. [PubMed ID: 25227786].
-
7.
Zandi K, Eghbali SS, Hamkar R, Ahmadi S, Ramedani E, Deilami I, et al. Prevalence of various human papillomavirus (HPV) genotypes among women who subjected to routine Pap smear test in Bushehr city (south west of Iran) 2008-2009. Virol J. 2010;7:65. [PubMed ID: 20302680]. https://doi.org/10.1186/1743-422X-7-65.
-
8.
Mohamadian Roshan N, Jafarian A, Ayatollahi H, Ghazvini K, Tabatabaee SA. Correlation of laryngeal squamous cell carcinoma and infections with either HHV-8 or HPV-16/18. Pathol Res Pract. 2014;210(4):205-9. [PubMed ID: 24417904]. https://doi.org/10.1016/j.prp.2013.12.001.
-
9.
Gheit T, Tommasino M. Detection of high-risk mucosal human papillomavirus DNA in human specimens by a novel and sensitive multiplex PCR method combined with DNA microarray. Methods Mol Biol. 2011;665:195-212. [PubMed ID: 21116803]. https://doi.org/10.1007/978-1-60761-817-1_12.
-
10.
Pakzad R, Rafiemanesh H, Ghoncheh M, Sarmad A, Salehiniya H, Hosseini S, et al. Prostate Cancer in Iran: Trends in Incidence and Morphological and Epidemiological Characteristics. Asian Pac J Cancer Prev. 2016;17(2):839-43. [PubMed ID: 26925689].
-
11.
Lin Y, Mao Q, Zheng X, Yang K, Chen H, Zhou C, et al. Human papillomavirus 16 or 18 infection and prostate cancer risk: a meta-analysis. Ir J Med Sci. 2011;180(2):497-503. [PubMed ID: 21400096]. https://doi.org/10.1007/s11845-011-0692-6.
-
12.
Taylor ML, Mainous A3, Wells BJ. Prostate cancer and sexually transmitted diseases: a meta-analysis. Fam Med. 2005;37(7):506-12. [PubMed ID: 15988645].
-
13.
Groom HC, Warren AY, Neal DE, Bishop KN. No evidence for infection of UK prostate cancer patients with XMRV, BK virus, Trichomonas vaginalis or human papilloma viruses. PLoS One. 2012;7(3). e34221. [PubMed ID: 22470540]. https://doi.org/10.1371/journal.pone.0034221.
-
14.
Aghakhani A, Hamkar R, Parvin M, Ghavami N, Nadri M, Pakfetrat A, et al. The role of human papillomavirus infection in prostate carcinoma. Scand J Infect Dis. 2011;43(1):64-9. [PubMed ID: 20662618]. https://doi.org/10.3109/00365548.2010.502904.
-
15.
Whitaker NJ, Glenn WK, Sahrudin A, Orde MM, Delprado W, Lawson JS. Human papillomavirus and Epstein Barr virus in prostate cancer: Koilocytes indicate potential oncogenic influences of human papillomavirus in prostate cancer. Prostate. 2013;73(3):236-41. [PubMed ID: 22851253]. https://doi.org/10.1002/pros.22562.
-
16.
Pournourali M, Tarang AR, Yousefi M. The association between 1349T>G polymorphism of ApE1 gene and the risk of prostate cancer in northern Iran. Cell Mol Biol (Noisy-le-grand). 2015;61(4):21-4. [PubMed ID: 26255264].
-
17.
Khodabandehloo M, Hosseini W, Rahmani MR, Rezaee MA, Hakhamaneshi MS, Nikkhoo B, et al. No detection of xenotropic murine leukemia virus-related viruses in prostate cancer in Sanandaj, west of Iran. Asian Pac J Cancer Prev. 2013;14(11):6929-33. [PubMed ID: 24377628].