Abstract
Background:
Sarcopenia, defined as low muscle mass with reduced function, is frequently encountered in cirrhotic patients and is a major predictor of adverse events, including post-liver transplant (LT) outcome.Objectives:
This study assessed the impact of sarcopenia using computed tomography (CT)-based measurements on post-LT mortality and complications.Methods:
From January 2008 to June 2016, 646 adult patients underwent 613 LTs at our institution. We analyzed the postoperative outcome of 287 patients who had pathologically proven cirrhosis on the explanted liver and who had performed a CT examination three months before LT. Psoas muscle density (PMD) was detected for every patient using standard instruments present in the radiological workstation and was related to postoperative survival rates and complications. Statistical analysis was carried out using the appropriate tests.Results:
Postoperative mortality was 6.3%. At least one grade III-IV postoperative complication was experienced by 121 patients. Respiratory and infective complications occurred in 30 and 32 patients, respectively. Also, PMD was an independent predictor of postoperative mortality (P = 0.021), respiratory complications (P = 0.015), and infections (P = 0.010). The ROC analysis identified a PMD ≤ 43.72 HU as the best cutoff value for predicting 90-day mortality after LT.Conclusions:
Psoas muscle density accurately predicted post-LT mortality and complications. Its ease and low-cost determination can allow widespread use of this parameter to improve clinical care and help with the decision to give these patients some priority on the transplant waiting list.Keywords
Sarcopenia Malnutrition Liver Transplant Muscle Density Complications Infection MELD
1. Background
Liver transplant (LT) is the only curative option for patients with end-stage liver disease (ESLD).
Unfortunately, severe graft shortage prolongs the time on the waiting list, leading to advanced liver disease and worse patient clinical conditions at the same rate (1). Efforts to increase the donor pool have included adding donation after cardiac death to standard donation after brain death (2). Moreover, older donors and donors with fatty livers have been utilized in recent years (3). At the same time, the predicted decline in organ availability in the future, due to graft quality deterioration (4), confirms the strict selection of transplant candidates being of paramount importance.
Sarcopenia can be defined as low muscle mass associated with a lower muscle function (5). It is frequent in patients with ESLD, with a reported incidence up to 70% (6-9), and is a major predictor of adverse clinical outcome measures, including survival, quality of life, development of other complications of cirrhosis, and post-transplant outcomes (9-13). In particular, a recent study demonstrated that both short- and long-term post-LT survival were considerably poorer in the sarcopenic group than in the non-sarcopenic group (82% versus 98% and 53.6% versus 79.8%; P < 0.001) (14). In the study by Valero et al. (15), sarcopenia was an independent predictor of postoperative complications in multivariable analysis (Odds Ratio 3.06, 95%CI = 1.07 - 8.72, P = 0.03).
Sarcopenia is an objective measure of malnutrition in cirrhotic patients instead of serum (pre-) albumin, body mass index (BMI), and weight, which may be conditioned by liver impaired function or ascites. In the literature, computed tomography (CT) measurements of skeletal muscle mass include a wide variety of tools, such as Psoas muscle area (PMA) (16), PMA normalized for height (14), psoas thickness normalized for height (17), and third lumbar vertebra (L3) muscle index standardized by the squared height (8, 17). Due to many clinical definitions, the European Working Group on Sarcopenia in 2010 stated that “more research is urgently needed to obtain good reference values” (5). In particular, no precise cut-off value is available to diagnose sarcopenia in patients affected by ESLD. Moreover, this definition recommends the use of a practical clinical definition and a consensus diagnostic criterion.
The main limit in finding a cut-off agreed upon by the scientific community is that many authors use different measurements, some of which requiring expensive software that is not readily available in every center (8, 14, 16, 17).
2. Objectives
In the present study, we assessed the impact of sarcopenia on post-LT mortality and complications, using single-slice CT-based measurements of Psoas muscle density (PMD).
3. Methods
3.1. Study Design
From January 1, 2008, to June 15, 2016, 646 adult patients underwent 613 LTs from AB0-identical or AB0-compatible brain-death-deceased donors at our institution. From the 646 transplanted patients, we designated 287 patients with pathologically proven cirrhosis on the explanted liver, for whom a CT examination was available (three months before LT). The availability of recent imaging depended on the oncologic follow-up in patients with HCC (18) and modifications of patient clinical conditions, which needed a CT scan evaluation (i.e., sudden liver decompensation, acute abdominal pain, etc.).
The clinical features of both donors and recipients, including biochemical and surgical parameters, were stored in the institutional database. Since 2004, a two-center regional allocation based on the model for end-stage liver disease (MELD) score has been used, with additional points assigned for different indications (hepatocellular carcinoma (HCC), metabolic diseases, etc.), with periodical re-evaluation between the two centers involved (18, 19). At present, malnutrition determines an assignation of extra points only in patients affected by the polycystic hepatic disease, for whom the loss of weight was exclusively related to the mass effect imposed by massive hepatomegaly (20). The results were assessed considering post-transplant survival rates and complications. Since sarcopenic patients are at higher risk of developing respiratory or infective postoperative complications (15, 21), we performed an additional analysis of these issues.
This single-center study, carried out at our tertiary care center, was approved by the institutional review board and written informed consent was obtained from each patient.
3.2. Definitions
The postoperative course was designated as the period comprising 90 days after LT, as previously done (19, 22). Postoperative complications were graded according to the Dindo-Clavien classification (23). Only were grade III-IV complications analyzed, as previously performed (19). Acute-on-chronic Liver Failure (ACLF) was defined as a rapid decompensation in a chronic setting of liver disease, with a potential organ failure(s) predefined by the CLIF-SOFA score (24). Hepatorenal syndrome (HRS) was diagnosed as per the International Club of Ascites definition (25). Prolonged post-LT mechanical ventilation (MV) was defined as MV for at least 72 h postoperatively (26). Postoperative respiratory complications included pneumonia, pulmonary edema, and respiratory failure (defined as prolonged MV, the need for re-intubation for respiratory failure, and the need for tracheotomy) (27). Infections were defined following the standard criteria recommended by the centers for disease control and prevention (28).
3.3. Imaging Analysis
The CT examinations were performed in all patients, following a previously described protocol (29). A more than 10-year experienced radiologist in the abdominal imaging field, blinded to all of the information, including clinical history, assessed the images. All the images were retrieved from and evaluated on our institutional picture archiving and communication system (Carestream PACS®, version 1.4; Kodak, Rochester, NY). All the measurements (size, area, or density evaluation) were performed using standard instruments present in all the imaging workstations without the use of dedicated software. We decided to perform three different evaluations: (1) transversal psoas muscle thickness (TPMT) measurement; (2) cross-sectional PMA measurement; and (3) PMD measurement. All the measurements were performed on both the right and the left psoas muscles, and their means were calculated by halving the sum of right and left assessments (Figure 1).
Transversal psoas muscle thickness. (A) In a sagittal CT image passing through the spinal column, the red line was positioned at the umbilicus to obtain a single axial image (B). (C) Transversal psoas muscle thickness measurement (dotted line) was perpendicular to the axial psoas muscle thickness measurement, which corresponds to the largest diameter of the psoas muscle on an axial view (continuous line). Cross-sectional psoas muscle area and psoas muscle density. (D) In a sagittal CT image, the red line was positioned at L3 to obtain a single axial image (E). (F) Psoas muscle profile was contoured using the free-hand density measurement tool. Thanks to this instrument, both psoas muscle area and density were simultaneously calculated. All the measurements were performed on the right and left psoas muscle.
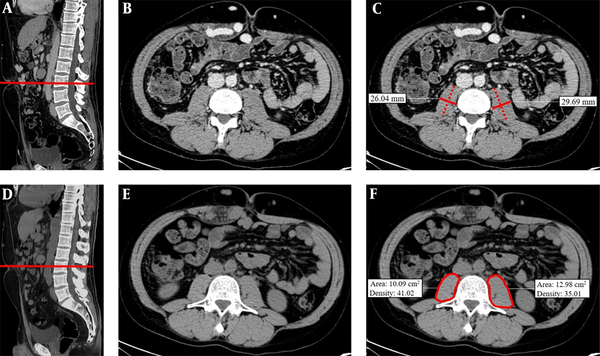
Measurements of TPMT on CT were performed, according to the previously described method by Durand et al. (17). Besides, TPMT was normalized for stature divided by height (TPMTh). Measurements of PMA and PMD were performed on a single axial image of basal CT scan at the level of L3. The radiologist traced the contour of the muscle using the freehand instrument for density measurement, automatically obtaining the following values: -area (mm2) and -density (Hounsfield Unit, HU). Then, PMA was normalized for Body Surface Area (BSA) (14). Normal density for skeletal muscle is defined as 30 - 100 HU (30); a lower density is related to “fatty replacement” of muscle tissue due to patient age, obesity, and concomitant diseases, such as neurological chronic conditions, type 2 diabetes, malignancy, or cirrhosis (30-32).
3.4. Statistical Analysis
Continuous variables were expressed as median and range and compared using a two-tailed unpaired Student’s t test. Categorical variables were compared using χ2 or Fisher analysis. Patient survival was calculated from the date of LT to the last date of follow-up or death. Actuarial survivals were computed with the Kaplan-Meier method, and the differences between the groups were compared by the log-rank test.
To identify the predictors of 90-day mortality and 90-day postoperative complications, a univariable analysis was performed. A multilinear regression was also performed, including those variables significantly correlated to the outcome at univariable analysis. In this way, it evaluated the correlation between some of these identified predictors. In particular, since ACLF definition, according to the CLIF-SOFA score, already included the MELD-score, and HRS was diagnosed based on the serum creatinine level, which is one factor used to calculate the MELD-score, only was the variable with the higher chi-square value included in the multivariable analysis. In this way, the rule of thumb of one variable for 10 events was fulfilled.
Since our principal goal was to better understand which recipient characteristics will most impact post-LT survival and considering that we have already demonstrated comparable post-transplant outcomes regardless of donor characteristics (19), the latter were not analyzed as possible predictors of postoperative survival and complications.
The prognostic value of PMD measurement in predicting 90-day postoperative mortality was assessed using receiver operating characteristic (ROC) curve analysis. The area under the curve (AUC), sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios for the cut-off point obtained were reported. A P-value less than 0.05 was considered statistically significant. Statistical analysis was performed with SPSS version 17.0 software (SPSS, Inc., Chicago, IL). The ROC analysis was performed with MedCalc version 12.5.0 (MedCalc Software, Ostend, Vlaanderen, Belgium). The statistical review of the study was performed by a biomedical statistician.
4. Results
4.1. Recipient Characteristics and Postoperative Course
The baseline characteristics of the study population are summarized in Table 1. After a median follow-up of 40 months (0 - 109 months), 58 (20.2%) patients died. The one- and five-year overall survival rates were 87.1% and 78.9%, respectively.
| Variables | Patients (N = 287) |
|---|---|
| Age | 54 (18 - 71) |
| Male gender | 213 (74.2) |
| BMI, kg/m2 | 24.7 (11.8 - 37.2) |
| BSA, m2 | 1.86 (1.37 - 2.40) |
| Liver disease | |
| Post-necrotic cirrhosis | 177 (61.7) |
| Alcoholic cirrhosis | 46 (16.0) |
| Cholestatic disease | 21 (7.3) |
| Other | 43 (15.0) |
| Hepatocellular carcinoma | 127 (44.3) |
| HCV infection | 130 (45.3) |
| HBV infection | 82 (28.6) |
| MELD score at LT | 20 (6 - 49) |
| ACLF pre-LT | 60 (20.9) |
| Pre-LT in-hospital stay | 102 (35.5) |
| Pre-LT hepatorenal syndrome | 84 (29.3) |
| Pre-LT CVVH/HD | 8 (2.8) |
| Pre-LT mechanical ventilation | 10 (3.5) |
| Pre-LT use of norepinephrine | 7 (2.4) |
| Transversal psoas muscle thickness/height, mm/m | 18.06 (4.98 - 26.99) |
| Psoas muscle area/BSA, mm2/m2 | 342.17 (25.34 - 832.08) |
| Psoas muscle density, HU | 44.69 (12.99 - 59.78) |
| Postoperative course | |
| ICU stay, d | 6 (0 - 277) |
| In-hospital stay, d | 17 (0 - 370) |
| 90-day mortality | 18 (6.3) |
| Postoperative complications | 142 (49.5) |
| Grade III-IV complications | 121 (42.2) |
| Infective complications | 32 (11.1) |
| Respiratory complications | 30 (10.5) |
Men made up 74.2% of the cohort. Notable characteristics included a median BMI of 24.7 kg/m2 (range: 11.8 - 37.2) and post-necrotic cirrhosis as the cause of liver dysfunction in 61.7% of the cases. Respecting the markers of liver disease severity, the median pre-LT MELD score was 20 (6 - 49), and HRS and ACLF were diagnosed in 29.3% and 20.9% of the cases, respectively. Postoperative mortality was 6.3% (18 patients). The main cause of postoperative death was infection/multi-organ failure (MOF) (61.1%, 11 patients), followed by hemorrhagic shock (22.2%, four patients). At least one grade III-IV postoperative complication was observed in 121 (42.2%) patients. Respiratory and infective complications occurred in 30 and 32 patients (10.5% and 11.1%), respectively.
4.2. Psoas Muscle Density and 90-day Mortality and Complications
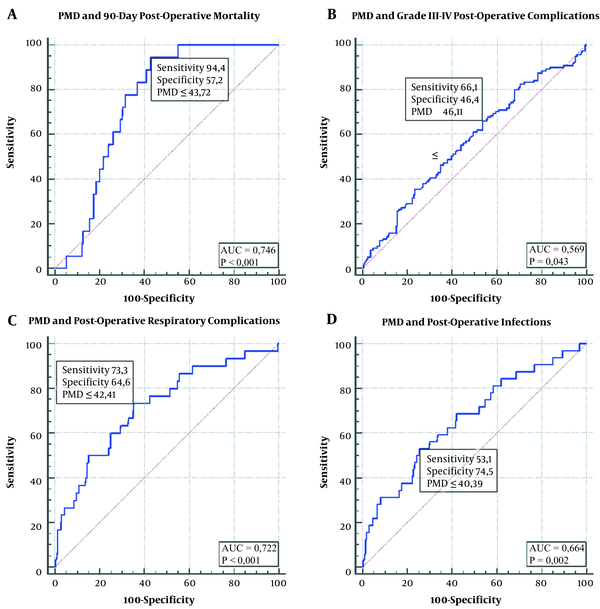
The ROC analysis identified a PMD ≤ 43.72 HU as the best cut-off value for predicting 90-day mortality after LT (AUC = 0.746, 95%C.I. = 0.691–0.795; sensitivity = 94.4%; specificity = 57.3%; positive predictive value [PPV] = 12.4%; negative predictive value [NPV] = 99.4%; positive likelihood ratio [PLR] = 2.21; negative likelihood ratio [NLR] = 0.097; P < 0.001] (Figure 2A). Seventeen (94.4%) of the 18 patients who died in the postoperative period had PMD ≤ 43.72 HU, while only one (5.6%) had higher PMD (P < 0.001).
We used the ROC analysis to find the PMD cut-off value also for grade III-IV complications, respiratory complications, and infections. According to this statistical analysis, the three cut-off values respectively were:
PMD ≤ 46.11 HU (AUC = 0.569, 95%C.I. = 0.510-0.628; sensitivity = 66.1%; specificity = 46.4%; PPV = 12.1%; NPV = 92.5%; PLR = 1.23; NLR = 0.73; P = 0.043) (Figure 2B);
PMD ≤ 42.41 HU (AUC = 0722, 95%C.I. = 0.666-0.773; sensitivity = 73.3%; specificity = 64.6%; PPV = 18.7%; NPV = 95.6%; PLR = 2.07; NLR = 0.41; P < 0.001) (Figure 2C);
PMD ≤ 40.39 HU (AUC = 0.664, 95%C.I. = 0.606-0.719; sensitivity = 53.1%; specificity = 74.5%; PPV = 18.8%; NPV = 93.5%; PLR = 2.08; NLR = 0.63; P = 0.002) (Figure 2D).
ROC analysis of psoas muscle density for predicting: A, 90-day postoperative mortality; B, Grade III-IV postoperative complications; C, Postoperative respiratory complications; D, Postoperative infections.
4.3. Analysis of Factors Affecting 90-day Mortality and Complications
The following factors were analyzed for their impact on postoperative mortality and complications: Recipient age, gender, BMI, and BSA; type of liver disease, presence of HCC, hepatitis C virus (HCV) or hepatitis B virus (HBV) infection; MELD score, the onset of ACLF or HRS; pre-LT in-hospital stay, the need for continuous venovenous hemofiltration (CVVH), MV or norepinephrine; TPMTh, PMA normalized for BSA, and PMD.
A higher MELD score, onset of ACLF, and lower PMD were correlated with higher 90-day mortality in univariable analysis (Table 2). The only independent predictor of 90-day mortality was PMD (Exp(B) = 0.929, 95% CI = 0.872 - 0.989; P = 0.021). Grade III-IV complications were significantly related to the MELD score, onset of ACLF, diagnosis of HRS, and hospitalization at the time of LT (Table 3). In multivariable analysis, only could the MELD score independently predict postoperative complications (Exp(B) = 1.051, 95% CI = 1.015 - 1.087; P = 0.004).
Comparison of Preoperative Characteristics of Patients Deceased 90-day post-LT with Those of Patients Who Surviveda
| Variables | 90-Day Survivors (N = 269) | 90-Day Deceased Patients (N = 18) | P | Exp(B) | C.I. | P |
|---|---|---|---|---|---|---|
| Age | 54 (14 - 71) | 53 (21 - 65) | 0.377 | - | - | - |
| Male gender | 202 (75.1) | 11 (61.1) | 0.189 | - | - | - |
| BMI, kg/m2 | 24.7 (11.8 - 37.22) | 24.5 (21.2 - 36.7) | 0.071 | - | - | - |
| BSA, m2 | 1.86 (1.37 - 2.40) | 1.84 (1.57 - 2.12) | 0.590 | - | - | - |
| Liver disease | ||||||
| Post-necrotic cirrhosis | 167 (62.1) | 10 (55.6) | 0.581 | - | - | - |
| Alcoholic cirrhosis | 43 (16.0) | 3 (16.7) | 0.575 | - | - | - |
| Cholestatic disease | 19 (7.1) | 2 (11.1) | 0.386 | - | - | - |
| Other | 41 (15.2) | 2 (11.1) | 0.476 | - | - | - |
| Hepatocellular carcinoma | 122 (45.4) | 5 (27.8) | 0.146 | - | - | - |
| HCV infection | 121 (45) | 9 (50.0) | 0.679 | - | - | - |
| HBV infection | 79 (29.4) | 3 (16.7) | 0.190 | - | - | - |
| MELD score at LT | 19 (6 - 49) | 25 (7 - 42) | 0.017 | - | - | - |
| Pre-LT ACLF | 52 (19.3) | 8 (44.4) | 0.011 | 2.712 | 0.994-7.398 | 0.051 |
| Pre-LT in-hospital stay | 93 (34.6) | 9 (50.0) | 0.186 | - | - | - |
| Pre-LT hepatorenal syndrome | 77 (28.6) | 7 (38.9) | 0.354 | - | - | - |
| Pre-LT CVVH/HD | 6 (2.2) | 2 (11.1) | 0.083 | - | - | - |
| Pre-LT MV | 8 (3.0) | 2 (11.1) | 0.124 | - | - | - |
| Pre-LT use of norepinephrine | 6 (2.2) | 1 (5.6) | 0.368 | - | - | - |
| Transversal psoas muscle thickness/height | 18.01 (4.98 - 26.99) | 18.55 (9.26 - 22.72) | 0.927 | - | - | - |
| Psoas muscle area/BSA, mm2/m2 | 342.17 (25.34 - 832.08) | 351.91 (181.92 - 639.27) | 0.815 | - | - | - |
| Psoas muscle density (HU) | 45.28 (12.99 - 59.78) | 39.52 (31.21 - 45.91) | 0.005 | 0.929 | 0.872 - 0.989 | 0.021 |
| Psoas muscle density ≤ 43.72 HU | 115 (42.8) | 17 (94.4) | < 0.001 | - | - | - |
Comparison of Preoperative Characteristics of Patients Who Experienced Postoperative Grade III-IV Complications with Those of Patients Without Postoperative Complicationsa
| Variables | No Grade III-IV Complications (N = 166) | Grade III-IV Complications (N = 121) | P | Exp(B) | C.I. | P |
|---|---|---|---|---|---|---|
| Age | 54 (18 - 71) | 54 (18 - 69) | 0.691 | - | - | - |
| Male gender | 118 (71.1) | 95 (78.5) | 0.155 | - | - | - |
| BMI, kg/m2 | 24.6 (11.7 - 37.2) | 25.0 (15.3 - 36.7) | 0.529 | - | - | - |
| BSA, m2 | 1.86 (1.37 - 2.40) | 1.87 (1.47 - 2.30) | 0.500 | - | - | - |
| Liver disease | ||||||
| Post-necrotic cirrhosis | 103 (62.0) | 74 (61.2) | 0.878 | - | - | - |
| Alcoholic cirrhosis | 21 (12.7) | 25 (20.7) | 0.068 | - | - | - |
| Cholestatic disease | 16 (9.6) | 5 (4.1) | 0.077 | - | - | - |
| Other | 27 (16.3) | 16 (13.2) | 0.476 | - | - | - |
| Hepatocellular carcinoma | 81 (48.8) | 46 (38.0) | 0.069 | - | - | - |
| HCV infection | 74 (44.6) | 56 (46.3) | 0.775 | - | - | - |
| HBV infection | 50 (30.1) | 32 (26.4) | 0.496 | - | - | - |
| MELD score at LT | 18 (6 - 39) | 22 (6 - 49) | < 0.001 | 1.051 | 1.015 - 1.087 | 0.004 |
| Pre-LT ACLF | 28 (16.9) | 32 (26.4) | 0.049 | - | - | - |
| Pre-LT in-hospital stay | 48 (28.9) | 54 (44.6) | 0.006 | 1.277 | 0.717 - 2.277 | 0.406 |
| Pre-LT hepatorenal syndrome | 31 (18.7) | 53 (43.8) | < 0.001 | |||
| Pre-LT CVVH/HD | 2 (1.2) | 6 (5.0) | 0.062 | - | - | - |
| Pre-LT MV | 6 (3.6) | 4 (3.3) | 0.579 | - | - | - |
| Pre-LT use of norepinephrine | 3 (1.8) | 4 (3.3) | 0.331 | - | - | - |
| Transversal psoas muscle thickness/height | 18.04 (4.98 - 25.87) | 18.06 (9.26 - 26.99) | 0.849 | - | - | - |
| Psoas muscle area/BSA, mm2/m2 | 343.94 (68.49 - 617.64) | 341.01 (25.34 - 832.08) | 0.867 | - | - | - |
| Psoas muscle density, HU | 45.37 (12.99 - 59.78) | 43.23 (22.61 - 59.78) | 0.071 | - | - | - |
| Psoas muscle density ≤ 46.11 HU | 89 (53.6) | 80 (66.1) | 0.022 | - | - | - |
Postoperative respiratory complications occurred more frequently in older patients, with higher MELD score, pre-LT diagnosis of HRS and ACLF, and lower PMD (Table 4). In multivariable analysis, recipient age (Exp(B) = 1.060, 95% CI = 1.005 - 1.119; P = 0.032), MELD score (Exp(B) = 1.088, 95% CI = 1.034 - 1.145; P = 0.001), and PMD (Exp(B) = 0.930, 95% CI = 0.878 - 0.986; P = 0.015) independently predicted the occurrence of post-LT respiratory complications.
Comparison of Preoperative Characteristics of Patients Who Experienced Postoperative Respiratory Complications with Those of Patients Without Postoperative Respiratory Complicationsa
| Variables | No Respiratory Complications (N = 257) | Respiratory Complications (N = 30) | P | Exp(B) | C.I. | P |
|---|---|---|---|---|---|---|
| Age | 53 (14 - 71) | 59 (39 - 69) | 0.011 | 1.060 | 1.005 - 1.119 | 0.032 |
| Male gender | 192 (74.7) | 21 (70.0) | 0.577 | - | - | - |
| BMI, kg/m2 | 24.7 (11.7 - 37.2) | 25.0 (15.8 - 32.5) | 0.855 | - | - | - |
| BSA, m2 | 1.86 (1.37 - 2.40) | 1.84 (1.48 - 2.24) | 0.365 | - | - | - |
| Liver disease | ||||||
| Post-necrotic cirrhosis | 162 (63.0) | 15 (50.0) | 0.165 | - | - | - |
| Alcoholic cirrhosis | 38 (14.8) | 8 (26.7) | 0.093 | - | - | - |
| Cholestatic disease | 21 (8.2) | 0 (0) | 0.090 | - | - | - |
| Other | 37 (14.4) | 6 (20.0) | 0.416 | - | - | - |
| Hepatocellular carcinoma | 117 (45.5) | 10 (33.3) | 0.203 | - | - | - |
| HCV infection | 118 (45.9) | 12 (40.0) | 0.538 | - | - | - |
| HBV infection | 76 (29.6) | 6 (20.0) | 0.272 | - | - | - |
| MELD score at LT | 19 (6 - 39) | 25 (10 - 49) | < 0.001 | 1.088 | 1.034 - 1.145 | 0.001 |
| Pre-LT ACLF | 47 (18.3) | 13 (43.3) | 0.001 | - | - | - |
| Pre-LT in-hospital stay | 87 (33.8) | 15 (50.0) | 0.080 | - | - | - |
| Pre-LT hepatorenal syndrome | 67 (26.1) | 17 (56.7) | 0.001 | - | - | - |
| Pre-LT CVVH/HD | 6 (2.3) | 2 (6.7) | 0.199 | - | - | - |
| Pre-LT MV | 9 (3.5) | 1 (3.3) | 0.719 | - | - | - |
| Pre-LT use of norepinephrine | 6 (2.3) | 1 (3.3) | 0.542 | - | - | - |
| Transversal psoas muscle thickness/height | 18.26 (4.98 - 26.99) | 16.32 (9.26 - 24.51) | 0.058 | - | - | - |
| Psoas muscle area/BSA, mm2/m2 | 345.72 (68.49-832.08) | 335.47 (25.34 - 626.72) | 0.326 | - | - | - |
| Psoas muscle density (HU) | 45.28 (12.99 - 59.78) | 39.05 (22.61 - 57.44) | < 0.001 | 0.930 | 0.878 - 0.986 | 0.015 |
| Psoas muscle density ≤ 42.41 HU | 91 (35.4) | 22 (73.3) | < 0.001 | - | - | - |
Post-LT infections arose more frequently in patients with higher MELD scores, pre-LT diagnosis of HRS, and lower PMD (Table 5). Multivariable analysis found HRS and PMD as independent predictors of postoperative infections (Exp(B) = 3.688, 95% CI = 1.698 - 8.013; P = 0.001 and Exp(B) = 0.934, 95% CI = 0.887 - 0.984; P = 0.010, respectively).
Comparison of Preoperative Characteristics of Patients Who Experienced Postoperative Infections with Those of Patients Without Postoperative Infectionsa
| Variables | No Infections (N = 255) | Postoperative Infections (N = 32) | P | Exp(B) | C.I. | P |
|---|---|---|---|---|---|---|
| Age | 54 (14 - 71) | 56 (35 - 69) | 0.370 | - | - | - |
| Male gender | 190 (74.5) | 23 (71.9) | 0.748 | - | - | - |
| BMI, kg/m2 | 24.5 (11.7 - 37.2) | 26.6 (18.0 - 34.4) | 0.052 | - | - | - |
| BSA, m2 | 1.86 (1.37 - 2.40) | 1.83 (1.52 - 2.26) | 0.899 | - | - | - |
| Liver disease | ||||||
| Post-necrotic cirrhosis | 160 (62.7) | 17 (53.1) | 0.291 | - | - | - |
| Alcoholic cirrhosis | 41 (16.1) | 5 (15.6) | 0.947 | - | - | - |
| Cholestatic disease | 20 (7.8) | 1 (3.1) | 0.293 | - | - | - |
| Other | 35 (13.7) | 8 (25.0) | 0.092 | - | - | - |
| Hepatocellular carcinoma | 118 (46.2) | 9 (28.1) | 0.051 | - | - | - |
| HCV infection | 116 (45.5) | 14 (43.8) | 0.852 | - | - | - |
| HBV infection | 75 (29.4) | 7 (21.9) | 0.374 | - | - | - |
| MELD score at LT | 19 (6 - 49) | 24 (8 - 39) | 0.038 | - | - | - |
| Pre-LT ACLF | 51 (20.0) | 9 (28.1) | 0.287 | - | - | - |
| Pre-LT in-hospital stay | 88 (34.5) | 14 (43.8) | 0.303 | - | - | - |
| Pre-LT hepatorenal syndrome | 65 (25.5) | 19 (59.4) | < 0.001 | 3.688 | 1.698 - 8.013 | 0.001 |
| Pre-LT CVVH/HD | 6 (2.4) | 2 (6.2) | 0.220 | - | - | - |
| Pre-LT MV | 9 (3.5) | 1 (3.1) | 0.691 | - | - | - |
| Pre-LT use of norepinephrine | 5 (2.0) | 2 (6.2) | 0.177 | - | - | - |
| Transversal psoas muscle thickness/height | 18.01 (4.98 - 25.87) | 18.48 (9.45 - 26.99) | 0.302 | - | - | - |
| Psoas muscle area/BSA, mm2/m2 | 340.92(25.34 - 832.08) | 361.87(169.08 - 639.27) | 0.489 | - | - | - |
| Psoas muscle density, HU | 44.99 (12.99 - 59.78) | 40.25 (22.61 - 55.47) | 0.001 | 0.934 | 0.887 - 0.984 | 0.010 |
| Psoas muscle density ≤ 40.39 HU | 65 (25.5) | 17 (53.1) | 0.002 | - | - | - |
4.4. Analysis of Factors Affecting 90-day Mortality of Sarcopenic Patients
To better understand the impact of sarcopenia on post-LT mortality, we performed a further sub-analysis, considering only the patients with PMD value ≤ 43.72 HU (n = 132, 46.0% of the cases), searching for possible differences between survivors (115 patients, 87.1%) and deceased patients (17 patients, 12.9%) in this sarcopenic subgroup. By analyzing the same variables reported above, a higher median MELD score (22 versus 27; P = 0.012) and age (55 versus 61 years; P = 0.041) were associated with 90-day mortality in univariable analysis. In multivariable analysis, both MELD score and recipient age independently predicted 90-day postoperative mortality (Exp(B) = 1.095, 95% CI = 1.022 - 1.174; P = 0.010 and Exp(B) = 1.098, 95% CI = 1.010 - 1.193; P = 0.028, respectively).
5. Discussion
The identification of patients listed for LT with an increased risk of postoperative morbidity and mortality is crucial to improve candidate selection, organ allocation, and optimal graft-to-recipient matching. Malnutrition and sarcopenia have been correlated with mortality in cirrhotic patients, waiting-list mortality, post-transplant severe infections, and longer hospital stay (9-16). Considering that prolonged MV, bacterial infections, intensive care, and hospital long stay are well-known negative predictors of post-LT survival (19, 33, 34), the impact of malnutrition and muscle reduction on these variables is fundamental.
At present, different methods are available to evaluate body composition and estimate muscle mass in patients with cirrhosis; however, most of these modalities present some limitations, primarily owing to limited reproducibility. In this setting, the muscle thickness assessment based on cross-sectional CT imaging has been proposed as a quantitative index of nutritional status in patients with cirrhosis, particularly owing to its objective nature (8, 13-17, 32).
Englesbe et al. (16) first introduced the concept of sarcopenia to LT using an objective and recordable value, namely PMA at level L4. In fact, one-year post-LT survival ranged from 49.7% for the quartile with the smallest PMA to 87.0% for the quartile with the largest PMA, with a hazard ratio of 3.7/1,000 mm2 decrease in PMA (P < 0.001). Subsequently, many other authors used PMA or TPMT to find a relationship between these values and post-LT survival or complications (14, 15, 17). The principal limitation of these studies is that PMA and TPMT, even if normalized for height or BSA, could vary significantly due to gender, ethnicity, age, height, weight, and other clinical conditions, leading to potential confounding results (30-32). In addition, the location of the umbilicus, as proposed by Durand et al. for TPMT (17), may change owing to ascites or obesity. Thus, some measurements may be recorded at different levels.
Montano-Loza et al. (8) demonstrated a correlation between sarcopenia, defined using abdominal muscle density, and mortality in cirrhotic patients (HR = 2.21, P = 0.008). As previously reported, muscle density is independent of gender, height, weight, or ethnicity, and thus appears to be more objective than muscle area or thickness (31). Interestingly, the frequency of sepsis-related death was significantly higher in sarcopenic patients than in non-sarcopenic patients (22% versus 8%; P = 0.02), supporting the hypothesis of impaired immunity in these patients (34-36). Moreover, the same authors more recently demonstrated that sarcopenia predicted post-LT outcomes independently of MELD score; in fact, if present, sarcopenia was equivalent to adding 10 points to the MELD score (37). The principal limitations of these studies were the need for specifically dedicated software and the use of cut-off values taken from a population of non-cirrhotic patients (38). In this sense, the measurement of PMD seemed to be a more rapid and low-cost method. Regarding the cut-off value for PMD, interestingly, Kalafateli et al. (32) reported a 43.14 HU of psoas muscle density as the best predictor of 12-month mortality in a population of 98 cirrhotic patients, which is similar to ours. Our study demonstrated that PMD was the best predictor of postoperative mortality after LT (P = 0.021); in fact, 94% of the patients who died within 90 days after LT had a PMD lower than 43.72 HU, which is the cut-off we applied to define sarcopenic patients. Among 132 sarcopenic patients, the variables that independently influenced 90-day mortality were higher MELD score and recipient age. These data are congruent with our analyses of factors associated with postoperative complications. In fact, while the MELD score independently predicted serious postoperative complications (P = 0.004), older recipient age (P = 0.032) and higher MELD score (P = 0.001) were independently associated with the occurrence of respiratory complications following LT.
Moreover, we proved the ability of PMD to predict both postoperative respiratory complications and infections, supporting the results of previous studies from other authors (8, 12, 15). Our results confirmed the hypothesis that undernutrition is associated with immunosuppression, due to a reduction in circulating immune cell number and in pro-inflammatory cytokine release, leading to an increased susceptibility to infection (39).
The present study must be considered within the context of its limitations. One limitation is the small sample size, which restricted the type of analyses that we were able to perform. The principal limitation is the retrospective design of the study, which determined a potential selection bias. In particular, the sample may have been biased toward sicker patients, as these patients are more likely to more often undergo abdominal CT scans. Since sarcopenia was associated with cirrhosis severity (7-10, 37), our results are probably exaggerated by focusing on sicker patients.
Nevertheless, this study was innovative in its approach by using a simple and rapid way to check sarcopenia. Actually, the strength of this method is that every radiologist could measure PMD, with no need for specifically dedicated software. Another advantage of using PMD to assess sarcopenia is that muscle density is independent of gender, height, weight, or ethnicity compared to muscle area or thickness and thus appears more objective. In this sense, PMD should better contextualize the nutritional status of patients affected by nonalcoholic steatohepatitis, who are often malnourished (40); in these patients, a low PMD should guide clinicians to prescribe specific nutritional programs.
5.1. Conclusions
Further prospective studies on the utility of using PMD in prognostication and care of high-risk LT patients are needed. A better diagnosis of sarcopenia in cirrhotic patients should improve clinical care and help with the decision to give these patients some priority on the transplant waiting list.
References
-
1.
Yi Z, Mayorga ME, Orman ES, Wheeler SB, Hayashi PH, Barritt A. Trends in Characteristics of Patients Listed for Liver Transplantation Will Lead to Higher Rates of Waitlist Removal Due to Clinical Deterioration. Transplantation. 2017;101(10):2368-74. [PubMed ID: 28858174]. [PubMed Central ID: PMC5667556]. https://doi.org/10.1097/TP.0000000000001851.
-
2.
Morrissey PE, Monaco AP. Donation after circulatory death: current practices, ongoing challenges, and potential improvements. Transplantation. 2014;97(3):258-64. [PubMed ID: 24492420]. https://doi.org/10.1097/01.TP.0000437178.48174.db.
-
3.
Wong TC, Fung JY, Chok KS, Cheung TT, Chan AC, Sharr WW, et al. Excellent outcomes of liver transplantation using severely steatotic grafts from brain-dead donors. Liver Transpl. 2016;22(2):226-36. [PubMed ID: 26359934]. https://doi.org/10.1002/lt.24335.
-
4.
Orman ES, Mayorga ME, Wheeler SB, Townsley RM, Toro-Diaz HH, Hayashi PH, et al. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21(8):1040-50. [PubMed ID: 25939487]. [PubMed Central ID: PMC4566853]. https://doi.org/10.1002/lt.24160.
-
5.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412-23. [PubMed ID: 20392703]. [PubMed Central ID: PMC2886201]. https://doi.org/10.1093/ageing/afq034.
-
6.
Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65(6):1232-44. [PubMed ID: 27515775]. [PubMed Central ID: PMC5116259]. https://doi.org/10.1016/j.jhep.2016.07.040.
-
7.
Marasco G, Serenari M, Renzulli M, Alemanni LV, Rossini B, Pettinari I, et al. Clinical impact of sarcopenia assessment in patients with hepatocellular carcinoma undergoing treatments. J Gastroenterol. 2020;55(10):927-43. [PubMed ID: 32748172]. [PubMed Central ID: PMC7519899]. https://doi.org/10.1007/s00535-020-01711-w.
-
8.
Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10(2):166-73. 173 e1. [PubMed ID: 21893129]. https://doi.org/10.1016/j.cgh.2011.08.028.
-
9.
Alberino F, Gatta A, Amodio P, Merkel C, Di Pascoli L, Boffo G, et al. Nutrition and survival in patients with liver cirrhosis. Nutrition. 2001;17(6):445-50. [PubMed ID: 11399401]. https://doi.org/10.1016/s0899-9007(01)00521-4.
-
10.
Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16(1):95-131. [PubMed ID: 22321468]. [PubMed Central ID: PMC4383161]. https://doi.org/10.1016/j.cld.2011.12.009.
-
11.
Shiraki M, Nishiguchi S, Saito M, Fukuzawa Y, Mizuta T, Kaibori M, et al. Nutritional status and quality of life in current patients with liver cirrhosis as assessed in 2007-2011. Hepatol Res. 2013;43(2):106-12. [PubMed ID: 23409849]. https://doi.org/10.1111/hepr.12004.
-
12.
Nam NH, Kaido T, Uemoto S. Assessment and significance of sarcopenia in liver transplantation. Clin Transplant. 2019;33(12). [PubMed ID: 31651060]. https://doi.org/10.1111/ctr.13741.
-
13.
van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, I. Jzermans JN. Systematic Review and Meta-Analysis of the Impact of Computed Tomography-Assessed Skeletal Muscle Mass on Outcome in Patients Awaiting or Undergoing Liver Transplantation. Am J Transplant. 2016;16(8):2277-92. [PubMed ID: 26813115]. https://doi.org/10.1111/ajt.13732.
-
14.
Golse N, Bucur PO, Ciacio O, Pittau G, Sa Cunha A, Adam R, et al. A new definition of sarcopenia in patients with cirrhosis undergoing liver transplantation. Liver Transpl. 2017;23(2):143-54. [PubMed ID: 28061014]. https://doi.org/10.1002/lt.24671.
-
15.
Valero V3, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19(2):272-81. [PubMed ID: 25389056]. [PubMed Central ID: PMC4332815]. https://doi.org/10.1007/s11605-014-2680-4.
-
16.
Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271-8. [PubMed ID: 20670867]. [PubMed Central ID: PMC2914324]. https://doi.org/10.1016/j.jamcollsurg.2010.03.039.
-
17.
Durand F, Buyse S, Francoz C, Laouenan C, Bruno O, Belghiti J, et al. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60(6):1151-7. [PubMed ID: 24607622]. https://doi.org/10.1016/j.jhep.2014.02.026.
-
18.
Ravaioli M, Grazi GL, Piscaglia F, Trevisani F, Cescon M, Ercolani G, et al. Liver transplantation for hepatocellular carcinoma: results of down-staging in patients initially outside the Milan selection criteria. Am J Transplant. 2008;8(12):2547-57. [PubMed ID: 19032223]. https://doi.org/10.1111/j.1600-6143.2008.02409.x.
-
19.
Bertuzzo VR, Cescon M, Odaldi F, Di Laudo M, Cucchetti A, Ravaioli M, et al. Actual Risk of Using Very Aged Donors for Unselected Liver Transplant Candidates: A European Single-center Experience in the MELD Era. Ann Surg. 2017;265(2):388-96. [PubMed ID: 28059967]. https://doi.org/10.1097/SLA.0000000000001681.
-
20.
Drenth JP, Chrispijn M, Nagorney DM, Kamath PS, Torres VE. Medical and surgical treatment options for polycystic liver disease. Hepatology. 2010;52(6):2223-30. [PubMed ID: 21105111]. https://doi.org/10.1002/hep.24036.
-
21.
Underwood PW, Cron DC, Terjimanian MN, Wang SC, Englesbe MJ, Waits SA. Sarcopenia and failure to rescue following liver transplantation. Clin Transplant. 2015;29(12):1076-80. [PubMed ID: 26358578]. https://doi.org/10.1111/ctr.12629.
-
22.
Mayo SC, Shore AD, Nathan H, Edil BH, Hirose K, Anders RA, et al. Refining the definition of perioperative mortality following hepatectomy using death within 90 days as the standard criterion. HPB (Oxford). 2011;13(7):473-82. [PubMed ID: 21689231]. [PubMed Central ID: PMC3133714]. https://doi.org/10.1111/j.1477-2574.2011.00326.x.
-
23.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-13. [PubMed ID: 15273542]. [PubMed Central ID: PMC1360123]. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
-
24.
Moreau R, Jalan R, Gines P, Pavesi M, Angeli P, Cordoba J, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426-37. 1437 e1-9. [PubMed ID: 23474284]. https://doi.org/10.1053/j.gastro.2013.02.042.
-
25.
Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62(4):968-74. [PubMed ID: 25638527]. https://doi.org/10.1016/j.jhep.2014.12.029.
-
26.
Adams RC, Gunter OL, Wisler JR, Whitmill ML, Cipolla J, Lindsey DE, et al. Dynamic changes in respiratory frequency/tidal volume may predict failures of ventilatory liberation in patients on prolonged mechanical ventilation and normal preliberation respiratory frequency/tidal volume values. Am Surg. 2012;78(1):69-73. [PubMed ID: 22273318].
-
27.
Brooks-Brunn JA. Postoperative atelectasis and pneumonia: risk factors. Am J Crit Care. 1995;4(5):340-9. quiz 350-1. [PubMed ID: 7489036].
-
28.
Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128-40. [PubMed ID: 2841893]. https://doi.org/10.1016/0196-6553(88)90053-3.
-
29.
Renzulli M, Buonfiglioli F, Conti F, Brocchi S, Serio I, Foschi FG, et al. Imaging features of microvascular invasion in hepatocellular carcinoma developed after direct-acting antiviral therapy in HCV-related cirrhosis. Eur Radiol. 2018;28(2):506-13. [PubMed ID: 28894901]. https://doi.org/10.1007/s00330-017-5033-3.
-
30.
Buettner S, Wagner D, Kim Y, Margonis GA, Makary MA, Wilson A, et al. Inclusion of Sarcopenia Outperforms the Modified Frailty Index in Predicting 1-Year Mortality among 1,326 Patients Undergoing Gastrointestinal Surgery for a Malignant Indication. J Am Coll Surg. 2016;222(4):397-407 e2. [PubMed ID: 26803743]. https://doi.org/10.1016/j.jamcollsurg.2015.12.020.
-
31.
Goodpaster BH, Thaete FL, Kelley DE. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci. 2000;904:18-24. [PubMed ID: 10865705]. https://doi.org/10.1111/j.1749-6632.2000.tb06416.x.
-
32.
Kalafateli M, Karatzas A, Tsiaoussis G, Koutroumpakis E, Tselekouni P, Koukias N, et al. Muscle fat infiltration assessed by total psoas density on computed tomography predicts mortality in cirrhosis. Ann Gastroenterol. 2018;31(4):491-8. [PubMed ID: 29991895]. [PubMed Central ID: PMC6033770]. https://doi.org/10.20524/aog.2018.0256.
-
33.
Kritikos A, Manuel O. Bloodstream infections after solid-organ transplantation. Virulence. 2016;7(3):329-40. [PubMed ID: 26766415]. [PubMed Central ID: PMC4871682]. https://doi.org/10.1080/21505594.2016.1139279.
-
34.
Ravaioli M, Neri F, Lazzarotto T, Bertuzzo VR, Di Gioia P, Stacchini G, et al. Immunosuppression Modifications Based on an Immune Response Assay: Results of a Randomized, Controlled Trial. Transplantation. 2015;99(8):1625-32. [PubMed ID: 25757214]. https://doi.org/10.1097/TP.0000000000000650.
-
35.
Bertuzzo VR, Giannella M, Cucchetti A, Pinna AD, Grossi A, Ravaioli M, et al. Impact of preoperative infection on outcome after liver transplantation. Br J Surg. 2017;104(2):e172-81. [PubMed ID: 28121031]. https://doi.org/10.1002/bjs.10449.
-
36.
Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8(11):979-85. [PubMed ID: 20621200]. https://doi.org/10.1016/j.cgh.2010.06.024.
-
37.
Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JX, et al. Inclusion of Sarcopenia Within MELD (MELD-Sarcopenia) and the Prediction of Mortality in Patients With Cirrhosis. Clin Transl Gastroenterol. 2015;6. e102. [PubMed ID: 26181291]. [PubMed Central ID: PMC4816259]. https://doi.org/10.1038/ctg.2015.31.
-
38.
Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-35. [PubMed ID: 18539529]. https://doi.org/10.1016/S1470-2045(08)70153-0.
-
39.
Alwarawrah Y, Kiernan K, MacIver NJ. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front Immunol. 2018;9:1055. [PubMed ID: 29868016]. [PubMed Central ID: PMC5968375]. https://doi.org/10.3389/fimmu.2018.01055.
-
40.
Carias S, Castellanos AL, Vilchez V, Nair R, Dela Cruz AC, Watkins J, et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol. 2016;31(3):628-33. [PubMed ID: 26399838]. [PubMed Central ID: PMC6615558]. https://doi.org/10.1111/jgh.13166.