Abstract
Background:
In countries with unavailable tenofovir, a combination of lamivudine (LMV) and adefovir (ADV) is recommended for the treatment of LMV-resistant chronic hepatitis B (CHB). Considering that telbivudine (L-dT) was demonstrated to be superior to LMV in previous studies, L-dT and ADV combination therapy is expected to show better antiviral efficacy than the combination of LMV and ADV in patients with LMV-resistant CHB.Methods:
This was a prospective randomized multicenter study. The primary endpoint was Hepatitis B Virus (HBV) DNA reduction after 52 weeks of treatment. The secondary endpoints were HBV DNA undetectability, hepatitis B e antigen seroconversion, the incidence of virological and biochemical breakthroughs, and safety during the study period.Results:
A total of 43 LMV-resistant CHB patients were enrolled. Twenty-one were treated with LMV + ADV and 22 with L-dT + ADV. After 52 weeks of antiviral treatment, the HBV DNA reduction showed no significant intergroup difference (-4.54 ± 1.23 log IU/mL in the LMV + ADV group, -4.24 ± 1.46 log IU/mL in the L-dT + ADV group, P = 0.475). There were no significant intergroup differences in HBV DNA undetectability rates, mean HBV DNA level, or hepatitis B e antigen seroconversion rate at 13, 26, 39, and 52 weeks of treatment. In terms of safety, the mean creatine phosphokinase level was significantly higher in the L-dT + ADV group.Conclusions:
In the treatment of LMV-resistant CHB, the combination of L-dT and ADV did not show any clinical benefit compared to the combination of LMV and ADV.Keywords
Adefovir Hepatitis B Lamivudine Resistance Rescue Therapy Telbivudine
1. Background
Chronic hepatitis B (CHB) is a serious health issue affecting 300 million people worldwide (1). Chronic hepatitis B virus (HBV) infection increases the risk of liver cirrhosis, liver failure, and hepatocellular carcinoma, accounting for approximately one million deaths annually (2, 3). Therefore, CHB treatment aims to eradicate HBV or inhibit viral replication by administering appropriate antiviral agents and thereby improve the patient survival rate by preventing complications such as cirrhosis or hepatocellular carcinoma.
Lamivudine (LMV) is the first approved antiviral agent for chronic HBV infection and was shown to be efficacious in suppressing viral replication (4-6). Recently, lamivudine was reported to improve metabolic derangement in CHB patients (7). However, the long-term administration of LMV involves a high rate of drug resistance (up to 54% in three years, 70% or more in over five years) (8-11). The emergence of LMV-resistant mutations reduces the virological response to LMV and increases the risk of severe exacerbation of HBV infection and progression of liver cirrhosis (12-15).
Adefovir dipivoxil (ADV) is commonly used when a virological breakthrough occurs due to LMV-resistant HBV. The ADV administration in nucleos(t)ide analog (NA) treatment-naïve patients can be expected to show an antiviral effect and histological improvement similar to that obtained with LMV. Besides, ADV has also been shown to reduce serum HBV DNA levels of LMV-resistant mutant viruses, similar to that of wild-type viruses (16-18). However, when ADV is used in LMV-resistant patients, the incidence of ADV-resistant mutations is reportedly as high as 18% in one year and 25% in two years (19-21). To prevent ADV-resistant mutations, treatment with LMV and ADV combination in patients with the LMV-resistant mutant significantly reduces the incidence of ADV-resistant mutations to 1 - 2% over the same period (19-21). Therefore, a combination of LMV and ADV can be recommended to treat LMV-resistant CHB (19).
Tenofovir disoproxil fumarate (TDF) monotherapy was recently reported to be a highly effective treatment for LMV-resistant CHB (22), and clinical practice guidelines recommend it as first-line therapy in this population (23, 24). Unfortunately, TDF remains unavailable in several countries, including Asia-Pacific or South American countries. Therefore, ADV-based therapy should be considered for LMV-resistant CHB in areas of TDF unavailability.
Telbivudine (L-dT) is an L-nucleoside analog with an antiviral effect reportedly better than LMV and significantly lower incidence of resistance in treatment-naïve CHB patients (25). To date, there has been no well-designed clinical trial of combined treatment with L-dT and ADV in LMV-resistant CHB. Considering that L-dT was demonstrated to be superior to LMV in previous reports, L-dT and ADV combination therapy may have better antiviral efficacy than LMV and ADV combination therapy in patients with LMV-resistant CHB.
2. Objectives
This study was designed to investigate this hypothesis and suggest more appropriate treatment options for LMV-resistant CHB patients in areas with unavailable TDF.
3. Methods
3.1. Ethics Statement
The protocol was approved by the institutional review board at all clinical trial sites, and the study was conducted following the ethical principles of the Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT01804387).
3.2. Study Design
This investigator-initiated, prospective, multicenter, randomized, comparative, open-label pilot study of the treatment of LMV-resistant CHB patients was designed in May 2011 and approved by the Ministry of Food and Drug Safety in June 2012 in Korea. Patients were recruited from 10 hospitals affiliated with eight universities. Patients with LMV-resistant CHB who met the inclusion and exclusion criteria were prospectively enrolled from January 2013 to May 2014. Written informed consent was obtained from all patients. Randomization tables were generated by the nQuery Advisor program (version 6.01; Statistical Solutions Ltd., Cork, Ireland) with a block size of 4. The randomization was performed by opening serially numbered sealed envelopes, which were stratified by the study site and distributed in advance. After enrolment, LMV + ADV or L-dT + ADV therapy was initiated according to a random sequence.
3.3. Inclusion and Exclusion Criteria
Inclusion criteria were as follow: (1) age 18 - 70 years; (2) hepatitis B e antigen (HBeAg)-positive or negative CHB diagnosed based on a positive serum hepatitis B surface antigen (HBsAg) test for more than six months; (3) LMV treatment for at least six months and maintenance therapy by the time of screening; (4) LMV-resistant mutations (rtM204V or rtM204I) identified by a restriction fragment mass polymorphism assay; (5) virological breakthrough defined by an increase of HBV DNA level by more than 10 times the lowest level; (6) HBV DNA ≥ 20,000 IU/mL in HBeAg-positive or ≥ 2,000 IU/mL in HBeAg-negative patients at the time of screening; and (7) voluntary agreement to participate in this study. Exclusion criteria were as follow: (1) presence of ADV-resistant mutation (rtA181T, rtA181V, or rtN236T); (2) laboratory findings of serum AFP > 100 ng/mL, serum phosphorus < 2.4 mg/dL, serum creatinine > 1.5 mg/dL, or creatinine clearance < 50 mL/min; (3) decompensated liver cirrhosis with ascites, hepatic encephalopathy, esophagogastric variceal bleeding, jaundice, or Child-Pugh-Turcotte score exceeding 7 points; (4) history of more than four weeks of administration of an NA other than LMV acting on HBV; (5) history of immunomodulatory drug administration such as interferon or thymosin-alpha1 within 24 weeks of screening; (6) previous history of liver transplantation; (7) positive antibody test for human immune deficiency virus, hepatitis C virus, or hepatitis D virus; (8) concomitant metabolic liver disease with elevated Alanine Aminotransferase (ALT) levels; (9) consumption of alcohol more than 140 g/week for men and 70 g/week for women; (10) taking medications affecting ALT or HBV DNA level (corticosteroid, immunosuppressant, or nonsteroidal anti-inflammatory drugs); (11) women of childbearing age unwilling to use proper contraceptive measures; (12) women currently pregnant or lactating; and (13) history of hepatocellular carcinoma or other untreated malignancies.
3.4. Data Collection
We measured serum HBV DNA level using real-time polymerase chain reaction, HBeAg and antibody against HBeAg (anti-HBe) level, HBsAg level, serum ALT level, serum creatinine level, and Creatine Phosphokinase (CPK) level at 13, 26, 39, and 52 weeks of treatment. The incidences of virological and biochemical breakthroughs and any drug side effects were assessed at every visit.
3.5. Definitions
The virological response was defined as a decrease in the HBV DNA level to less than 20 IU/mL based on a real-time polymerase chain reaction. The biochemical response was defined as a decrease in serum ALT level to ≤ 40 IU/L in either sex. Serologic responses included HBeAg loss or seroconversion of HBeAg to anti-HBe. Besides, HBsAg loss was defined as a positive to negative HBsAg status shift regardless of the appearance of hepatitis B surface antibodies. Definitions of genotypic resistance, virological breakthrough, and biochemical breakthrough followed the guidelines given by the American Association for the Study of Liver Diseases (24).
3.6. Statistical Analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (version 18.0; IBM Inc, Armonk, NY, USA). Categorical variables, such as HBV DNA undetectability, ALT normalization, HBeAg seroconversion, and HBsAg loss rates, were analyzed using the chi-square test or Fisher’s exact test as appropriate. Continuous variables, such as mean HBV DNA and ALT levels, were compared between the groups using the Student’s t-test or the Mann-Whitney U test as appropriate. Serum HBV DNA levels were converted to a logarithmic scale before analysis. Data are expressed as mean ± standard deviation.
4. Results
4.1. Baseline Characteristics
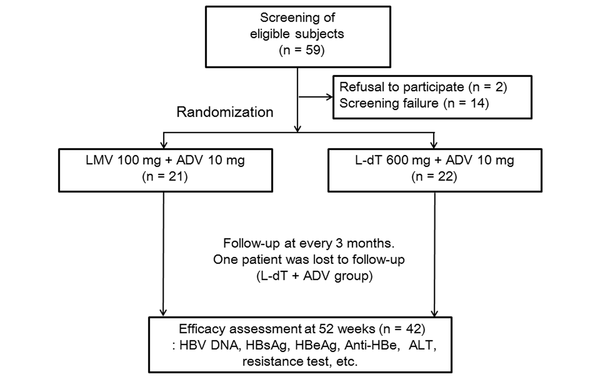
Forty-three patients who met the inclusion and exclusion criteria were included (Figrue 1). Twenty-one patients received LMV + ADV therapy, while 22 patients received L-dT + ADV therapy, with a mean patient age of 45.3 ± 8.7 and 49.3 ± 8.9 years, respectively. There were 13 (61.9%) males and eight (38.1%) females in the LMV + ADV group and 13 (59.1%) males and nine (40.1%) females in the L-dT + ADV group. The HBV DNA levels were 6.72 ± 1.48 log10 IU/mL and 6.53 ± 1.56 log10 IU/mL (P = 0.70) in the groups, respectively. Baseline lamivudine-resistant mutations were not significantly different between the groups (P = 0.37). besides, HBeAg-positive patients comprised 61.9% and 63.6% of the groups, respectively (P = 0.91). Serum ALT (86.5 ± 74.3 vs. 131.1 ± 214.0, p = 0.37) and creatinine (0.99 ± 0.22 mg/dL vs. 0.87 ± 0.22 mg/dL, P = 0.05) levels showed no significant intergroup differences (Table 1).
Study flow and patient disposition (ADV, adefovir dipivoxil; ALT, alanine aminotransferase; Anti-HBe, antibody to HBeAg; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; L-dT, telbivudine; LMV, lamivudine).
Patients’ Baseline Characteristics
| Variables | Total (n = 43) | LMV + ADV (n = 21) | L-dT + ADV (n = 22) | P Value |
|---|---|---|---|---|
| Male | 26 (60.5) | 13 (61.9) | 13 (59.1) | 0.85 |
| Age (y) | 47.3 ± 9.4 | 45.3 ± 9.7 | 49.3 ± 8.9 | 0.60 |
| BMI (kg/m2) | 23.88 ± 2.50 | 23.70 ± 2.64 | 24.04 ± 2.42 | 0.67 |
| Glucose (FBS, mg/dL) | 100.88 ± 20.61 | 103.52 ± 18.54 | 98.36 ± 22.55 | 0.42 |
| Total cholesterol | 174.0 ± 34.4 | 176.44 ± 32.61 | 171.68 ± 36.67 | 0.68 |
| HBV DNA (log10 IU/mL) | 6.63 ± 1.52 | 6.72 ± 1.48 | 6.54 ± 1.56 | 0.70 |
| HBeAg positivity | 27/43 (62.8) | 13/21 (61.9) | 14/22 (63.6) | 0.91 |
| Platelet (× 103/µL) | 166.81 ± 49.78 | 180.81 ± 52.18 | 153.45 ± 44.49 | 0.07 |
| ALT (IU/L) | 109.3 ± 161.4 | 86.5 ± 74.3 | 131.1 ± 214.0 | 0.37 |
| CPK (mg/dL) | 96.0 ± 46.4 | 98.8 ± 56.50 | 93.05 ± 34.03 | 0.70 |
| Creatinine (mg/dL) | 0.93 ± 0.22 | 0.99 ± 0.20 | 0.87 ± 0.22 | 0.05 |
| Total bilirubin (mg/dL) | 0.84 ± 0.41 | 0.76 ± 0.29 | 0.92 ± 0.49 | 0.19 |
| Albumin (g/dL) | 4.37 ± 0.38 | 4.41 ± 0.37 | 4.33 ± 0.38 | 0.45 |
| INR | 1.05 ± 0.10 | 1.04 ± 0.08 | 1.07 ± 0.12 | 0.34 |
| Na (mmol/L) | 141.15 ± 2.24 | 140.76 ± 2.14 | 141.55 ± 2.33 | 0.27 |
| Phosphorus (mg/dL) | 3.37 ± 0.31 | 3.31 ± 0.32 | 3.42 ± 0.30 | 0.24 |
| Liver cirrhosis | 4/43 (9.3) | 1/21 (4.8) | 3/22 (13.6) | 0.61 |
| Baseline HBV mutation | 0.37 | |||
| rtM204I | 11 (25.6) | 7 (33.3) | 4 (18.2) | |
| rtL180M + rtM204V | 18 (41.9) | 9 (42.9) | 9 (40.9) | |
| rtL180M + rtM204I | 12 (27.9) | 5 (23.8) | 7 (31.8) | |
| rtL180M + rtM204I/V | 2 (4.7) | 0 (0) | 2 (9.1) |
4.2. Virological and Biochemical Responses
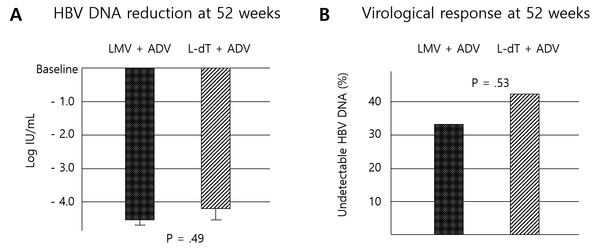
After 52 weeks of antiviral treatment, the LMV + ADV group showed a reduction in HBV DNA levels of -4.54 ± 1.23 log IU/mL, while the L-dT + ADV group showed a -4.24 ± 1.46 log IU/mL reduction. There was no significant difference between the two groups (p = 0.49) (Figure 2A). The virological response rate at 52 weeks showed no significant differences between the two groups (LMV + ADV group, 33.3%; L-dT + ADV group, 42.9%; P = 0.53) (Figure 2B). Further, the virological response rates at 13, 26, and 39 weeks of treatment did not differ significantly (Table 2). After 52 weeks of treatment, the mean HBV DNA levels were 2.18 ± 0.90 log IU/mL and 2.51 ± 1.45 log IU/mL in the LMV + ADV and L-dT + ADV groups, respectively (P = 0.384) (Table 3). None of the patients in either group showed HBeAg seroconversion or HBeAg or HBsAg loss (Table 2). Twenty (95.2%) patients showed ALT normalization in the LMV + ADV group versus 19 (90.5%) patients in the L-dT + ADV group. There was no significant intergroup difference (Table 2).
Antiviral responses. A, changes in HBV DNA levels after 52 weeks of treatment; B, virological response rates after 52 weeks of treatment (ADV, adefovir dipivoxil; HBV, hepatitis B virus; L-dT, telbivudine; LMV, lamivudine).
Virological and Biochemical Responses by Time Point
| Variables | LMV + ADV; No. (%) | L-dT + ADV; No. (%) | P Value |
|---|---|---|---|
| Virological response | |||
| 13 weeks | 4/21 (19.0) | 6/22 (27.3) | 0.52 |
| 26 weeks | 4/21 (19.0) | 5/21 (23.8) | 0.71 |
| 39 weeks | 8/21 (38.1) | 7/21 (33.3) | 0.75 |
| 52 weeks | 7/21 (33.3) | 9/21 (42.9) | 0.53 |
| HBeAg loss | |||
| 13 weeks | 0/12 | 0/12 | - |
| 26 weeks | 0/12 | 0/12 | - |
| 39 weeks | 0/12 (0.0) | 1/12 (8.3) a | > 0.99 |
| 52 weeks | 0/12 | 0/12 | - |
| HBsAg loss | |||
| 13 weeks | 0/21 | 0/22 | - |
| 26 weeks | 0/21 | 0/21 | - |
| 39 weeks | 0/21 | 0/21 | - |
| 52 weeks | 0/21 | 0/21 | - |
| Biochemical response | |||
| 13 weeks | 14/21 (66.7) | 15/22 (68.2) | 0.92 |
| 26 weeks | 18/21 (85.7) | 18/21 (85.7) | > 0.99 |
| 39 weeks | 19/21 (90.5) | 18/21 (85.7) | 0.96 |
| 52 weeks | 20/21 (95.2) | 19/21 (90.5) | 0.55 |
HBV DNA Levels During Treatment by Time Point
| Variables | LMV + ADV (Log IU/mL) | L-dT + ADV (Log IU/mL) | P Value |
|---|---|---|---|
| Baseline | 6.72 ± 1.48 | 6.54 ± 1.56 | 0.70 |
| 13 weeks | 3.27 ± 1.51 | 3.14 ± 1.91 | 0.82 |
| 26 weeks | 2.56 ± 1.05 | 2.74 ± 1.54 | 0.66 |
| 39 weeks | 2.27 ± 1.03 | 2.78 ± 1.72 | 0.25 |
| 52 weeks | 2.18 ± 0.90 | 2.51 ± 1.45 | 0.38 |
4.3. Antiviral Resistance and Safety
No patients showed virological breakthrough in the LMV + ADV group while two (9.5%) patients did in the L-DT + ADV group during 52 weeks, but the difference was insignificant (P = 0.488). No newly developed ADV-resistant mutation was observed in either group.
Finally, ADV combination therapy caused renal injury and hypophosphatemia. In addition, LMV and L-dT induced muscle injury and increased creatine phosphokinase. Changes in serum creatinine levels from baseline to week 52 did not differ between the groups. However, the mean changes in the serum CPK level from baseline to week 52 were significantly higher in the L-dT + ADV group than in the LMV + ADV group (1.00 ± 38.69 vs. 119.39 ± 207.90, p = 0.03) (Table 4).
Changes in Creatinine and Creatine Phosphokinase Levels After 52 Weeks of Treatment
| Variables | LMV + ADV | L-dT + ADV | P Value |
|---|---|---|---|
| Change in Cr (mg/dL) | 0.013 ± 0.100 | -0.006 ± 0.100 | 0.55 |
| Change in CPK (mg/dL) | 1.00 ± 38.69 | 119.39 ± 207.90 | 0.03 |
5. Discussion
Recently, European and American clinical practice guidelines for managing CHB have recommended entecavir and tenofovir (TDF or tenofovir alafenamide) as first-line antiviral agents for treatment-naïve patients (23, 24). Tenofovir is recommended as the best option in the case of antiviral-resistant CHB, such as LMV resistance. As a result, the use of LMV, ADV, and L-dT for treatment-naïve CHB patients has decreased worldwide, and the usefulness of ADV for LMV-resistant CHB has also diminished. However, LMV + ADV remains an important treatment option for LMV-resistant CHB in countries where tenofovir is not approved or unavailable (e.g., Argentina, Malaysia, Indonesia, and Myanmar). Hence, clinical practice guidelines in the Asia-Pacific region on managing HBV infection reserved the combination of LMV and ADV as a second treatment option to a switch to TDF for LMV resistance (26). Although entecavir (ETV) monotherapy is not recommended as a rescue therapy since LMV-resistant mutations confer cross-resistance to ETV (27), switching ETV + ADV combination therapy is an effective treatment option in LMV-resistant HBV infection (28), although it is more expensive and thus, has not been recommended strongly.
Previous studies revealed that LMV + ADV showed a favorable effect in LMV-resistant chronic HBV infection (16-18). However, according to a recent study, LMV + ADV showed a low virological response rate of 38.4% at 12 months of treatment, especially in patients with high viral concentrations (19, 20). Nonetheless, research on new combination therapies to overcome this problem is lacking.
Few studies have examined the effect of L-dT and ADV combination treatment for LMV-resistant CHB. Lin et al. reported that L-dT + ADV showed a reasonable virological response rate compared to LMV + ADV treatment (29). However, the study was not randomized and had limited participants. On the other hand, Xu and Nie reported that L-dT and ADV combination therapy improved renal function of CHB patients (30). Hence, we expected a beneficial effect of L-dT + ADV on virological response and renal safety.
L-dT is generally a well-tolerated and safe drug for CHB treatment. Furthermore, it is effective for preventing HBV reactivation during immunosuppression or chemotherapy and mother-to-child transmission of hepatitis B, although the influence of prenatal L-dT exposure on neonates is under debate (31-34). The CPK level elevation (asymptomatic), myopathy, and neuropathy are the well-known side effects of L-dT (35). Many studies reported that the male sex and a low estimated glomerular filtration rate were significant risk factors for CPK level elevation during L-dT treatment (35, 36). However, few studies have been on the effects of L-dT and ADV combination therapy on CPK levels. In this study, the L-dT + ADV group showed a significant CPK level elevation compared to baseline, although most patients were asymptomatic. Further, none of the patients stopped taking their medication due to this side effect; however, CPK monitoring was necessary.
In the present study, the degree of HBV DNA level reduction and virological responses did not differ significantly between the groups. Creatinine levels tended to be lower in the L-dT + ADV group after 52 weeks of treatment, but there was no significant difference from the LMV + ADV group. Therefore, L-dT + ADV therapy showed no advantages concerning virological response or renal function and carried a risk of muscle-related problems.
This study is the first randomized controlled trial to evaluate the effect of L-dT and ADV combination therapy on LMV-resistant CHB. The major limitation of the present study would be the small number of patients, which limited the study's strength. Despite such a limitation due to the nature of a pilot study, the results showed that L-dT and ADV did not exhibit a better effect than LMV and ADV combination therapy and that side effects such as elevated CPK levels were observed. At the same time, there were no significant muscle-related symptoms that required discontinuation of therapy. However, both rescue regimens are not the current first-line treatment options in the current international guidelines. The recommended antiviral agents, which had excellent efficacy and high genetic barriers, such as ETV, TDF, and tenofovir alafenamide, were widely available in most countries. This is another major limitation of the present study. However, LMV, ADV, and L-dT are still being produced and prescribed to some CHB patients. Hence, both regimens can only be considered in limited situations of tenofovir unavailability.
In summary, the combination of L-dT and ADV showed no significant differences from the combination of LMV and ADV concerning HBV DNA reduction, virological response, viral breakthrough rate, and serologic and biochemical response rates at 52 weeks of LMV-resistant CHB treatment. Conversely, the level of muscle enzymes, such as CPK, increased more in the L-dT + ADV group.
In conclusion, the combination of L-dT and ADV for the treatment of LMV-resistant CHB showed no clinical benefit over the combination of LMV and ADV, and the additional monitoring of CPK levels was considered necessary during combination therapy with L-dT and ADV. Hence, combining LMV and ADV for LMV-resistant CHB patients in regions with unavailable tenofovir would be more appropriate.
Acknowledgements
References
-
1.
Lee WM. Hepatitis B virus infection. N Engl J Med. 1997;337(24):1733-45. [PubMed ID: 9392700]. https://doi.org/10.1056/NEJM199712113372406.
-
2.
Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942-56. [PubMed ID: 2834034]. https://doi.org/10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j.
-
3.
World Health Organization. Hepatitis B. Geneva, Switzerland: World Health Organization; 2021, [updated 27th Jul 2021; cited 13th Oct 2021]. Available from: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b.
-
4.
Dienstag JL, Cianciara J, Karayalcin S, Kowdley KV, Willems B, Plisek S, et al. Durability of serologic response after lamivudine treatment of chronic hepatitis B. Hepatology. 2003;37(4):748-55. [PubMed ID: 12668966]. https://doi.org/10.1053/jhep.2003.50117.
-
5.
Dienstag JL, Schiff ER, Wright TL, Perrillo RP, Hann HW, Goodman Z, et al. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341(17):1256-63. [PubMed ID: 10528035]. https://doi.org/10.1056/NEJM199910213411702.
-
6.
Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339(2):61-8. [PubMed ID: 9654535]. https://doi.org/10.1056/NEJM199807093390201.
-
7.
Mohamed AA, Abdo S, Said E, El Agawy W, Awad P, Ghanem AIM, et al. Serum Vitamin D Levels in Chronic Hepatitis B Patients Before and During Treatment. Infect Disord Drug Targets. 2020;20(6):840-7. [PubMed ID: 31721718]. https://doi.org/10.2174/1871526519666191112112903.
-
8.
Liaw YF, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. Effects of extended lamivudine therapy in Asian patients with chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. Gastroenterology. 2000;119(1):172-80. [PubMed ID: 10889166]. https://doi.org/10.1053/gast.2000.8559.
-
9.
Chien RN, Liaw YF, Atkins M. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30(3):770-4. [PubMed ID: 10462384]. https://doi.org/10.1002/hep.510300313.
-
10.
Hadziyannis SJ, Vassilopoulos D. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology. 2001;34(4 Pt 1):617-24. [PubMed ID: 11584355]. https://doi.org/10.1053/jhep.2001.27834.
-
11.
Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, et al. Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology. 2001;33(6):1527-32. [PubMed ID: 11391543]. https://doi.org/10.1053/jhep.2001.25084.
-
12.
Kim JW, Lee HS, Woo GH, Yoon JH, Jang JJ, Chi JG, et al. Fatal submassive hepatic necrosis associated with tyrosine-methionine-aspartate-aspartate-motif mutation of hepatitis B virus after long-term lamivudine therapy. Clin Infect Dis. 2001;33(3):403-5. [PubMed ID: 11438912]. https://doi.org/10.1086/321879.
-
13.
Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin Infect Dis. 2003;36(6):687-96. [PubMed ID: 12627352]. https://doi.org/10.1086/368083.
-
14.
Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105-17. [PubMed ID: 12512035]. https://doi.org/10.1053/gast.2003.50013.
-
15.
Mutimer D, Pillay D, Shields P, Cane P, Ratcliffe D, Martin B, et al. Outcome of lamivudine resistant hepatitis B virus infection in the liver transplant recipient. Gut. 2000;46(1):107-13. [PubMed ID: 10601065]. [PubMed Central ID: PMC1727773]. https://doi.org/10.1136/gut.46.1.107.
-
16.
Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, et al. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32(1):129-34. [PubMed ID: 10869300]. https://doi.org/10.1053/jhep.2000.8626.
-
17.
Xiong X, Flores C, Yang H, Toole JJ, Gibbs CS. Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology. 1998;28(6):1669-73. [PubMed ID: 9828233]. https://doi.org/10.1002/hep.510280629.
-
18.
Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, et al. Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open-label pilot study. Lancet. 2001;358(9283):718-23. [PubMed ID: 11551579]. https://doi.org/10.1016/s0140-6736(01)05840-8.
-
19.
Lampertico P, Vigano M, Manenti E, Iavarone M, Sablon E, Colombo M. Low resistance to adefovir combined with lamivudine: a 3-year study of 145 lamivudine-resistant hepatitis B patients. Gastroenterology. 2007;133(5):1445-51. [PubMed ID: 17983801]. https://doi.org/10.1053/j.gastro.2007.08.079.
-
20.
Yeon JE, Yoo W, Hong SP, Chang YJ, Yu SK, Kim JH, et al. Resistance to adefovir dipivoxil in lamivudine resistant chronic hepatitis B patients treated with adefovir dipivoxil. Gut. 2006;55(10):1488-95. [PubMed ID: 16461777]. [PubMed Central ID: PMC1856440]. https://doi.org/10.1136/gut.2005.077099.
-
21.
Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, et al. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43(6):1385-91. [PubMed ID: 16729316]. https://doi.org/10.1002/hep.21189.
-
22.
Corsa AC, Liu Y, Flaherty JF, Mitchell B, Fung SK, Gane E, et al. No resistance to tenofovir disoproxil fumarate through 96 weeks of treatment in patients with lamivudine-resistant chronic hepatitis B. Clin Gastroenterol Hepatol. 2014;12(12):2106-12 e1. [PubMed ID: 24929235]. https://doi.org/10.1016/j.cgh.2014.05.024.
-
23.
European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370-98. [PubMed ID: 28427875]. https://doi.org/10.1016/j.jhep.2017.03.021.
-
24.
Terrault NA, Lok ASF, McMahon BJ, Chang KM, Hwang JP, Jonas MM, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology. 2018;67(4):1560-99. [PubMed ID: 29405329]. [PubMed Central ID: PMC5975958]. https://doi.org/10.1002/hep.29800.
-
25.
Lai CL, Gane E, Liaw YF, Hsu CW, Thongsawat S, Wang Y, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med. 2007;357(25):2576-88. [PubMed ID: 18094378]. https://doi.org/10.1056/NEJMoa066422.
-
26.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1-98. [PubMed ID: 26563120]. [PubMed Central ID: PMC4722087]. https://doi.org/10.1007/s12072-015-9675-4.
-
27.
Chao DC, Hu KQ. Update on rescue therapies in patients with lamivudine-resistant chronic hepatitis B. Drug Des Devel Ther. 2013;7:777-88. [PubMed ID: 23990707]. [PubMed Central ID: PMC3753145]. https://doi.org/10.2147/DDDT.S33947.
-
28.
Ha M, Zhang G, Diao S, Lin M, Wu J, Sun L, et al. Rescue therapy for lamivudine-resistant chronic hepatitis B: adefovir monotherapy, adefovir plus lamivudine or entecavir combination therapy. Intern Med. 2012;51(12):1509-15. [PubMed ID: 22728482]. https://doi.org/10.2169/internalmedicine.51.7329.
-
29.
Lin MT, Yen YH, Tsai MC, Tseng PL, Chang KC, Wu CK, et al. Comparison of the Efficacies and Safety of Combined Therapy between Telbivudine Plus Adefovir and Lamivudine Plus Adefovir in Patients with Hepatitis B Virus Infection in Real-World Practice. PLoS One. 2016;11(11). e0165416. [PubMed ID: 27806120]. [PubMed Central ID: PMC5091898]. https://doi.org/10.1371/journal.pone.0165416.
-
30.
Xu Y, Nie ZW. Telbivudine and adefovir dipivoxil combination therapy improves renal function in patients with chronic hepatitis B: A STROBE-compliant article. Medicine (Baltimore). 2018;97(48). e13430. [PubMed ID: 30508954]. [PubMed Central ID: PMC6283053]. https://doi.org/10.1097/MD.0000000000013430.
-
31.
Abdel-Noor R, Watany M, Abd-Elsalam S, ElKhalawany W, Soliman S, Badawi R. Is Hepatitis B Surface Antigen (HB s Ag) Enough Alone as a Screening Test for HBV Infection in Rheumatic Disease Patients Before Starting Immunosuppressive Therapies? A Cross-sectional Study. Infect Disord Drug Targets. 2020;20(6):878-83. [PubMed ID: 31830889]. https://doi.org/10.2174/1871526519666191212094141.
-
32.
Chen CJ, Yu HC, Chang CW, Bair MJ, Lin CC, Lin YS, et al. Efficacy of telbivudine and entecavir against virus reactivation in HBeAg-patients undergoing chemotherapy. Medicine (Baltimore). 2020;99(22). e20330. [PubMed ID: 32481407]. https://doi.org/10.1097/MD.0000000000020330.
-
33.
Ren C, Wang L, Sun W, Ma L, Dong Z, Hao A, et al. Efficacy and safety of telbivudine treatment for the prevention of HBV perinatal transmission. Medicine (Baltimore). 2020;99(24). e20583. [PubMed ID: 32541488]. [PubMed Central ID: PMC7302680]. https://doi.org/10.1097/MD.0000000000020583.
-
34.
Zhou C, Yu Y, Yang Q, Wang H, Hou M, Jin L, et al. Motor development delay in offspring is associated with prenatal telbivudine exposure. Medicine (Baltimore). 2018;97(9). e0053. [PubMed ID: 29489662]. [PubMed Central ID: PMC5851739]. https://doi.org/10.1097/MD.0000000000010053.
-
35.
Ambang T, Tan JS, Ong S, Wong KT, Goh KJ. Clinicopathological Features of Telbivudine-Associated Myopathy. PLoS One. 2016;11(9). e0162760. [PubMed ID: 27611456]. [PubMed Central ID: PMC5017711]. https://doi.org/10.1371/journal.pone.0162760.
-
36.
Zheng J, Deng M, Qiu X, Chen Z, Li D, Deng X, et al. Rhabdomyolysis, lactic acidosis, and multiple organ failure during telbivudine treatment for hepatitis B: a case report and review of the literature. J Med Case Rep. 2017;11(1):331. [PubMed ID: 29179767]. [PubMed Central ID: PMC5704524]. https://doi.org/10.1186/s13256-017-1498-6.