Abstract
Background:
This study investigated clinical characteristics and chronic factors of drug-induced liver injury (DILI) among patients with chronic hepatitis B virus (HBV) infection.Methods:
DILI patients were enrolled and divided into a DILI group and an HBV+DILI group. Laboratory indicators were recorded and analyzed. Multivariate logistic regression and the receiver operating characteristic (ROC) curve were used to determine risk factors and the predictive value for chronic DILI.Results:
Of all the 114 patients, 87 were in the DILI group and 27 were in the HBV+DILI group. Baseline total bilirubin (TBIL), direct bilirubin (DBIL), and incidence of chronicity were significantly higher in the HBV+DILI group than in the DILI group (P = 0.017, P = 0.037, P = 0.045, respectively). However, platelet (PLT) and prothrombin activity (PTA) were significantly lower in the HBV+DILI group than in the DILI group (P = 0.022, P = 0.013, respectively). HBV infection, baseline aspartate aminotransferase (AST) > 200 U/L, and TBIL > 34.2 μmol/L were predictors of chronic DILI (OR = 4.481 [95% CI, 1.298 - 15.470], P = 0.018; OR = 8.478 [95% CI, 2.079 - 34.566], P = 0.003; OR = 7.358 [95% CI, 2.215 - 24.446], P = 0.001). The area under ROC curve (AUC) of joint diagnosis for chronic DILI was 0.814 (95% CI, 0.704 - 0.925, P < 0.001), which was significantly higher than that of single parameter prediction. Also, the sensitivity, specificity, positive predictive value, and negative predictive value of joint diagnosis were 81.0%, 73.1%, 40.5%, and 94.4%, respectively.Conclusions:
HBV infection aggravated liver injury. HBV infection, baseline AST > 200 U/L, and TBIL > 34.2 μmol/L were predictors of chronic DILI, and their joint diagnosis could be used to predict chronic DILI effectively.Keywords
Independent Risk Factors Chronicity Chronic HBV Infection Drug-induced Liver Injury Joint Diagnosis
1. Background
Drug-induced liver injury (DILI) is the most common and severe adverse effect of drugs, which is caused by the drug itself or its metabolites (intrinsic hepatotoxicity) to the liver, or the hypersensitivity reaction and intolerance of our body (idiosyncratic hepatotoxicity) (1). There are about 1000 kinds of drugs approved for marketing and traditional drugs that have so far been proved potentially hepatotoxic and may induce DILI (2, 3). The annual incidence of DILI in the general population in China is estimated to be 23.80/100000 (4). Meanwhile, China is a high prevalence area of hepatitis B virus (HBV), and there are 70 million patients with chronic HBV infection (5). Some of them under treatment with drugs for other diseases can inevitably cause DILI. Previous studies have reported that patients with chronic HBV infection complicated with DILI have more severe liver damage and a higher proportion of poor prognoses such as chronicity and liver failure (6, 7). However, studies exploring prognostic factors of patients with chronic HBV infection complicated with DILI are still limited.
2. Objectives
We conducted this analysis to discuss the clinical characteristics and chronic factors of DILI in chronic hepatitis B infection patients.
3. Methods
3.1. Patients and Study Design
Patients who were clinically diagnosed as DILI according to the Roussel Uclaf Causality Assessment Method (RUCAM) score (1) and histopathologically supported afterward by liver biopsy were retrospectively enrolled from January 2018 to March 2019 in the Second Department of Hepatology, the Beijing Ditan Hospital. Entry standards were as follows: (1) patients with abnormalities in liver function tests after admission to our hospital including any one of the following: ALT ≥ 5 fold elevation above the upper limit of normal (ULN) or ALP ≥ 2 × ULN in the absence of known bone pathology or TBIL > 2 × ULN and ALT ≥ 3 × ULN; (2) having a definite history of drug use (herbal medicine and dietary supplements, non-steroidal anti-inflammatory drugs [NSAIDs], anti-infective drugs, hormone drugs, cardio-cerebrovascular drugs, and other drugs) ; (3) the RUCAM score ≥ 6; and (4) a DILI diagnosis supported by the liver biopsy result and no marked liver inflammatory damage (below G2) caused by other diseases. Also, exclusion criteria were as follows: (1) prior known cases of viral hepatitis (apart from hepatitis B) and cytomegalovirus, Epstein-Barr virus, human immunodeficiency virus (HIV), and other non-hepatitis virus infection; and (2) combination with other liver diseases, such as alcoholic hepatitis, autoimmune hepatitis, metabolic hepatitis, nonalcoholic fatty liver disease, Wilson's disease, liver cirrhosis, and liver cancer. The enrolled patients were divided into two groups based on the status of HBV infection: DILI and HBV+DILI, with hepatitis B surface antigen (HBsAg) ≥ 0.05 IU/mL lasting for more than six months and no history of antiviral drug use. Combined with the liver biopsy results, patients with chronic HBV infection referred to the patients in the immune tolerance phase (chronic HBV carrier status) or the immune control phase (inactive HBsAg carrier status).
All the patients were followed for six months (180 days) after their liver injuries were diagnosed. During the first admission to our hospital for DILI diagnosis and follow-up periods, information on demographics (age, sex), serum HBV markers (HBsAg), hepatic panel (including alanine aminotransferase [ALT], aspartate aminotransferase [AST], total bilirubin [TBIL], alkaline phosphatase [ALP]), blood routine, coagulation index (platelet [PLT], prothrombin activity [PTA]), liver imaging examination as well as types of drugs that caused liver injury and clinical outcomes were recorded. The clinical characteristics of DILI in chronic hepatitis B infection patients were analyzed by comparing the baseline laboratory indicators. For clinical outcomes, univariate and multivariate logistic regression were used to analyze the independent risk factors for DILI chronicity, and the receiver operating characteristic (ROC) curve was used to analyze the predictive value of the joint diagnosis.
The study was designed and performed according to the Helsinki criteria after evaluation by the Ethical Committee of the Beijing Ditan Hospital. This was a retrospective study that did not need informed consent.
3.2. Definition
R-value (ALT/ULN ÷ ALP/ULN) was calculated according to the patient's liver tests at the time of the DILI diagnosis. The patterns of DILI were defined as follows: The hepatocellular pattern as ALT levels ≥ 3 × ULN and R-value ≥ 5; cholestatic pattern as ALP ≥ 2 × ULN and R-value ≤ 2; mixed pattern as ALT ≥ 3 × ULN and ALP ≥ 2 × ULN with R-value between 2 and 5. Laboratory and clinical data were applied to classify the severity of the liver injury. The severities of DILI were defined as follows (1) Grade-0 (no liver injury): The patients could tolerate the exposed drugs, and there was no hepatotoxic reaction; Grade-1 (mild): Recoverably elevated serum ALT and/or ALP levels (TBIL < 2.5 × ULN [2.5 mg/dL or 42.75 μmol/L] with the international normalized ratio [INR] < 1.5). Most of the patients could adapt, with or without fatigue, weakness, nausea, anorexia, right upper abdominal pain, jaundice, pruritus, rash, weight loss, and other symptoms; Grade-2 (moderate): Elevated serum ALT and/or ALP levels (TBIL > 12.5 × ULN, or no increase in TBIL but INR ≥ 1.5). The above symptoms may be aggravated; Grade-3 (moderately severe): Elevated serum ALT and/or ALP levels (TBIL > 5 × ULN [5 mg/dL or 85.5 μmol/L] with or without INR ≥ 1.5). The patients’ symptoms worsened and required hospitalization or prolonged hospitalization; Grade-4 (severe): Elevated serum ALT and/or ALP levels (TBIL ≥ 10 × ULN [10 mg/dL or 171 μmol/L] or increased daily ≥ 1.0 mg/dL [17.1 μmol/L], INR ≥ 2 or PTA < 40%), which could be accompanied with clinical manifestations as (1) ascites or hepatic encephalopathy and (2) other organ failure related to DILI; Grade-5 (fatal): The patient died or underwent liver transplantation because of a DILI event within six months of onset (1). In this article, the severity of DILI was classified as mild, moderate, severe and above (including moderately severe, severe, and fatal).
Clinical outcomes were described in our study as cure (ALT, TBIL, and INR returned to normal range) and chronicity (ALT, AST, ALP, and TBIL were persistently abnormal, or there were radiological and histological pieces of evidence of portal hypertension or chronic liver injury six months after the diagnosis of DILI) (1). In order to support the diagnosis of chronic DILI and provide proper treatment in time, liver biopsy was performed again on patients with persistently abnormal biochemical indicators (ALT, AST, ALP, and TBIL) six months after the diagnosis of DILI, especially those with a history of chronic HBV infection.
3.3. Laboratory Assays
A Hitachi automatic biochemistry analyzer was used to detect liver function (Wako Pure Chemical Industries, Ltd., Japan). Routine blood testing was assayed using a SYSMEX XE5000 system (Sysmex Corp., Japan). Also, a STAGO STR automatic analyzer (Stago group, France) was used to perform coagulation testing. The HBsAg was detected using the Abbott Architac i2000 chemiluminescence reagent.
3.4. Liver Biopsy
The pathological slides of the liver biopsy were observed with the Nikon Eclipse 80i Microscope (Nikon, Inc., Japan). All stained liver biopsy specimens were reviewed and diagnosed by three experienced pathologists from the Beijing Ditan Hospital, who were blinded to any other clinical and laboratory data of the patients.
3.5. Statistical Analysis
The statistical analysis was performed using SPSS 21.0 software (IBM Inc., Armonk, NY). Quantitative data with normal distributions were denoted as means ± standard deviations (SD), and the Student's t test was used to compare the differences. Abnormally distributed quantitative data were denoted as medians (lower quartile, upper quartile [Q1, Q3]), and the Mann-Whitney U test was performed to compare the differences. Categorical variables were expressed as frequency and percentage. The rank variables of the categorical variables were compared using the Mann-Whitney U test, and the rest of the categorical variables were compared using the chi-square test or Fisher's exact test, as appropriate. The odds ratio (OR) and 95% confidence interval (95% CI) of variables with theoretical clinical importance for chronic outcome were calculated, and those that achieved a P-value of < 0.100 in the univariate analysis were included in the multivariate logistic regression analysis. A conditional forward method was used to select independent risk factors for chronic DILI. ROC and the area under ROC (AUC) were used to evaluate the predictive value of joint diagnosis for chronic DILI. A two-tailed P-value of < 0.050 was considered statistically significant.
4. Results
4.1. Baseline Characteristics
Among 126 patients who were suspected of DILI according to the RUCAM scoring system and further supported by the liver biopsy, 12 were excluded (eight for missing data, two for liver cancer, and two for HIV infection). A total of 114 patients were enrolled, of whom 87 were recognized as the DILI group, and the other 27 were recognized as the HBV+DILI group. The mean age of the enrolled patients, comprising 31 males and 83 females, was 43.77 ± 13.76 years. We found that the proportion of female patients was higher than that of male patients in both groups (DILI: 75.86% [66/87] vs. 24.14% [21/87]; HBV+DILI: 62.96% [17/27] vs. 37.04% [10/27]), and there were no significant differences in demographic characteristics between the two groups, as shown in Table 1.
| Baseline Characteristics | Total (N = 114) | DILI Group (N = 87) | HBV+DILI Group (N = 27) | χ2/Z/t | P-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 31 (27.19) | 21 (24.14) A | 10 (37.04) A | 1.732 | 0.188 |
| Age (y) | 43.77 ± 13.76 | 43.60 ± 14.09 | 44.33 ± 12.89 | -0.242 | 0.810 |
| Drug history | 1.039 | 0.959 | |||
| Herbal medicine and dietary supplements | 53 (46.49) | 41 (47.13) A | 12 (44.44) A | ||
| NSAIDs | 26 (22.81) | 19 (21.84) A | 7 (25.93) A | ||
| Anti-infective drugs | 14 (12.28) | 10 (11.49) A | 4 (14.81) A | ||
| Hormone drugs | 8 (7.02) | 6 (6.90) A | 2 (7.41) A | ||
| Cardio-cerebrovascular drugs | 5 (4.39) | 4 (4.60) A | 1 (3.70) A | ||
| Other drugs | 8 (7.02) | 7 (8.05) A | 1 (3.70) A | ||
| Type | 7.919 | 0.019 | |||
| Hepatocellular pattern | 92 (80.70) | 75 (86.21) A | 17 (62.96) B | ||
| Cholestatic pattern | 14 (12.28) | 9 (10.34) A | 5 (18.52) A | ||
| Mixed pattern | 8 (7.02) | 3 (3.45) A | 5 (18.52) B | ||
| Degree | -1.749 | 0.080 | |||
| Mild | 72 (63.16) | 58 (66.67) A | 14 (51.85) A | ||
| Moderate | 24 (21.05) | 19 (21.84) A | 5 (18.52) A | ||
| Severe and above | 18 (15.79) | 10 (11.49) A | 8 (29.63) B |
In the DILI group and the HBV+DILI group, the top three implicated agents causing liver injury were: herbal medicine and dietary supplements (DILI: 41/87 [47.13%] and HBV+DILI: 12/27 [44.44%]) and NSAIDs (DILI: 19/87 [21.84%] and HBV+DILI: 7/27 [25.93%]) and anti-infective drugs (DILI: 10/87 [11.49%] and HBV+DILI: 4/27 [14.81%]), respectively. Patients in the HBV+DILI group showed no statistical difference in types of drugs causing liver injury compared to the DILI group, as shown in Table 1.
According to the liver function tests at the time of DILI diagnosis, 92/114 (80.70%), 14/114 (12.28%), and 8/114 (7.02%) patients were classified as hepatocellular, cholestatic, and mixed patterns of DILI, respectively. The patterns of liver injury in both groups were hepatocellular injury, cholestatic injury, and mixed injury in order of proportion. There was a statistical difference between the two groups regarding liver injury type (χ2 = 7.919, P = 0.019). The proportion of mixed type was higher in the HBV+DILI group than in the DILI group (18.52% [5/27] vs. 3.45% [3/87], P = 0.025), while the proportion of hepatocellular injury type was higher in the DILI group than in the HBV+DILI group (86.21% [75/87] vs. 62.96% [17/27], P = 0.008). The severity of the liver injury was mainly mild in both groups, which accounted for 66.67% (58/87) and 51.85% (14/27) of the patients, respectively. Patients in the HBV+DILI group had a higher proportion of severe and above liver injury (29.63% [8/27] vs. 11.49% [10/87], P = 0.034), although not significant, as shown in Table 1.
4.2. Laboratory Tests at Baseline
Baseline TBIL (39 vs. 22 μmol/L, P = 0.017), DBIL (26 vs. 10 μmol/L, P = 0.037), and HGB (135.52 vs. 127.82 G/L, P = 0. 039) were significantly higher in the HBV+DILI group than in the DILI group. However, baseline PLT (152 vs. 187 × 109/L, P = 0.022) and PTA (78.39 vs. 88.80 %, P = 0.013) were significantly lower in the HBV+DILI group than in the DILI group, as shown in Table 2.
The Baseline Laboratory Indicators of the DILI and HBV+DILI Group Patients
| Baseline Laboratory Indicators | Total (N = 114) | DILI Group (N = 87) | HBV+DILI Group (N = 27) | Z/t | P-Value |
|---|---|---|---|---|---|
| ALT (U/L) | 450.5 (255,921) | 466 (274,853) | 363 (119,1123) | -1.193 | 0.233 |
| AST (U/L) | 228 (121,541) | 247 (144,541) | 188 (74,643) | -1.230 | 0.219 |
| TBIL (μmol/L) | 31 (15,59) | 22 (13,52) | 39 (23,122) | -2.393 | 0.017 |
| DBIL (μmol/L) | 17 (7,46) | 10 (6.3,40) | 26 (11,97) | -2.090 | 0.037 |
| γ-GT (U/L) | 165.5 (99,261) | 182 (101,264) | 150 (63,210.2) | -1.563 | 0.118 |
| ALP (U/L) | 134.5 (94,177) | 135 (96,197) | 121 (92,153) | -1.353 | 0.176 |
| ALB (g/L) | 39 (36,42) | 39 (36,42) | 39 (35,41) | -0.790 | 0.430 |
| TBA (μmol/L) | 31.5 (10,157) | 31 (10,147) | 37 (13,198) | -0.673 | 0.501 |
| PLT (× 109/L) | 185 (137,220) | 187 (152,231) | 152 (124,196) | -2.283 | 0.022 |
| PTA (%) | 86.34 ± 19.15 | 88.80 ± 16.75 | 78.39 ± 24.08 | 2.527 | 0.013 |
| WBC (× 109/L) | 4.68 (3.91,5.88) | 4.67 (3.92,6.23) | 5.00 (3.75,5.80) | -0.220 | 0.826 |
| EO (× 109/L) | 0.07 (0.04,0.13) | 0.07 (0.04,0.15) | 0.07 (0.04,0.12) | -0.063 | 0.949 |
| HGB (g/L) | 129.5 (121,141) | 127.82 ± 17.51 | 135.52 ± 13.72 | -2.092 | 0.039 |
4.3. Clinical Outcomes
A total of 21 patients still had persistent hepatochemical abnormalities six months after DILI diagnosis. Liver biopsy results in all the patients suggested the marked presence of chronic liver inflammation (above G2) caused by DILI. In those DILI patients with a history of HBV infection, liver biopsy results suggested marked chronic liver inflammation accompanied by no (G0) or only mild (G1) inflammation which was caused by HBV infection. Therefore, a total of 21 patients were diagnosed with chronic DILI, and the incidence of DILI chronicity in the HBV+DILI group was higher than that in the DILI group (33.33% [9/27] vs. 13.79% [12/87]), and the difference was statistically significant (χ2 = 4.016, P = 0.045), as shown in Table 3.
| Clinical Outcomes | Total (N = 114) | DILI Group (N = 87) | HBV+DILI Group (N = 27) | χ2 | P-Value |
|---|---|---|---|---|---|
| Cure | 93 (81.58) | 75 (86.21) | 18 (66.67) | 4.016 | 0.045 |
| Chronicity | 21 (18.42) | 12 (13.79) | 9 (33.33) |
4.4. Prediction of Chronic DILI Based on Clinical Parameters
Univariate analysis and multivariate regression analysis were used to determine factors associated with the outcomes of the patients. Continuous variables, such as ALT, AST, TBIL, ALP, PLT, and PTA, were converted into categorical variables, as shown in Table 4. The results of univariate analysis and multivariate regression analysis revealed that HBsAg-positive (+), baseline AST > 200 U/L, and baseline TBIL > 34.2 μmol/L were independent risk factors for chronic DILI (OR = 4.481 [95% CI, 1.298 - 15.470], P = 0.018; OR = 8.478 [95% CI, 2.079 - 34.566], P = 0.003; OR = 7.358 [95% CI, 2.215 - 24.446], P = 0.001), as shown in Table 5. These three variables were combined to calculate a joint predictor, ie, the variable "joint diagnosis," which was an index with a numerical value. Based on the above three parameters, the following logistic regression model (LRM) of joint diagnosis was established:
Y = -8.686 + 1.500 × HBsAg + 2.137 × AST + 1.996 × TBIL
If the patient was HBsAg (+) or AST > 200 U/L or TBIL > 34.2 μmol/L, the value at HBsAg or AST or TBIL in the formula was 2; If not, the value 1 was taken into the formula. It is worth noting that the numerical value of joint diagnosis results from Y in the above formula.
The Laboratory Indicator Variable Assignment Form
| Variable | Classified Variable | Assignment Instructions |
|---|---|---|
| ALT (U/L) | 1: ≤ 200 ; 2: > 200 | Based on the definition of DILI, the point is 200 |
| AST (U/L) | 1: ≤ 200 ; 2: > 200 | Based on the definition of DILI, the point is 200 |
| TBIL (μmol/L) | 1: ≤ 34.2 ; 2: > 34.2 | Based on the definition of DILI, the point is 34.2 |
| ALP (U/L) | 1: ≤ 250 ; 2: > 250 | Based on the definition of DILI, the point is 250 |
| PLT (× 109/L) | 1: ≤ 100 ; 2: > 100 | Based on the PLT’s lower limit of normal, the point is 100 |
| PTA (%) | 1: ≤ 70 ; 2: > 70 | Based on the PTA’s lower limit of normal, the point is 70 |
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-Value | OR | 95% CI | P-Value | |
| HBsAg (+) | 3.125 | 1.143~8.544 | 0.026 | 4.481 | 1.298~15.470 | 0.018 |
| ALT > 200 (U/L) | 2.771 | 0.596~12.873 | 0.193 | - | - | - |
| AST > 200 (U/L) | 3.655 | 1.142~11.694 | 0.029 | 8.478 | 2.079~34.566 | 0.003 |
| TBIL > 34.2 (μmol/L) | 5.553 | 1.868~16.504 | 0.002 | 7.358 | 2.215~24.446 | 0.001 |
| ALP > 250 (U/L) | 0.308 | 0.038~2.493 | 0.270 | - | - | - |
| PLT > 100 (× 109/L) | 0.894 | 0.176~4.551 | 0.893 | - | - | - |
| PTA > 70 (%) | 0.296 | 0.100~0.882 | 0.029 | - | - | - |
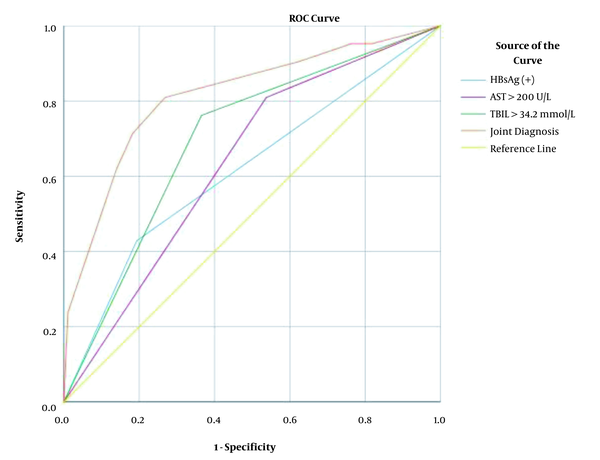
The AUC of HBsAg (+), AST > 200 U/L, and TBIL > 34.2 μmol/L for predicting DILI chronicity were 0.618 (95% CI, 0.476-0.759), 0.636 (95% CI, 0.513-0.759) and 0.698 (95% CI, 0.577-0.819), respectively. Compared with single parameter prediction, the joint diagnosis of HBsAg (+), baseline AST > 200 U/L, and baseline TBIL > 34.2 μmol/L had a higher AUC (0.814 [95% CI, 0.704 - 0.925], P < 0.001) for predicting chronic DILI, as shown in Table 6 and Figure 1. By using a cut-off value ≥ 0.170 in the LRM of joint diagnosis for predicting chronic DILI, the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were 81.0%, 73.1%, 40.5%, and 94.4%, respectively. The AUC value, sensitivity, PPV, and NPV of joint diagnosis were higher than those of single diagnosis in predicting chronic DILI.
The Efficacy Analysis of HBsAg (+), AST > 200 U/L, TBIL > 34.2 μmol/L, and Joint Diagnosis in Diagnosing Chronic DILI a
| Factors | AUC (95% CI) | Cut-off Value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| HBsAg (+) | 0.618 (0.476~0.759) | - | 42.9 | 80.6 | 33.3 | 86.2 |
| AST > 200 U/L | 0.636 (0.513~0.759) | - | 81.0 | 46.2 | 25.4 | 91.5 |
| TBIL > 34.2 μmol/L | 0.698 (0.577~0.819) | - | 76.2 | 63.4 | 32.0 | 92.2 |
| Joint diagnosis | 0.814 (0.704~0.925) | 0.170 | 81.0 | 73.1 | 40.5 | 94.4 |
The AUCs of HBsAg (+), AST > 200 U/L, TBIL > 34.2 μmol/L, and joint diagnosis for diagnosing chronic DILI
5. Discussion
Our study showed that females were more likely to suffer DILI than males, which was consistent with a previous study (8, 9). Also, there were more male patients in the HBV+DILI group than in the DILI group, which may be related to the fact that males are more likely to develop chronic HBV infection status (10). We also found a significant difference in the HGB level between the DILI group and the HBV+DILI group, possibly due to the same reason for gender, as the HGB level is generally higher in males than in females within the physiological range (11).
We found that baseline TBIL and DBIL levels were significantly higher in the HBV+DILI group than in the DILI group, which is similar to that reported by Chen et al. (6). Compared with patients with DILI alone, chronic HBV infection may increase the production of inflammatory cytokines, accompanied by the free radicals produced by drugs or its metabolites to trigger a more robust immune response, which leads to further severe damage to the liver (12). Meanwhile, the mixed DILI was more common in the HBV+DILI group than in the other group. The mixed DILI showed a large amount of cholestasis due to the aggravation of cholangiocyte inflammation, manifested by the significantly elevated bilirubin level (13), which is consistent with the above results.
The baseline PLT level in the HBV+DILI group was significantly lower than that in the DILI group, which may be since patients with chronic HBV infection suffered more severe liver damage and produced less thermoplastic polyolefin (TPO). It may also be associated with increased platelet destruction caused by hypersplenism and the loss of hematopoietic function of the bone marrow caused by HBV infection (14, 15). Our study showed that the baseline level of PTA was statistically lower in the HBV+DILI group than in the DILI group, which was due to the decrease of coagulation factors mainly synthesized by the liver, indicating the more serious hepatocellular damage (16).
The HBV+DILI group had a higher proportion in the type of severe liver injury than the DILI group, although not statistically significant. The difference may be attributed to the inflammatory environment caused by chronic HBV infection reducing the activities of P450 enzyme and glucuronosyltransferase, and the level of glutathione, which resulted in the disorder of drug metabolism and the accumulation of a large number of toxic substances.
Recent studies have revealed that about 20% of acute DILI patients would progress to chronic outcomes (2). In this study, 18.42% of the patients developed chronic DILI, and the incidence of the chronic outcome in the HBV+DILI group was significantly higher than that in the DILI group, which might be due to more severe liver damage and poor hepatocellular regeneration caused by chronic HBV infection. Women, advanced age, dyslipidemia, and the severity of acute attack have been stated to be the risk factors for chronic DILI (17). Some studies have also revealed that diclofenac (18), anti-infective drugs (19), and statins (20) are associated with a higher risk of chronic DILI. However, current blood biomarkers are not ideal enough in predicting DILI chronicity. Studies (21) have shown that total keratin 18 and the macrophage colony-stimulating factor receptor combined with the end-stage liver disease model are more accurate in evaluating disease progression or chronic outcome. Meanwhile, osteopontin has so far been the best predictor of adverse outcomes, including liver failure (21). The serum microRNA-122 level has been proposed to predict adverse outcomes such as subsequent development of DILI to liver failure (22). Zhu et al. (23) have reported that prolonger T0.5TBil (total bilirubin decreased from peak to half of the peak), an index of cholestasis, is an early and independent predictor of chronic DILI. These findings need to be further validated by larger prospective studies. Previous studies have demonstrated that DILI patients with chronic HBV infection are associated with a higher likelihood of chronic DILI (6, 7), and serum AST and TBIL levels can be predictors of the incidence of liver disease-related adverse outcomes (24, 25). In our study, although HBsAg (+), baseline AST > 200 U/L, and baseline TBIL > 34.2 μmol/L were independently associated with DILI chronicity, the AUCs for predicting DILI chronicity were only 0.618, 0.636, and 0.698, respectively. These results suggested that using a single parameter was challenging to predict chronic outcomes in DILI patients. In order to establish an index model to predict chronic DILI, the parameters of categorical variables, including HBsAg (+), baseline AST > 200 U/L, and baseline TBIL > 34.2 μmol/L, were combined to create a new index. Joint diagnosis and its diagnostic power were tested by ROC later. Our findings indicated that the LRM of the joint diagnosis we developed could be used to predict chronic outcomes effectively in DILI patients.
Several limitations of this study should be noted. First, it was a retrospective study performed at a single center with a small sample. Second, the data of HBV viral load were unavailable, and hence, we could not analyze the impact of different HBV viral loads on DILI patients with chronic HBV infection.
In conclusion, our study demonstrated that chronic HBV infection could aggravate liver injury and increase the incidence of chronic DILI. HBsAg (+), baseline AST > 200 U/L, and baseline TBIL > 34.2 μmol/L were independent risk factors of DILI chronicity. The LRM of joint diagnosis had a higher AUC value, sensitivity, PPV, and NPV, which could be used in clinical prediction of early chronic DILI and strengthen follow-up of corresponding patients.
References
-
1.
Yu YC, Mao YM, Chen CW, Chen JJ, Chen J, Cong WM, et al. CSH guidelines for the diagnosis and treatment of drug-induced liver injury. Hepatol Int. 2017;11(3):221-41. [PubMed ID: 28405790]. [PubMed Central ID: PMC5419998]. https://doi.org/10.1007/s12072-017-9793-2.
-
2.
European Association for the Study of the Liver, Clinical Practice Guideline Panel, Panel members, EASL Governing Board representative. EASL Clinical Practice Guidelines: Drug-induced liver injury. J Hepatol. 2019;70(6):1222-61. [PubMed ID: 30926241]. https://doi.org/10.1016/j.jhep.2019.02.014.
-
3.
Bjornsson HK, Bjornsson ES, Avula B, Khan IA, Jonasson JG, Ghabril M, et al. Ashwagandha-induced liver injury: A case series from Iceland and the US Drug-Induced Liver Injury Network. Liver Int. 2020;40(4):825-9. [PubMed ID: 31991029]. [PubMed Central ID: PMC8041491]. https://doi.org/10.1111/liv.14393.
-
4.
Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156(8):2230-2241 e11. [PubMed ID: 30742832]. https://doi.org/10.1053/j.gastro.2019.02.002.
-
5.
Liu J, Liang W, Jing W, Liu M. Countdown to 2030: eliminating hepatitis B disease, China. J Bull World Health Organ. 2019;97(3):230-8. [PubMed ID: 30992636]. [PubMed Central ID: PMC6453311]. https://doi.org/10.2471/BLT.18.219469.
-
6.
Chen L, Bao D, Gu L, Gu Y, Zhou L, Gao Z, et al. Co-infection with hepatitis B virus among tuberculosis patients is associated with poor outcomes during anti-tuberculosis treatment. BMC Infect Dis. 2018;18(1):295. [PubMed ID: 29970037]. [PubMed Central ID: PMC6029116]. https://doi.org/10.1186/s12879-018-3192-8.
-
7.
Mo X, Xu X, Ren Z, Guan J, Peng J. Patients with tuberculous meningitis and hepatitis B co-infection have increased risk for antituberculosis drug-induced liver injury and poor outcomes. Infect Dis (Lond). 2020;52(11):793-800. [PubMed ID: 32619380]. https://doi.org/10.1080/23744235.2020.1788223.
-
8.
Hou FQ, Zeng Z, Wang GQ. Hospital admissions for drug-induced liver injury: clinical features, therapy, and outcomes. Cell Biochem Biophys. 2012;64(2):77-83. [PubMed ID: 22806342]. https://doi.org/10.1007/s12013-012-9373-y.
-
9.
George N, Chen M, Yuen N, Hunt CM, Suzuki A. Interplay of gender, age and drug properties on reporting frequency of drug-induced liver injury. Regul Toxicol Pharmacol. 2018;94:101-7. [PubMed ID: 29407200]. https://doi.org/10.1016/j.yrtph.2018.01.018.
-
10.
Wang SH, Chen PJ, Yeh SH. Gender disparity in chronic hepatitis B: Mechanisms of sex hormones. J Gastroenterol Hepatol. 2015;30(8):1237-45. [PubMed ID: 25708186]. https://doi.org/10.1111/jgh.12934.
-
11.
Murphy WG. The sex difference in haemoglobin levels in adults - mechanisms, causes, and consequences. Blood Rev. 2014;28(2):41-7. [PubMed ID: 24491804]. https://doi.org/10.1016/j.blre.2013.12.003.
-
12.
Eisenberg-Lerner A, Kimchi A. PKD is a kinase of Vps34 that mediates ROS-induced autophagy downstream of DAPk. Cell Death Differ. 2012;19(5):788-97. [PubMed ID: 22095288]. [PubMed Central ID: PMC3321617]. https://doi.org/10.1038/cdd.2011.149.
-
13.
Geier A, Fickert P, Trauner M. Mechanisms of Disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(10):574-85. https://doi.org/10.1038/ncpgasthep0602.
-
14.
Ishikawa T, Ichida T, Matsuda Y, Sugitani S, Sugiyama M, Kato T, et al. Reduced expression of thrombopoietin is involved in thrombocytopenia in human and rat liver cirrhosis. J Gastroenterol Hepatol. 1998;13(9):907-13. [PubMed ID: 9794189]. https://doi.org/10.1111/j.1440-1746.1998.tb00760.x.
-
15.
Kajihara M, Okazaki Y, Kato S, Ishii H, Kawakami Y, Ikeda Y, et al. Evaluation of platelet kinetics in patients with liver cirrhosis: similarity to idiopathic thrombocytopenic purpura. Hepatol Int. 2007;22(1):112-8. [PubMed ID: 17201890]. https://doi.org/10.1111/j.1440-1746.2006.04359.x.
-
16.
Amitrano L, Guardascione MA, Brancaccio V, Balzano A. Coagulation disorders in liver disease. Semin Liver Dis. 2002;22(1):83-96. [PubMed ID: 11928081]. https://doi.org/10.1055/s-2002-23205.
-
17.
Bessone F, Robles-Diaz M, Hernandez N, Medina-Caliz I, Lucena MI, Andrade RJ. Assessment of Serious Acute and Chronic Idiosyncratic Drug-Induced Liver Injury in Clinical Practice. Semin Liver Dis. 2019;39(3):381-94. [PubMed ID: 31049898]. https://doi.org/10.1055/s-0039-1685519.
-
18.
Mazeika PK, Ford MJ. Chronic active hepatitis associated with diclofenac sodium therapy. Br J Clin Pract. 1989;43(3):125-6.
-
19.
Qiqi C, Huihui L, Fangfang S, Zhan Z, Lu Z, Yao L. Analysis of chronic factors in patients with drug-induced liver injury. Chinese J Liver Dis. 2020;12(1):68-75.
-
20.
Russo MW, Hoofnagle JH, Gu J, Fontana RJ, Barnhart H, Kleiner DE, et al. Spectrum of statin hepatotoxicity: experience of the drug-induced liver injury network. Hepatol Int. 2014;60(2):679-86. [PubMed ID: 24700436]. [PubMed Central ID: PMC4110177]. https://doi.org/10.1002/hep.27157.
-
21.
Church RJ, Kullak-Ublick GA, Aubrecht J, Bonkovsky HL, Chalasani N, Fontana RJ, et al. Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort. Hepatology. 2019;69(2):760-73. [PubMed ID: 29357190]. [PubMed Central ID: PMC6054900]. https://doi.org/10.1002/hep.29802.
-
22.
Russo MW, Steuerwald N, Norton HJ, Anderson WE, Foureau D, Chalasani N, et al. Profiles of miRNAs in serum in severe acute drug induced liver injury and their prognostic significance. Liver Int. 2017;37(5):757-64. [PubMed ID: 27860186]. [PubMed Central ID: PMC5502673]. https://doi.org/10.1111/liv.13312.
-
23.
Zhu W, Wang L, Zhao X, Wang T, Shi X, Ou X, et al. Prolonged interval of total bilirubin decline is an early independent predictive factor of chronic persistent drug-induced liver injury. Hepatol Res. 2020;50(2):224-32. [PubMed ID: 31652370]. https://doi.org/10.1111/hepr.13435.
-
24.
Xie K, Chen CH, Tsai SP, Lu PJ, Wu H, Zeng Y, et al. Loss of Life Expectancy by 10 Years or More From Elevated Aspartate Aminotransferase: Finding Aspartate Aminotransferase a Better Mortality Predictor for All-Cause and Liver-Related than Alanine Aminotransferase. Am J Gastroenterol. 2019;114(9):1478-87. [PubMed ID: 31425154]. https://doi.org/10.14309/ajg.0000000000000332.
-
25.
Stephens C, Robles-Diaz M, Medina-Caliz I, Garcia-Cortes M, Ortega-Alonso A, Sanabria-Cabrera J, et al. Comprehensive analysis and insights gained from long-term experience of the Spanish DILI Registry. J Hepatol. 2021;75(1):86-97. [PubMed ID: 33539847]. https://doi.org/10.1016/j.jhep.2021.01.029.