Abstract
Introduction:
Chinese herbal medicine (CHM) has been widely used by patients in China and results in unpredictable nephrotoxicity and hepatotoxicity effects.Case Presentation:
We report the case of a postoperative 69-year-old female patient with ascending colon cancer who rapidly developed liver cirrhosis after 18 months of continued CHM administration. The patient underwent right hemicolectomy at the Fourth Hospital of Hebei Medical University in August 2019 due to ascending colon cancer; at that time, the patient had no signs of liver cirrhosis based on computed tomography (CT) and routine blood examination. Postoperatively, the patient continued CHM administration for 18 months. The patient then visited our hospital with complaints of jaundice, abdominal distension, and edema in both lower limbs. CT imaging showed cirrhosis of the liver, while gastroscopy showed mild esophageal varices. Blood examinations including routine blood, coagulation function, and liver function tests, and biomarkers of hepatic fibrosis also supported the diagnosis of liver cirrhosis. To the best of our knowledge, this is the first report of CHM-induced liver cirrhosis.Conclusions:
CHM administration possibly induces rapid liver cirrhosis within 18 months.Keywords
1. Introduction
Cancer is the leading cause of human mortality worldwide, with an age-standardized incidence of 401.2 per 100,000 persons and mortality of 205.8 per 100,000 persons in China. Treatment options for cancer include surgical resection, radiotherapy, chemotherapy, molecular targeted therapy, and immunotherapy (1). Nonetheless, Chinese herbal medicine (CHM) is often chosen by patients with advanced cancer in China based on the belief that CHM is natural, safe, and effective. However, the components of CHM are complex and can result in renal injury, liver damage, and various toxic insults (2). In a retrospective study of drug-induced liver injury (DILI) in mainland China, the annual incidence in the general population was estimated to be 23.80 per 100,000 persons with traditional Chinese medicines, herbal and dietary supplements, and antituberculosis drugs as the leading causes (3). However, there have been no reports of CHM-induced liver cirrhosis. Virus infection, autoimmunity, and alcohol intake are the common causes of liver cirrhosis. Chronic hepatitis from hepatitis B virus (HBV) and hepatitis C virus (HCV) is a slowly progressive disease characterized by persistent hepatic inflammation. HCV infection induces the onset of cirrhosis in approximately 10 - 20% of chronic hepatitis patients over the course of 20 - 30 years, whereas HBV-induced cirrhosis accounts for 10% of chronic hepatitis patients during a mean follow-up of 11 years (4, 5). Here, we report a case of CHM administration that possibly induces rapid cirrhosis within 18 months.
2. Case Presentation
A 69-year-old woman presented to our hospital with chief complaints of jaundice, abdominal distension, and edema in both lower extremities on February 9, 2021. The patient had no medical history of hepatic disease, obesity, diabetes mellitus, autoimmune diseases, atopy, or alcohol intake. She underwent radical resection of the colon for ascending colon carcinoma at the Fourth Hospital of Hebei Medical University (Shijiazhuang, China) 18 months prior (pT4aN0M0 II B), with an unremarkable liver on CT scan (Figure 1A - C) and normal results of blood examinations including routine blood, coagulation function, and hepatorenal function tests (data not shown). Post-operation, the patient self-medicated with oral CHM remedies for antipyretic, detoxification, and benefiting vitality every day as adjuvant treatment, and continuously took approximately 20 types of CHM in conventional dosages for 18 months without routine hospital examination. The types of CHM and the dosages were adjusted by the patient’s CHM doctor (Table 1).
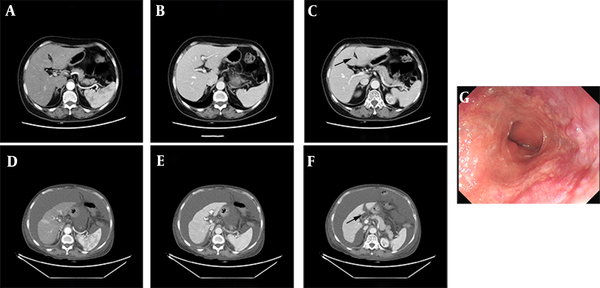
Comparison of abdominal CT scan before and after oral administration of CHM. A, B, C, the edge of the liver was smooth, hepatic crack was normal (black arrow), the proportion of each lobe was balanced before oral administration of CHM; D, E, F, the liver volume shrank, hepatic crack broadened (black arrow), the proportion of each lobe unbalanced, and ascites appeared after CHM administration; G, the gastroscopic showed mild esophageal varices.
| Chinese Herbal Medicine Name | Chinese Herbal Medicine Dosage (g) |
|---|---|
| Red peony root | 15 |
| Radix bupleuri | 15 |
| Angelica sinensis | 12 |
| Actractylodes macrocephala koidz | 20 |
| Poria cocos | 15 |
| Coix seed | 20 |
| Salvia miltiorrhiza | 15 |
| Radix seudostellariae | 20 |
| Rhizoma sparganii | 10 |
| Curcuma zedoary | 10 |
| Woodlouse | 10 |
| Sculellaria barbata | 15 |
| Fiveleaf gynostemma herb | 15 |
| Putchuck | 10 |
| Liquorice | 6 |
| Interior eardamom | 6 |
| Codonopsis | 20 |
| Hawthorn | 20 |
| Medicated leaven | 20 |
| Sojutsuvar | 20 |
| Airpotato yam rhizome | 15 |
| Chinese dwarf cherry seed | 20 |
| Christina loosestrife | 20 |
| Japanese climbing fern spore | 12 |
| Officinal magnolia bark | 12 |
| Inner membrane of chicken gizzard | 12 |
| Hedyotis | 20 |
| Heterophylly falsestarwort root | 20 |
| Muskroot-like semiaquilegia root | 20 |
| Germinated barley | 20 |
| Catclaw buttercup root | 15 |
| Cablin patchouli herb | 15 |
| Rice bean | 15 |
| Platycodon root | 15 |
| Common selfheal fruit-spike | 15 |
| Common pleione pseudobulb | 10 |
| Immature tangerine peel | 15 |
| Medicinal cyathula root | 15 |
| Rhizoma corydalis | 20 |
| Virgate wormwood herb | 15 |
| Chinese yam | 20 |
| Nightshade | 15 |
| Yangtao actinidia root | 15 |
| Cardamon fruit | 15 |
| Pubescent holly root | 15 |
| Ligusticum chuanxiong Hort | 12 |
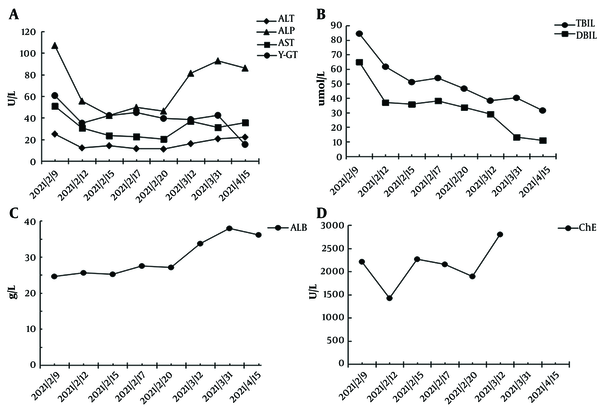
After admission to our hospital, routine blood tests showed decreased white blood cells (3.12 × 109/L) and platelet count (93 × 109/L), and an elevated international normalized ratio (INR 1.56). Liver function tests revealed elevated aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), total bilirubin (TBIL), and γ-glutamyl transpeptidase (γ-GT) levels, and decreased serum albumin (ALB) and cholinesterase (ChE) levels (Figure 2). With a suspicion for liver cirrhosis, we measured the serum biomarkers of hepatic fibrosis including hyaluronic acid, procollagen type III, laminin, and collagen type IV subsequently. The levels of these markers were significantly elevated, with hyaluronic acid at 1266.64 ng/mL from a normal value (NV) of 0 - 120 ng/mL, procollagen type III at 33.49 ng/mL (NV: 0 - 15 ng/mL), laminin at 302.39 ng/mL (NV: 0 - 130 ng/mL), and collagen type IV at 279.06 ng/mL (NV: 0 - 95 ng/mL). To rule out other etiologies of cirrhosis, serological tests for antibodies specific for hepatitis A, B, C, and E, cytomegalovirus, and Epstein–Barr were performed, with negative results. Autoimmune antibody spectrum tests including anti-smooth muscle antibody, anti-mitochondrial and anti-liver kidney microsome antibodies, anti-liver cytosol type 1 antibody, and anti-soluble liver antigen antibodies displayed negative results. However, anti-nuclear antibody, with elevated levels at 115.12 U/mL (NV: < 40 U/mL), and SSA/Ro60KD were positive based on our test.
Graphical illustration of serological indicators over time. A, Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), γ-glutamyl transpeptidase (γ-GT), B, serum bilirubin; C, serum albumin (ALB); D, cholinesterase (ChE).
An enhanced CT of the patient revealed liver cirrhosis with narrow liver volume, uneven surface of the liver, broadening of the hepatic crack, and presence of ascites (Figure 1D - F). We cross-checked this with the CT image before the operation and noted no sign of cirrhosis in the first scan done (Figure 1A - C). Subsequent gastroscopy also revealed mild esophageal varices (Figure 1G).
We diagnosed this patient with CHM-induced liver cirrhosis (Child-Pugh C) confirmed by clinical factors including serological indicators (cytopenia, coagulation dysfunction, impaired liver function, and elevated biomarkers of liver fibrosis), radiographic liver morphology, and evidence of portal hypertension (ascites and esophageal varices), while other causes of cirrhosis were ruled out. Therefore, we discontinued CHM and prescribed the patient with compound glycyrrhizin and acetylcysteine for liver protection, and an albumin infusion and diuretics for reduction of edema. The AST, ALT, and TBIL levels decreased significantly 3 days after the treatment, and the laboratory examination improved gradually over the following 2 weeks (Figure 2). The patient was discharged from the hospital on February 23, 2021, with prescribed home medications of oral Yinzhihuang and ursodeoxycholic acid. Seven weeks after discharge, the TBIL decreased to 31.5 umol/L, ALT to 22.1 U/L, and AST to 35.5 U/L, and ALB resumed to normal levels of 36.12 g/L (Figure 2); however, anti-nuclear antibody (at elevated levels of 128.5 U/mL; NV < 40 U/mL) and SSA/Ro60KD were still positive.
3. Discussion
It is regrettable that we were not able to obtain the liver pathology results of this patient. The poor coagulation function and massive ascites were high-risk factors for liver biopsy. Moreover, the patient refused the biopsy. Although liver biopsy remains the current gold standard for quantifying and staging liver disease, it is invasive and has risks of morbidity and mortality (6). In addition, a biopsy is a cross-sectional assessment of liver fibrosis and presents difficulty in assessing the liver vertically. Noninvasive tests may be more closely associated with fibrosis staging, with precision as good as liver biopsy (7). We diagnosed this case of CHM-induced DILI as "highly probable" with the score of 10 using the Roussel Uclaf Causality Assessment Method scale (8), whereas, using the Maria and Victorino scale, we diagnosed it as "possible" with the score of 12 (9). The association was not as strong for the Maria and Victorino scale because we were not able to include a “time from withdrawal of the drug until normalization of laboratory values” score of 3, which warranted a follow-up after 6 months.
The incidence of herbal drug-induced liver injury (HILI) is unknown due to the lack of a demography-based survey and difficulty in identifying the medications used. The DILI percentage for HILI varied from 26 to 81%, based on different reports (10, 11). Compared with Western medicines, liver damage caused by CHM develops slowly, with clinical symptoms appearing within one week to one month. The clinical manifestations vary from fatigue (67.3%), jaundice (60.3%), anorexia (58.0%), nausea (35.9%), and fever (19.3%); other symptoms have also been reported, such as rash, pruritus, and clay-colored stools (12).
The pathogenesis of CHM-induced liver injury is not well understood. Some CHMs contain toxic metabolites that can directly cause liver damage. The occurrence and severity of this type of liver injury are positively correlated with drug dosage within a short incubation time, coexisting with injury to other organs (13). Some herbal medicines contain toxic minerals, such as mercury, arsenic, and lead. Radix bupleuri, Poria cocos, air potato rhizome, and selfheal fruit-spike were active ingredients in the previous CHM of this patient, which contained hepatotoxic components. In addition, many cases have reported that liver injury caused by CHM has autoimmune features, such as the presence of autoantibodies as well as a pronounced hepatic infiltration of immune-competent cells (14). Hepatocytes are capable of resolving the herbs into drug-protein adducts to stimulate CD4 and CD8 lymphocytes and natural killer cell response as antigens (15, 16). Although anti-nuclear and SSA/Ro60KD antibodies were present in this patient, there were no clinical manifestations of autoimmune diseases such as dryness of the mouth and eye, arthralgia, and rash. There is a possibility that CHM-induced autoantibodies accelerate cirrhosis progression.
The patient developed liver cirrhosis as a result of oral CHM intake within 18 months, a much shorter duration than the decades required for viral infection-induced cirrhosis. Moreover, the severity of liver injury caused by CHM is even more serious than that caused by chemotherapy, and many patients with advanced cancer have maintained chemotherapy for more than two years. To the best of our knowledge, there have been no reports of chemotherapy-induced cirrhosis.
In summary, we report a case of CHM-induced liver cirrhosis.
References
-
1.
Wu C, Li M, Meng H, Liu Y, Niu W, Zhou Y, et al. Analysis of status and countermeasures of cancer incidence and mortality in China. Sci China Life Sci. 2019;62(5):640-7. [PubMed ID: 30900169]. https://doi.org/10.1007/s11427-018-9461-5.
-
2.
Gorey JD, Wahlqvist ML, Boyce NW. Adverse reaction to a Chinese herbal remedy. Med J Aust. 1992;157(7):484-6. [PubMed ID: 1406399]. https://doi.org/10.5694/j.1326-5377.1992.tb137315.x.
-
3.
Shen T, Liu Y, Shang J, Xie Q, Li J, Yan M, et al. Incidence and Etiology of Drug-Induced Liver Injury in Mainland China. Gastroenterology. 2019;156(8):2230-2241 e11. [PubMed ID: 30742832]. https://doi.org/10.1053/j.gastro.2019.02.002.
-
4.
Westbrook RH, Dusheiko G. Natural history of hepatitis C. J Hepatol. 2014;61(1 Suppl):S58-68. [PubMed ID: 25443346]. https://doi.org/10.1016/j.jhep.2014.07.012.
-
5.
Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130(3):678-86. [PubMed ID: 16530509]. https://doi.org/10.1053/j.gastro.2005.11.016.
-
6.
Karanjia RN, Crossey MM, Cox IJ, Fye HK, Njie R, Goldin RD, et al. Hepatic steatosis and fibrosis: Non-invasive assessment. World J Gastroenterol. 2016;22(45):9880-97. [PubMed ID: 28018096]. [PubMed Central ID: PMC5143756]. https://doi.org/10.3748/wjg.v22.i45.9880.
-
7.
Lurie Y, Webb M, Cytter-Kuint R, Shteingart S, Lederkremer GZ. Non-invasive diagnosis of liver fibrosis and cirrhosis. World J Gastroenterol. 2015;21(41):11567-83. [PubMed ID: 26556987]. [PubMed Central ID: PMC4631961]. https://doi.org/10.3748/wjg.v21.i41.11567.
-
8.
Danan G, Benichou C. Causality assessment of adverse reactions to drugs--I. A novel method based on the conclusions of international consensus meetings: Application to drug-induced liver injuries. J Clin Epidemiol. 1993;46(11):1323-30. [PubMed ID: 8229110]. https://doi.org/10.1016/0895-4356(93)90101-6.
-
9.
Maria VA, Victorino RM. Development and validation of a clinical scale for the diagnosis of drug-induced hepatitis. Hepatology. 1997;26(3):664-9. [PubMed ID: 9303497]. https://doi.org/10.1002/hep.510260319.
-
10.
Wong MCS, Huang JLW, George J, Huang J, Leung C, Eslam M, et al. The changing epidemiology of liver diseases in the Asia-Pacific region. Nat Rev Gastroenterol Hepatol. 2019;16(1):57-73. [PubMed ID: 30158570]. https://doi.org/10.1038/s41575-018-0055-0.
-
11.
Wang R, Qi X, Yoshida EM, Mendez-Sanchez N, Teschke R, Sun M, et al. Clinical characteristics and outcomes of traditional Chinese medicine-induced liver injury: A systematic review. Expert Rev Gastroenterol Hepatol. 2018;12(4):425-34. [PubMed ID: 29323538]. https://doi.org/10.1080/17474124.2018.1427581.
-
12.
Ma X, Peng JH, Hu YY. Chinese Herbal Medicine-induced Liver Injury. J Clin Transl Hepatol. 2014;2(3):170-5. [PubMed ID: 26355537]. [PubMed Central ID: PMC4521244]. https://doi.org/10.14218/JCTH.2014.00009.
-
13.
Wang J, Zhou C. [Research progress of Chinese herbal medicine and traditional Chinese medicine resulting in liver injury]. Zhongguo Zhong Yao Za Zhi. 2011;36(23):3371-4. Chinese. [PubMed ID: 22393753].
-
14.
Sebode M, Schulz L, Lohse AW. "Autoimmune(-Like)" Drug and Herb Induced Liver Injury: New Insights into Molecular Pathogenesis. Int J Mol Sci. 2017;18(9). [PubMed ID: 28895915]. [PubMed Central ID: PMC5618603]. https://doi.org/10.3390/ijms18091954.
-
15.
Ortega-Alonso A, Andrade RJ. Chronic liver injury induced by drugs and toxins. J Dig Dis. 2018;19(9):514-21. [PubMed ID: 29808546]. https://doi.org/10.1111/1751-2980.12612.
-
16.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J Hepatol. 2015;63(4):971-1004. [PubMed ID: 26341719]. https://doi.org/10.1016/j.jhep.2015.06.030.