Abstract
Background:
Golgi membrane glycoprotein 73 (GP73) serum level is a potential biomarker for diagnosing significant fibrosis and cirrhosis in chronic liver diseases.Objectives:
The current study aimed to evaluate the accuracy of GP73 serum levels as a biomarker in the diagnosis of significant liver fibrosis in patients with hepatitis B virus (HBV). A new promising algorithm was developed by combining LSM and GP73 to predict significant liver fibrosis.Methods:
Information on the following parameters were obtained from 165 patients with HBV: liver stiffness measurement (LSM), serum GP73 level, and some other fibrosis criteria approved for clinical practice. The area under the curve (AUC) and sensitivity and specificity of GP73 were compared with LSM, aminotransferase-to-platelet ratio index (APRI), and 4-factor based fibrosis index (FIB-4) for diagnosing significant fibrosis.Results:
Compared to the non-significant liver fibrosis patients, the HBV infected patients with significant fibrosis showed a higher level of serum GP73 [64.05 (24.41 - 144.39) versus 91.30 (31.81 - 200.05) ng/mL, P < 0.001]. Concerning significant fibrosis diagnosis, GP73 exhibited advantages regarding the AUC (0.702), sensitivity (69.3%), and specificity (66.0%). Besides, GP73 did not show any advantage over LSM and APRI, but it had a better performance than FIB-4 in significant fibrosis detection. For the newly developed algorithm combining GP73 with LSM, the AUC, sensitivity, and specificity were 0.848, 77.4%, and 83.5%, respectively; hence, it's superior to LSM (0.832, 72.6%, and 83.5%, respectively; P = 0.016) for diagnosing significant fibrosis.Conclusions:
This study demonstrated that GP73 can be considered as a new effective biomarker for diagnosing liver fibrosis. The accuracy of significant fibrosis detection in patients with HBV infection can be improved by the new algorithm that contains GP73 and LSM.Keywords
GP73 Chronic Hepatitis B Sensitivity Specificity Significant Fibrosis Liver Stiffness Measurement
1. Background
Accurate diagnostic assessment is a key step in the clinical management of liver fibrosis and preventing long-term consequences. Besides, it can provide valuable information to decide whether the antiviral treatment should be administrated in patients infected with chronic hepatitis B virus (HBV) or not. Significant fibrosis is a key criterion for diagnosing liver cirrhosis during the development of chronic HBV infection. According to the national Chinese guidelines, serum HBV DNA in patients with significant fibrosis (F ≥ 2) is a major indication for antiviral treatment of patients with chronic hepatitis B (CHB) virus infection (1, 2). Therefore, accurate diagnosis of significant hepatic fibrosis is key for making therapeutic decisions.
There are semi-quantitative scoring systems for organizing liver biopsy in clinical practices, including Metavir or Ishak, which are the gold standard for evaluating the degree of hepatic fibrosis. Regarding the post-procedure complications and invasive nature of the liver biopsy, its application in clinical practice is limited (3). Besides, because of the sampling bias and the variability of intra/inter-observer in liver biopsy, the cirrhosis and fibrosis of the liver may be neglected or underestimated (4). Given the drawbacks of liver biopsy, researchers are interested in developing and investigating non-invasive methods that combine several biochemical parameters to assess fibrosis stages, and progress has been achieved, such as the aspartate aminotransferase-to-platelet ratio index (APRI), the 4-factor based fibrosis index (FIB-4), and Fibro Test.
Recently, as a rapid and reproducible method, named liver stiffness measurement (LSM), assessed by transient elastography, has been introduced by guidelines on managing chronic HBV and HCV infections, based on its non-invasive merit in detecting liver fibrosis (5). It worth noting that several confounding factors (e.g., age, alanine aminotransferase (ALT), body mass index (BMI), and the interpretation of LSM values) may affect the final diagnosis (6-9). Several algorithms are proposed to increase the diagnostic accuracy in patients with significant fibrosis by improving LSM in combination with other serum biomarkers (10-13).
Golgi membrane glycoprotein 73 (GP73), a type II Golgi transmembrane protein (14), is reported as a promising serum biomarker for the primary diagnosis of hepatocellular carcinoma (HCC). Subsequent studies reported that patients with chronic liver disease have increased levels of serum GP73, which also causes inflammation, liver fibrosis, and other liver disease progression. Evidence provided during the last decade support the feasibility of GP73 serum level as an effective biomarker for diagnosing significant fibrosis and cirrhosis in patients with chronic liver disease (15). In the study by Cao et al., serum GP73 has been proposed as an accurate and promising marker for significant fibrosis detection in patients with CHB (16). However, future studies are needed to extend our knowledge about the diagnostic accuracy of serum GP73 in diagnosing significant fibrosis in patients with CHB.
2. Objectives
To provide evidence for the application of serum GP73 level in clinical practice, the current study, firstly, assessed the diagnostic accuracy of significant liver fibrosis in CHB patients, and, secondly, an improved algorithm, which contained combined LSM and GP73, was developed to predict significant liver fibrosis.
3. Methods
3.1. Study Population
All adult patients with CHB who received percutaneous liver biopsy at West China hospital from June 2017 to October 2018 were included in the present study. Before including patients, they were systematically examined by clinicians against the following inclusion criteria: 1- aged between 18 - 65 years; 2- presence of serum HBsAg (≥ 6 months); and 3- confirmed diagnosis of fibrosis according to histological assessment and availability of the results, and not receiving anti-HBV treatment. The exclusion criteria were as follows: 1- chronic liver diseases associated with other infections, including HCV, HDV, or HIV co-infection; 2- alcohol consumption (≥ 40 g/day); 3- other liver diseases, such as nonalcoholic steatohepatitis, primary biliary cirrhosis, autoimmune hepatitis, decompensated liver cirrhosis, and drug-induced hepatitis. The recruitment progress is shown in Figure 1.
Flowchart for patient recruitment
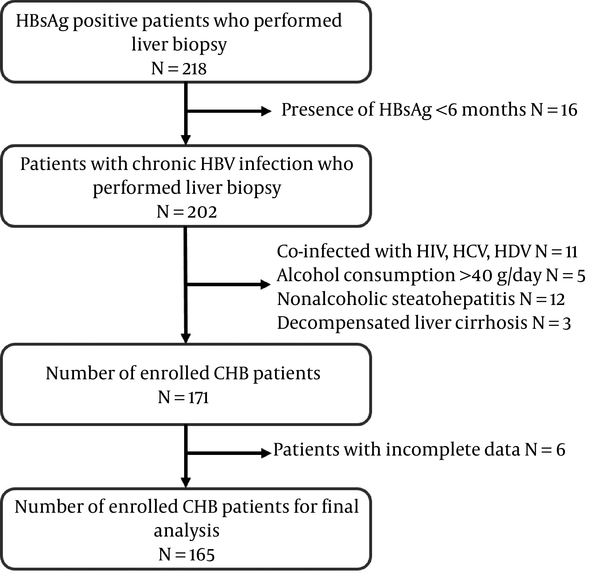
Eighteen CHB patients undergoing a 4-years NA treatment were included. FibroScan was performed at the entry and the 4th year. Of 18 patients who were receiving NA treatment, 2 were receiving a single oral daily dose of 600 mg telbivudine (LDT), and the other 16 were receiving 10 mg adefovir dipivoxil (ADV) in addition to daily LDT. The inclusion and exclusion criteria were the same as before.
Information on gender, age, and past medical history were collected for all patients. The biochemical parameters were also assessed, including aspartate transaminase (AST), alanine transaminase (ALT), albumin/globulin (A/G), γ-glutamyl-transpeptidase (γ-GT), direct bilirubin (DBIL), total bilirubin (TBIL), platelet count (PLT), and prothrombin time (PT). HBV biomarkers were detected using enzyme-linked immunosorbent assays (ELISA). Using a quantitative PCR assay, the serum HBV DNA quantification was performed. The serum GP73 level was detected by the ADICON diagnostics laboratory, according to the standard protocol of the ELISA test provided by the manufacturer (Hotgen Biotech, Beijing, China).
The liver biopsy and LSM were performed one month prior to being enrolled in the present study, and blood samples were collected for lab tests 15 days after registration. All examinations were performed before HBV management. All patients were explained about the process of the study and signed an official informed consent before participating. The West China hospital ethics committee approved the current study, and all processes were conducted according to the Declaration of Helsinki 1975 and its subsequent amendments.
3.2. Calculation of APRI and FIB-4
FIB-4 and APRI valued were calculated using the following formula (17, 18):
3.3. Liver Stiffness Measurement
Fibroscan (FS) was used to perform LSM, as described previously. To calculate the median value for each patient, at least 10 valid measurements were considered. Measurements with a rate of success ≤ of 60% or an interquartile range ≤ of 30% were considered failures and therefore excluded. The median LSM value obtained by two independent operators was calculated and recorded in kilopascal (kPa).
3.4. Liver Biopsy
The 16-G needle (1620; Bard, Murray Hill, United States) was used to perform the percutaneous liver biopsy. Liver diseases specialized pathologists, who were blinded to all other clinical information, performed the liver histology assessments. The specimen (≥ 15 mm) containing no less than 6 portal tracts was considered satisfactory. The METAVIR scoring system was used to perform the semi-quantitative evaluation of liver fibrosis according to the criteria.
3.5. Statistical Analysis
Data were analyzed using SPSS V17.0 (Chicago, United States) and MedCalc. Mann-Whitney test or student t-test was used for continuous variables comparison. MedCalc statistical software was used to perform the DeLong test for diagnostic performance evaluation and the areas under the receiver operating characteristic (ROC) curves were calculated. An optimal cut-off value was assessed according to the maximized Youden’s index. Diagnostic accuracy was assessed according to specificity, sensitivity, the area under the curve (AUC), and Youden’s index. All statistical results were obtained two-sided. Statistical significance was considered when P-Value < 0.05.
4. Results
4.1. CHB Patients with Significant Fibrosis Exhibited Higher Serum GP73 Level
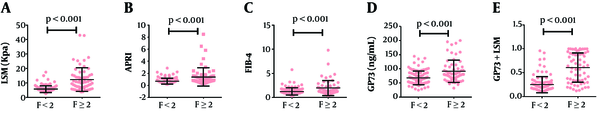
A total of 165 CHB patients (45 female cases) from West China Hospital were enrolled in the present study, and the serum levels of GP73, AST, ALT, PLT, INR, ALB, HBV DNA, HBeAg, FIB-4, LSM, and APRI were examined. Clinical characteristics and basic information of participants are provided in Table 1. According to the results of the METAVIR fibrosis stage, patients were separated into two groups: 103 cases (62.42%) were assigned to F0-F1 (no significant fibrosis), and 62 cases (37.58%) to F ≥ 2 (significant fibrosis). The median LSM was 5.20 kPa (range from 2.70 to 17.50 kPa) for no significant fibrosis and 10.15 kPa (range from 3.80 to 43.00 kPa) for significant fibrosis (Figure 2A). In CHB patients, the median GP73 level was 64.05 (ranged from 24.41 to 144.39) for those with no significant fibrosis (F0-F1) and 91.30 (ranged from 31.81 to 200.05) ng/mL for those with significant fibrosis (F ≥ 2) (P < 0.001) (Figure 2D). With the progression of fibrosis, serum GP73 levels increased in patients with CHB. Besides, coincident with GP73, the median values of APRI and FIB-4 also presented an upward trend with the fibrosis progression (Figures 2B and 2C).
| F < 2 (N = 103) | F ≥ 2 (N = 62) | Total (N = 165) | P-Value b | |
|---|---|---|---|---|
| Male/female | 69/34 | 51/11 | 120/45 | 0.033 |
| Age (y) | 32.66 ± 6.47 | 34.30 ± 10.51 | 33.33 ± 8.34 | < 0.001 |
| ALT (IU/L) | 38.5 (7 - 422) | 58 (15 - 584) | 45 (7 - 584) | < 0.001 |
| AST (IU/L) | 29.5 (12 - 155) | 47 (17 - 309) | 35 (12 - 309) | < 0.001 |
| Albumin (g/L) | 46.33 ± 3.03 | 45.94 ± 4.29 | 46.18 ± 3.55 | 0.005 |
| Total bilirubin (Umol/L) | 14.80 (3.70 -51.30) | 15.90(5.20-58.00) | 15.40(3.70-58.00) | <0.001 |
| INR | 0.94 ± 0.09 | 1.02 ± 0.09 | 0.97 ± 0.096 | < 0.001 |
| Platelet (109/L) | 152.06 ± 42.33 | 144.06 ± 53.12 | 149.05 ± 46.68 | 0.352 |
| E antigen positive (%) | 74 (71.84) | 33 (53.22) | 107 (64.84) | 0.126 |
| HBV DNA, Log10 copies/mL | 7.42 (2.70 - 9.96) | 5.54 (2.09 - 9.72) | 7.15 (2.09 - 9.96) | 0.012 |
| FIB-4 index | 1.05 (0.30 - 5.76) | 1.50 (0.37 - 9.80) | 1.23 (0.30 - 9.80) | < 0.001 |
| APRI | 0.52 (0.20 - 2.87) | 0.90 (0.23 - 8.49) | 0.65 (0.20 - 8.49) | < 0.001 |
| GP73 (ng/mL) | 64.05 (24.41 - 144.39) | 91.30 (31.81 - 200.05) | 68.58 (24.41 - 200.05) | < 0.001 |
| LSM (kPa) | 5.20 (2.70 - 17.50) | 10.15 (3.80 - 43.00) | 5.90 (2.70 - 43.00) | <0.001 |
Scatterplots of the data obtained with LSM, APRI, FIB-4, GP73, and LSM-GP73 against the fibrosis score; The LSM, APRI, FIB-4, GP73, and LSM-GP73 were higher in CHB patients with significant fibrosis (P < 0.001); Lines in scatterplots represent the median values of each parameter with 95% confidence interval (95% CI) bars.
4.2. Role of GP73 for Significant Fibrosis Prediction in CHB Patients
The AUC of GP73 in CHB patients was analyzed using receiver-operating characteristic, which was 0.702 for significant fibrosis (Figure 3 and Table 2). To diagnose significant fibrosis in CHB patients, the specificity and sensitivity of GP73 were 66.0% and 69.3%, respectively. The results did not show any advantage over LSM (AUC: 0.832, specificity: 83.5%, sensitivity: 72.6%) and APRI (AUC: 0.736, specificity: 55.3%, sensitivity: 83.9%). However, GP73 exhibited better performance compared to FIB-4 (AUC: 0.692, specificity: 65.1%, sensitivity: 67.7%) in the detection of significant fibrosis (Figure 3 and Table 2).
Comparison of diagnostic performance of LSM, APRI, FIB-4, GP73, and LSM-GP73. ROC analyses of LSM, APRI, FIB-4, GP73, and LSM-GP73 for diagnosis of significant fibrosis in CHB patients (F0-1 vs. F2-4).
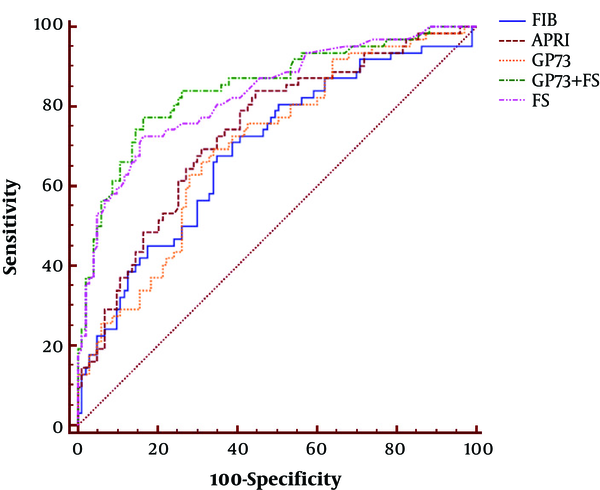
Diagnostic Performance of Fibrosis Markers in the Prediction of Significant Fibrosis (F ≥ 2) for CHB Patients
| AUC (95% CI) | Cut-Off | Se (95 % CI) | Sp(95% CI) | PPV (95% CI) | NPV (95% CI) | LR+ (95% CI) | LR-(95% CI) | Youden’s Index | |
|---|---|---|---|---|---|---|---|---|---|
| LSM (kPa) | 0.832 (0.766 - 0.885) | 6.8 | 72.6 (61.5 - 84.5) | 83.5 (74.9 - 90.1) | 72.(60.2 - 83.5) | 83. (75.7 - 90.8) | 4.4 (3.8 - 5.3) | 0.33 (0.2 - 0.6) | 0.56 |
| GP73 (ng/mL) | 0.702 (0.626 - 0.771) | 69.2 | 69.3 (56.3 - 80.4) | 66.0 (56.0 - 75.1) | 55.1 (43.4 - 66.5) | 78.2 (68.0 - 86.3) | 2.04 (1.6 - 2.5) | 0.46 (0.3 - 0.7) | 0.35 |
| FIB-4 | 0.692 (0.616 - 0.761) | 1.25 | 67.7 (54.7 - 79.1) | 65.1 (55.0 - 74.2) | 53.8 (42.1 - 65.3) | 77.0 (66.8 - 85.4) | 1.94 (1.6 - 2.4) | 0.50 (0.3 - 0.8) | 0.33 |
| APRI | 0.736 (0.662 - 0.802) | 0.55 | 83.9 (72.3 - 92.0) | 55.3 (45.2 - 65.1) | 53.1 (42.7 - 63.3) | 85.1 (74.2 - 92.7) | 1.88 (1.5 - 2.3) | 0.29 (0.2 - 0.5) | 0.39 |
| LSM + GP73 | 0.848 (0.784 - 0.899) | 0.31 | 77.4 (63.3 - 85.8) | 83.5 (74.9 - 90.1) | 73.8 (60.9 - 83.7) | 86.0 (76.7 - 91.4) | 4.69 (3.9 - 5.4) | 0.67 (0.2 - 0.8) | 0.61 |
4.3. GP73 and LSM Combined Model in Significant Fibrosis Prediction
By modeling the values of the serum GP73 and LSM, an algorithm in the combination of the two variables was constructed to predict significant fibrosis, using the following formula:
The AUC, specificity, and the sensitivity of the newly constructed model were, respectively, 0.848, 83.5%, and 77.4% for diagnosing significant fibrosis (Table 2), which was better than LSM in the diagnosis of significant fibrosis (P = 0.016). Using the improved algorithm, estimating the liver fibrosis level in CHB patients during the process of liver fibrosis development becomes possible.
4.4. Longitudinal GP73 and LSM Values 4 Years After the Start of Nucleoside Analog Treatment
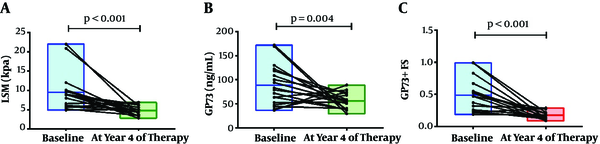
A longitudinal study was also recommended to evaluate the ability of the GP73-LSM algorithm to monitor dynamic changes in the fibrosis stages. We observed that the GP73-LSM algorithm significantly improved after 4 years of NA treatment. At baseline, the median LSM value was 8.85 (ranged from 4.9 to 22.0) kPa. After 4 years of NA treatment, the median LSM value was 4.7 (ranged from 2.8 to 6.9) kPa. LSM of CHB patients significantly improved after 4 years of NA treatment (P < 0.001). At baseline and 4 years after NA treatment, the median GP73 values were 80.91 (ranged from 36.58 to 171.85) ng/mL and 55.11 (ranged from 29.56 to 88.95) ng/mL, respectively (P = 0.004). Similarly, the predictive model based on serum GP73 level and LSM were 0.47 (ranged from 0.19 to 0.99) and 0.16 (ranged from 0.08 to 0.29) at baseline and year 4, respectively (P < 0.001). Both serum GP73 and the predictive model significantly improved after 4 years of NA treatment, which was coincident with the trend of LSM (Figure 4).
Longitudinal LSM values, GP73, and LSM-GP73 4 years after the start of nucleoside analog treatment. Each line connecting two plots represents an individual case before and after the nucleoside analog treatment. Floating bars represent the max and min values with the median lines.
5. Discussion
In the current study, CHB patients with significant fibrosis exhibited a higher serum GP73 level compared to those without significant fibrosis. Besides, serum GP73 level was also detected to have a positive correlation with the fibrosis progression in CHB patients. These results indicate that elevated serum GP73 can be an independent factor of significant hepatic fibrosis detection in CHB patients. Hence, serum GP73 can be considered as a promising biomarker for significant fibrosis diagnosis. In the study by Cao et al., serum GP73 exhibited good performance in diagnosing significant fibrosis (14, 16), which is consistent with the findings of the present study. In the current study, there was no advantage of serum GP73 compared to LSM and APRI, but it was superior to FIB-4 for the accuracy of significant fibrosis detection in CHB patients, which helped us to identify the significant fibrosis and monitor the fibrosis progression in CHB patients.
In the present study, an algorithm superior to LSM was developed, consisting of GP73 and LSM, in order to cover up the shortage of using LSM alone, which resulted in a practical model for predicting the incidence of significant fibrosis in CHB patients. By using easy to access factors of GP73 and LSM and having a simple formulation, the GP73-LSM based algorithm can be considered as a feasible clinical option. The modified algorithm in combination with serum GP73 and LSM can improve the accuracy of single LSM in detecting significant fibrosis in CHB patients. Hence, it can be suggested as a more accurate way to identify the antiviral treatments, which would have a brilliant prospect in future clinical practice.
We recommend performing a longitudinal study to evaluate the ability of the GP73-LSM algorithm in monitoring dynamic changes of the fibrosis stage. We observed that the GP73-LSM algorithm was statistically improved after a 4- year NAs treatment, which is coincident with the trends of LSM. Despite having some limitations, including a relatively small sample size, significant GP73-LSM algorithm changes were detected over different time points in the present study. However, both GP73 and the predictive model based on GP73 and LSM are useful in monitoring liver fibrosis during NAs treatment.
Serum GP73 can be detected by standard protocol with a commercially available ELISA kit. It costs approximately 8 USD for a single test of serum GP73 level in China, and it worth paying additional costs of an extra ELISA test considering the accuracy improvement of significant fibrosis prediction in CHB patients. Compared to the single LSM measurement, the combination of LSM and GP73 is more feasible, applicable, and cost-effective. Therefore, serum GP73 application is of great potential in clinical practice, especially in developing countries with limited non-invasive approaches.
Attention should also be paid to the limitations of the present study. Whether GP73 can be used as a fibrosis marker in other liver diseases still needs to be proven. In liver fibrosis caused by fatty liver disease, HCV infection, or other reasons, further investigations to detecting the feasibility of regarding GP73 as a biomarker are needed. The sensitivity and specificity to regard GP73 as a biomarker of liver fibrosis caused by other etiologies are yet to be detected. Besides, because of the relatively small number of HCC patients and healthy volunteers in this study, the predictive value of GP73 for HCC occurrence in CHB patients was limited.
5.1. Conclusions
In conclusion, this study demonstrated that GP73 can be regarded as a new biomarker to diagnose liver fibrosis effectively. Combining GP73 and LSM improved the accuracy of significant fibrosis detection in HBV infected patients. Further studies over longer periods with a larger size of populations will highly recommend examining the feasibility of GP73 as a biomarker to detect liver fibrosis caused by other etiologies.
References
-
1.
Hou JL, lai W; Chinese Society of Hepatology; Chinese Society of Infectious Diseases. [The guideline of prevention and treatment for chronic hepatitis B: a 2015 update]. Zhonghua Gan Zang Bing Za Zhi. 2015;23(12):888-905. Chinese. [PubMed ID: 26739464]. https://doi.org/10.3760/cma.j.issn.1007-3418.2015.12.002.
-
2.
WHO. Guidelines for the prevention, care and treatment of persons with chronic hepatitis b infection. Geneva: World Health Organization; 2015.
-
3.
Castera L, Negre I, Samii K, Buffet C. Pain experienced during percutaneous liver biopsy. Hepatology. 1999;30(6):1529-30. [PubMed ID: 10610352]. https://doi.org/10.1002/hep.510300624.
-
4.
Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449-57. [PubMed ID: 14647056]. https://doi.org/10.1016/j.hep.2003.09.022.
-
5.
Sarin SK, Kumar M, Lau GK, Abbas Z, Chan HL, Chen CJ, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int. 2016;10(1):1-98. [PubMed ID: 26563120]. [PubMed Central ID: PMC4722087]. https://doi.org/10.1007/s12072-015-9675-4.
-
6.
Yan LB, Zhu X, Bai L, Liang LB, Chen EQ, Du LY, et al. Impact of mild to moderate elevations of alanine aminotransferase on liver stiffness measurement in chronic hepatitis B patients during antiviral therapy. Hepatol Res. 2013;43(2):185-91. [PubMed ID: 22978384]. https://doi.org/10.1111/j.1872-034X.2012.01068.x.
-
7.
Chen XB, Zhu X, Chen LY, Chen EQ, Tang H. [Accuracy of fibroscan for the diagnosis of liver fibrosis influenced by serum alanine aminotransferase levels in patients with chronic hepatitis B]. Zhonghua Gan Zang Bing Za Zhi. 2011;19(4):286-90. Chinese. [PubMed ID: 21586228]. https://doi.org/10.3760/cma.j.issn.1007-3418.2011.04.013.
-
8.
Liang XE, Chen YP, Zhang Q, Dai L, Zhu YF, Hou JL. Dynamic evaluation of liver stiffness measurement to improve diagnostic accuracy of liver cirrhosis in patients with chronic hepatitis B acute exacerbation. J Viral Hepat. 2011;18(12):884-91. [PubMed ID: 21062388]. https://doi.org/10.1111/j.1365-2893.2010.01389.x.
-
9.
Chen YP, Liang XE, Dai L, Zhang Q, Peng J, Zhu YF, et al. Improving transient elastography performance for detecting hepatitis B cirrhosis. Dig Liver Dis. 2012;44(1):61-6. [PubMed ID: 21907650]. https://doi.org/10.1016/j.dld.2011.08.004.
-
10.
Chen YP, Dai L, Wang JL, Zhu YF, Feng XR, Hou JL. Model consisting of ultrasonographic and simple blood indexes accurately identify compensated hepatitis B cirrhosis. J Gastroenterol Hepatol. 2008;23(8 Pt 1):1228-34. [PubMed ID: 18637057]. https://doi.org/10.1111/j.1440-1746.2008.05421.x.
-
11.
Liang XE, Dai L, Yang SL, Zhong CX, Peng J, Zhu YF, et al. Combining routine markers improves the accuracy of transient elastography for hepatitis B cirrhosis detection. Dig Liver Dis. 2016;48(5):512-8. [PubMed ID: 26965782]. https://doi.org/10.1016/j.dld.2016.02.002.
-
12.
Lee MH, Cheong JY, Um SH, Seo YS, Kim DJ, Hwang SG, et al. Comparison of surrogate serum markers and transient elastography (Fibroscan) for assessing cirrhosis in patients with chronic viral hepatitis. Dig Dis Sci. 2010;55(12):3552-60. [PubMed ID: 20428950]. https://doi.org/10.1007/s10620-010-1219-0.
-
13.
Yan LB, Zhang QB, Zhu X, He M, Tang H. Serum S100 calcium binding protein A4 improves the diagnostic accuracy of transient elastography for assessing liver fibrosis in hepatitis B. Clin Res Hepatol Gastroenterol. 2018;42(1):64-71. [PubMed ID: 28688902]. https://doi.org/10.1016/j.clinre.2017.05.013.
-
14.
Weiskirchen R, Tacke F. Combining GP73 with liver stiffness measurements: A proof-of-concept for non-invasive fibrosis assessment in antiviral-naive HBV patients. Liver Int. 2017;37(11):1605-7. [PubMed ID: 29065256]. https://doi.org/10.1111/liv.13563.
-
15.
Yao M, Wang L, Leung PSC, Li Y, Liu S, Wang L, et al. The clinical significance of GP73 in immunologically mediated chronic liver diseases: Experimental data and literature review. Clin Rev Allergy Immunol. 2018;54(2):282-94. [PubMed ID: 29256057]. https://doi.org/10.1007/s12016-017-8655-y.
-
16.
Cao Z, Li Z, Wang Y, Liu Y, Mo R, Ren P, et al. Assessment of serum Golgi protein 73 as a biomarker for the diagnosis of significant fibrosis in patients with chronic HBV infection. J Viral Hepat. 2017;24 Suppl 1:57-65. [PubMed ID: 29082644]. https://doi.org/10.1111/jvh.12786.
-
17.
Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46(1):32-6. [PubMed ID: 17567829]. https://doi.org/10.1002/hep.21669.
-
18.
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518-26. [PubMed ID: 12883497]. https://doi.org/10.1053/jhep.2003.50346.