Abstract
Background:
Herbal extracts have recently received the greatest attention in the path of finding naturally occurring chemicals with antibacterial and therapeutic value; however, each type of herbal remedy may have its own side effects.Objectives:
The aim of the current experiment was to study the antibacterial effect of myrtle, parsley, mint, henna and chamomile extracts on Escherichia coli and their effects on colony formation and survival of spermatogonial stem cells (SSCs).Materials and Methods:
Spermatogonial stem cells were isolated by two-time enzymatic digestion from slaughterhouse origin ovine testis and plant extraction by deionized water. Comparisons between different treatments were performed using analysis of variance (ANOVA) followed by Duncan’s multiple range tests.Results:
The results showed that there was no significant difference between mint, henna and penicillin, on inhibition of Escherichia coli growth, however parsley, myrtle and chamomile were significantly different from penicillin (P < 0.05). The formation of SSC colonies was not affected by different herbal medicines. Myrtle and chamomile resulted in death of SSC colonies compared with the control, and the survival of spermatogonial and Sertoli cells were significantly affected by myrtle, mint, chamomile and parsley (P < 0.05).Conclusions:
The results of these experiments provide evidence that henna by antibacterial activity had no detrimental affect on SSC and Sertoli cells and is a good candidate for substitution of antibiotics.Keywords
Henna Antibacterial Herbal Medicine Spermatogonial Stem Cell Escherichia coli
1. Background
Resistance to antibiotics is an increasing worldwide problem, and has implications for morbidity, mortality, and healthcare both in hospitals and in the community that has necessitated the search for novel and effective antimicrobial compounds (1, 2). Herbal extracts have recently received the greatest attention in the path of finding naturally occurring chemicals with therapeutic value (2).
Different ingredients including phenols, peptides, alkaloid components, unsaturated long chain aldehydes, chloroform, ethanol, methanol and butanol soluble constituents and some essential oils are responsible for the antimicrobial efficiency of herbal extracts. Therefore, they have potential therapeutic application against bacteria, fungi or virus (3).
Myrtle plant with scientific name of Lagerstroemia indica is an aromatic and medicinal herb for which antibacterial, antifungal, antiviral, antioxidant and anti-mutagenicity properties have been reported (4). It contains oligomeric non-prenylated acylphloroglucinols as unique antioxidant agents and also semi-myrtucommulone and myrtucommulone A antioxidants, which protect linoleic acid against free radical attack (5). Parsley with scientific name of Petroselinum crispum with ingredients such as essential oils, myristicin, limonene alpha-thujene and eugenol prevents of tumor formation (especially lung cancer). There are reports that indicate parsley stimulates the activity of glutathione S-transferase, which is responsible for the prevention of cellular damage (6, 7). In the watery and alcoholic extract of parsley there exists some antioxidant constituents such as ascorbic acid, tocopherol, phenolic compounds, flavonoids (apiin, luteolin and apigenin-glycosides) and essential oils (apiol and myristicin) (8). Mint plant with scientific name of Mentha piperita showed maximum total phenols and antioxidant activity in its leaves (9). Seven compounds were isolated from henna leaves with five from the chloroformic fraction including lawsone as well as two compounds from the ethyl acetate fraction (10). Besides these, other constituents were fats, glucose, gallic acid, mannitol, resin, mucilage and traces of an alkaloid. These compounds had many properties such as, antidiabetic activity, immunomodulatory effect, hepatoprotective activity, antioxidant effect, antibacterial, antifungal, antiviral, antitrypanosomal, antiparasitic, molluscicidal, tuberculostatic, cytotoxic and antifertility activity (11). Different classes of bioactive medicinal components are present in chamomile, as well as 0.24 – 1.9% volatile oil, composed of a variety of separate oils, and around 120 secondary metabolites, including 28 terpenoids and 36 flavonoids. Among the flavonoids, apigenin is the most promising compound, which is mainly present in the form of various glycosides and very small quantities as free apigenin. Other flavonoids are apigenin, luteolin, patuletin and quercetin. Also azulenesse, farnesene and spiro-ether sesquiterpene lactones, glycosides, hydroxycoumarins, coumarins (herniarin and umbelliferone), terpenoids, and mucilage are considered to be the major bioactive ingredients (12).
The process of generation of sperm is called spermatogenesis (13). Spermatogenesis is a highly organized process that originates from SSCs (14). This kind of stem cell is unipotent and responsible for the maintenance of spermatogenesis throughout the entire life of a male. They are the only germ line stem cells in adults and may choose self-renewal or generate a daughter cell committed to differentiation (15). These cells are biotechnologically important because they are the only cells in adult stem cell systems capable of transmitting genetic information to future generations (13).
Optimization for isolation, culture, and transplantation of SSCs methods has facilitated the development of clinical applications for preserving human male fertility (16). In this regard, antioxidants are compounds that help in preventing or delaying damage of cells and tissues by inhibiting many oxidation reactions caused by free radicals. However, the antioxidant activities of vegetables varied largely and their activity correlated with active compounds phytochemicals such as phenols, tannins, flavonoids etc. (17) The superiority of natural antioxidants has been proven over synthetic ones in terms of safety and tolerance, without any toxicity and side effects. Thus, they are essential for maintaining a good state of health in our body (18).
2. Objectives
The purpose of the current study was to investigate the properties of antibacterial plant-derived extracts for their ability to stimulate colony formation and survival of SSCs. For these reasons, myrtle, parsley, mint, henna and chamomile extracts were studied for their antibacterial and their negative effects on SSCs.
3. Materials and Methods
3.1. Chemicals
All the culture media, growth factors, fetal bovine serum (FBS) and other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and the plastic ware were purchased from Falcon (Paignton, UK) unless stated otherwise.
3.2. Herbal Extracts
The stems and leaves of parsley and mint and the leaves of myrtle, henna and chamomile flowers were provided. Dirt was removed by early washing and in the second stage, plants were washed with distilled water. In the third stage, plants were immersed in 20% ethanol. Then the plants were placed in the oven for one day for drying. Dried plants were ground and prepared for extraction. The aqueous extract was extracted with Soxhlet apparatus so that 40 grams of each plant powder was placed in bags. The dilute extracts were obtained and placed in an evaporator and after evaporating excess water at a temperature of 67°C, 40 grams of dense extracts was obtained from each plant. The samples were then centrifuged (3000 rpm, five minutes) to separate impurities and suspended solids, and the purified extract was obtained. For identifying the extract dry matters, we dried and weighted a part of them and according to its dry matter of every herbal extract was set at 20 mg/mL.
3.3. Bacterial Culture
Escherichia coli (PTCC 1399) was purchased from the Persian type culture collection (PTCC), IROST, Iran. Bacteria were cultured in broth medium and used for assays.
3.4. Zone Inhibition
The antibacterial assay was based on the standard agar diffusion assay, where one colony of microorganism was picked from a stock plate and suspended in deionized water. An aliquot of microorganism suspension was swabbed on brain heart infusion (BHI) (Cat No: 110493) agar plates and the diameter of the growth inhibition was measured.
3.5. Collection of Testes
Sheep testes were collected from Mysam slaughterhouse (Tehran) immediately after slaughter and washed three to four times with normal saline solution (32 - 37°C), containing 0.1% streptomycin sulfate (Cat No: S9137). The washed testes were then place in a sample collection bottle containing normal saline and antibiotics. The collected testes were transported to the laboratory within two hours of slaughter. In the laboratory, the testes were rinsed twice with normal saline and were then trimmed to remove tunica vaginalis and washed properly with warm saline containing antibiotics five to six times.
3.6. Isolation of Spermatogonial Stem Cells by Enzymatic Digestion
Spermatogonial stem cells were isolated by a two-time enzymatic digestion process, as described by Izadyar et al. (2002) with some modifications (14). Briefly, after washing with Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, Cat No: 14190-250), the tubular tissue was washed with Dulbecco’s modified eagle medium (DMEM) (Cat No: 14190-250). For the first enzymatic digestion, minced seminiferous tissue was suspended in DMEM containing 1 mg/mL collagenase (Cat No: C9891), 1 mg/mL hyaluronidase type II (Cat No: H2126), 5 μg/mL DNase (Cat No: DN25) and 1 mg/mL trypsin-EDTA (0.25%) (Gibco, Cat No: 25200-056) and was incubated at 37°C in an incubator at 200 cycles/minute for 45 minutes. After this step, the dispersed tissue was collected and subjected to centrifugation at 1000 rpm for two minutes. The supernatant was discarded and the tissue pellet was washed once with DMEM. For the second enzymatic digestion, the tissue was suspended in DMEM containing 1 mg/mL collagenase, 1 mg/mL hyaluronidase type II and 5 μg/mL DNase and was incubated in a shaking incubator operated at 200 cycles/minute for 30 minutes. The tissue was then centrifuged at 1000 rpm for two minutes and the supernatant was collected in a 15-mL tube. The dispersed cells, present in the supernatant were expected to contain SSCs, Sertoli cells, myeloid cells and other contaminating cells of the seminiferous tubular tissue. One day after the second enzymatic digestion, differential plating was performed. Differential plating consists of an overnight in vitro culture of freshly isolated SSCs followed by a subculture of only non-adherent cells. Stem cells tend to remain in the suspension, while supporting cells like Sertoli cells and other testicular cells adhere to the culture dish.
3.7. Feeder Layer Preparation and Culture of Spermatogonial Stem Cells
For the preparation of feeder layer, primary Sertoli cells were inactivated by treatment with 10 mg/mL mitomycin-C (Cat No: m4287) for two hours after which the monolayer was trypsinized and the cells were washed three to four times with DPBS + 10% FBS + 50 mg/mL gentamicin sulfate (Cat No: G1264). The feeder layer was prepared by seeding these harvested cells in four well plates/tissue culture dishes one day prior to the culture of SSCs.
3.8. Analysis of Spermatogonial Stem Cells Colonies and Cell Counting
The number of colonies on each four well plate was counted under inverted microscopy (CKX41; Olympus). Cells were counted 72 hours after the treatment. To achieve this, the cells were dyed with trypan blue (Cat No: T8154), which turns viable cells blue and the cell number was counted using a hemocytometer under a light microscope (Ti-U, Nikon, Tokyo, Japan).
3.9. Statistical Analysis
Each experiment was repeated at least three times. Data were analyzed with a statistical software program (SPSS 11.5, 2004, IBM, USA). Comparisons between datasets were performed using one-way ANOVA followed by Duncan multiple-range test. Results are expressed as mean ± standard error of the mean (SEM), and statistical significance was accepted at P <0.05.
4. Results
4.1. Antibacterial Effect of Herbal Medicine
In this experiment, the effect of five herbal extracts on Escherichia coli was studied. For this reason the zone of inhibition assay was tested for its ability to demonstrate the antibacterial activity of these extracts. The results indicated that there was no significant difference between mint, henna and penicillin, however the effect of parsley, myrtle and chamomile was significantly lower than penicillin on the growth of Escherichia coli (P < 0.05) (Figure 1). The results indicated that either henna or mint is a possible alternative to penicillin that could be used for control of Escherichia coli growth.
The Effect of Parsley (2 mg/Well), Myrtle (2 mg/Well), Chamomile (2mg/Well), Mint (2 mg/Well), Henna (2 mg/Well) and Penicillin (10 mg/mL) on Escherichia coli

4.2. Effect of Herbal Medicine on Spermatogonial Stem Cells Colony Formation
In order to optimize the SSC culture system, the effect of parsley, myrtle, chamomile, mint and henna on the formation of colonies was evaluated. Based on the MTT assay on fibroblast cells, the highest concentration of herbal extracts (0.2 mg/mL) that had no significant negative effect on the viability of fibroblast cells were used in this study. The results showed that, supplementing the SSC media with each of myrtle, chamomile or mint herbal extracts significantly decreased the formation of SSC colonies compared with the control (P > 0.05). However, the formations of the colonies were not affected by parsley or henna (Figure 2). Thus, none of five herbal extracts improved the formation of SSC colonies.
The Effect of Parsley, Myrtle, Chamomile, Mint and Henna (0.2 mg/mL) on SSC Colony Formation

4.3. Effect of Herbal Medicine on Survival of Spermatogonial Stem Cells Colonies
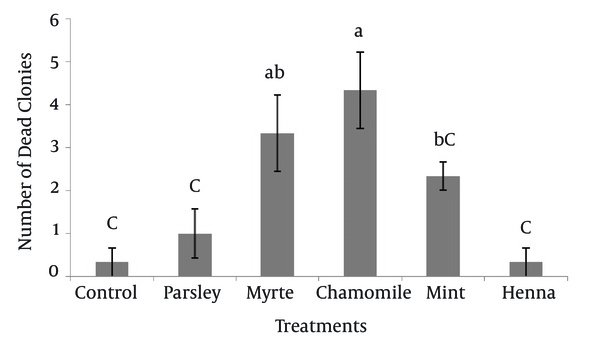
The numbers of dead SSC colonies in the presence of five herbal extracts are shown in Figure 3. The results indicated that, chamomile had the greatest effect (4.33 ± 0.88) on mortality of SSC colonies. The dead colonies of SSC in henna (0.33 ± 0.33) were significantly lower (P < 0.05) compared to myrtle and chamomile. Other results showed that there was no significant difference between myrtle with mint and myrtle with chamomile on survival of SSC colonies.
Effect of Parsley, Myrtle, Chamomile, Mint and Henna (0.2 mg/mL) on the Death of Spermatogonial Stem Cell Colonies
4.4. Effect of Herbal Medicine on Survival of Spermatogonial Stem Cells and Sertoli Cells
The results indicated that myrtle, mint, chamomile and parsley significantly increased the number of dead spermatogonial and Sertoli cells in comparison to henna and control groups (P < 0.05), and there was no significant difference between henna and the control (P > 0.05) (Figure 4).
Effect of Parsley, Myrtle, Chamomile, Mint and Henna (0.2 mg/mL) on the Number of Dead Spermatogonial Stem Cells and Sertoli Cells

5. Discussion
The emergence of multi-drug resistance in human and animal pathogenic microbes as well as undesirable side effects of certain antibiotics has triggered huge interest to study new antimicrobial drugs of plant origin. However, they have different mechanisms from antibiotics for inhibiting bacterial growth and also unknown effects on living cells; this makes additional researches on medicinal plants essential (19, 20).
This study aimed to investigate the effects of some antibacterial plants based on SSCs formation and survival. For these purposes, the antimicrobial activity was determined in the extracts using the agar disc diffusion method for Escherichia coli, after which the colony formation and survival of spermatogonial and Sertoli cells under different herbal treatments were investigated.
In agreement with our study, the antibacterial activity of henna and mint extract was reported by Malekzadeh and Shabestari (1989) and Davoodi et al. (2007), respectively (21, 22).
According to Cowan (1999), coumarins, flavonoids and tannin compounds are not responsible for antimicrobial activity of henna extracts and their activity is due to polyphenolic compounds. Lawsone, the major bioactive component of henna, is known for its antibacterial activity and possesses a wider spectrum of activity (19, 23). Also, phenols are responsible for the antimicrobial efficiency of mint (3, 9). However, there were contradictory reports about the antibacterial activity of medicinal plants (20, 24, 25). These discrepancies could be due to differences in the plant’s physiological state, seasonal variation, environmental condition, studied part of the plant, extraction procedure, concentration of crude extracts and strains of test bacteria (25).
The antibacterial effects of plant extracts and their side effects on different cell lines have been studied for years in the last three decades (25). However, to the best of our knowledge, a comparative study of antibacterial plant extracts on survival of SSCs has not been previously reported. The results of the current study revealed that henna resulted in less negative effects on SSC colonies and spermatogonial and Sertoli cells. Although none of the five herbs improved the formation of SSC colonies.
Chaudhary et al. reported that antioxidant activity in henna was higher when compared to vitamin E or tocopherol. The strong cytotoxic properties against cancerous cells could be due to its high antioxidant activities (11). The antioxidant activity of herbs is proportional with their phenolic components (9). Polyphenol content and correlation with antiradical activity was indicated by the protection of HepG2 (liver hepatocellular) cells from oxidative damage (26). Antioxidant mechanisms of polyphenolic compounds are based on hydrogen donation abilities. After donating a hydrogen atom, phenolic compounds become resonance-stabilized radicals, which do not easily participate in other radical reactions (27).
In conclusion, the results of this study provided evidence that henna by antibacterial activity had no detrimental affect on spermatogonial and Sertoli cells and is a good candidate for substitution of antibiotics.
Acknowledgements
References
-
1.
Mills-Robertson FC, Onyeka CI, Tay SCK, Walana W. In vitro antimicrobial activity of Antibact, an herbal medicinal product against standard and clinical bacterial isolates. J Med Plants Res. 2015;9(11):370-8.
-
2.
Jastaniah SD. The antimicrobial activity of some plant extracts, commonly used by Saudi people, against multidrug resistant bacteria. Life Sci J. 2014;11(8):78-84.
-
3.
Karimi F, Rezaei M, Shariatifar N, Sayadi M, Mohammadpourfard I, Malekabad E, et al. of Parsley (PetroselinumCrispum) Against FoodPathogenic Bacteria. World Appl Sci J. 2014;31(6):1147-50.
-
4.
Taheri A, Seyfan A, Jalalinezhad S, Nasery F. Antibacterial effect of Myrtus communis hydro-alcoholic extract on pathogenic bacteria. Zahedan J Res Med Sci. 2013;15(6):19-24.
-
5.
Rosa A, Deiana M, Casu V, Corona G, Appendino G, Bianchi F, et al. Antioxidant activity of oligomeric acylphloroglucinols from Myrtus communis L. Free Radic Res. 2003;37(9):1013-9. [PubMed ID: 14670009].
-
6.
Shan B, Cai YZ, Brooks JD, Corke H. The in vitro antibacterial activity of dietary spice and medicinal herb extracts. Int J Food Microbiol. 2007;117(1):112-9. [PubMed ID: 17449125]. https://doi.org/10.1016/j.ijfoodmicro.2007.03.003.
-
7.
Zhang H, Chen F, Wang X, Yao HY. Evaluation of antioxidant activity of parsley (Petroselinum crispum) essential oil and identification of its antioxidant constituents. Food Res Int. 2006;39(8):833-9. https://doi.org/10.1016/j.foodres.2006.03.007.
-
8.
Mahmood S, Hussain S, Malik F. Critique of medicinal conspicuousness of Parsley(Petroselinum crispum): a culinary herb of Mediterranean region. Pak J Pharm Sci. 2014;27(1):193-202. [PubMed ID: 24374449].
-
9.
Al-Juhaimi F, Ghafoor K. Total phenols and antioxidant activities of leaf and stem extracts from coriander, mint and parsley grown in Saudi Arabia. Pak J Bot. 2011;43(4).
-
10.
Mikhaeil BR, Badria FA, Maatooq GT, Amer MM. Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch C. 2004;59(7-8):468-76. [PubMed ID: 15813363].
-
11.
Chaudhary G, Goyal S, Poonia P. Lawsonia inermis Linnaeus: a phytopharmacological review. Int J Pharm Sci Drug Res. 2010;2(2):91-8.
-
12.
Srivastava JK, Shankar E, Gupta S. Chamomile: A herbal medicine of the past with bright future. Mol Med Rep. 2010;3(6):895-901. [PubMed ID: 21132119]. https://doi.org/10.3892/mmr.2010.377.
-
13.
Aponte PM, De Rooij DG. Biomanipulation of bovine spermatogonial stem cells. Anim Reprod. 2008;5(1):16-22.
-
14.
Izadyar F, Spierenberg GT, Creemers LB, Den Ouden K, De Rooij DG. Isolation and purification of type A spermatogonia from the bovine testis. Reprod. 2002;124(1):85-94.
-
15.
de Barros FRO, Visintin JA, Giassetti MI. Spermatogonial stem cells and animal transgenesis. INTECH Open Access Publisher; 2012.
-
16.
Kang HR, Lee YA, Kim YH, Lee DG, Kim BJ, Kim KJ, et al. Petasites japonicus Stimulates the Proliferation of Mouse Spermatogonial Stem Cells. PLoS One. 2015;10(7):e0133077. [PubMed ID: 26207817]. https://doi.org/10.1371/journal.pone.0133077.
-
17.
Salih NM, Bayar AH, Hindi MJ. Antioxidant Activity of Dietary Plants: Peppermint. Pakistan J Nutr. 2013;12(6):571.
-
18.
Ozkan AMG, Guray CG. A mediterranean: Myrtus communis L.(myrtle). Plant cult. 2009:159-68.
-
19.
Rahmoun MN, Benabdallah M, Villemin D, Boucherit K, Mostefa-Kara B, Ziani-Cherif C, et al. Antimicrobial screening of the Algerian Lawsonia inermis (henna). Pharm Chem. 2010;2(6):320-6.
-
20.
Tabari MA, RezaYoussefi M, Ghasemi F, Tabari RG, Esmaili RH, Behzadi MY. Comparison of antibacterial effects of Eucalyptus essence, mint essence and combination of them on Staphylococcus aureus and Escherichia coli isolates. World Appl Sci J. 2012;16(10):1473-7.
-
21.
Malekzadeh F, Shabestari PP. Therapeutic effects and in vitro activity of an extract from Lawsonia inermis. J Sci Islamic Repub Iran. 1989;1(1):7-12.
-
22.
Davoodi P, Sharifi B, Mohamadi S. Antimicrobial activity studies of (Mentha piperita L). J Sci IndRes. 2007;99:125-32.
-
23.
Cowan MM. Plant products as antimicrobial agents. Clin microbiol rev. 1999;12(4):564-82.
-
24.
Mahboubi M. Comparison of Respitol B containing mint and eucalyptus essence with Menthophin. Irainian J Microbiol. 2007;1:39-44.
-
25.
Oskay M, Oskay D, Kalyoncu F. Activity of some plant extracts against multi-drug resistant human pathogens. Iranian J Pharm Res. 2010;8(4):293-300.
-
26.
Gramza-Michałowska A, Abramowski Z, Jovel E, Hes M. Antioxidant potential of herbs extracts and impact on HepG2 cells viability. Acta Sci. Pol., Technol. Aliment. 2008;7(4):61-72.
-
27.
Zohourian TH, Quitain AT, Sasaki M, Goto M. Polyphenolic contents and antioxidant activities of Lawsonia inermis leaf extracts obtained by microwave-assisted hydrothermal method. J Microw Power Electromagn Energy. 2011;45(4):193-204. [PubMed ID: 24428109].