Abstract
Background:
Extracellular matrix (ECM) functions as a scaffold for tissue morphogenesis and regeneration, promotes the maintenance of differentiated tissue and plays an extremely indispensable role in cell proliferation and differentiation. As a corollary of this importance, studying the cell behaviors without considering the ECM is to some extent impossible. Deletion of cells from ECM obtains natural three dimensional matrices that ape the in vivo functions of ECM.Objectives:
The current study aimed to verify whether acellular dermal matrix (ADM) is a suitable 3D-matrix for blastema tissue cells originated from pinna of rabbit and study different behaviors of these cells on ADM.Materials and Methods:
To prepare ADM, small pieces of skin were decellularized by repeated snap freeze-thaw and treatment with sodium dodecyl sulfate as a detergent. Decellularization was verified and proved by histological tests. Blastema rings were prepared by double punching the pinnas of male New Zealand White rabbits and cultured in vitro after assembling with ADM. Specimens were studied after 5, 10, 15, 20 and 25 days of culturing.Results:
Migration of cells started on the fifth day. As a result of this migration, most cells were located on the surface of the scaffold and formed epiderm-like structure and some penetrated into the scaffold. By day 25, cells which had penetrated into the scaffold had various destinies such as becoming elongated spindle-shaped cells and creating blood vessel-like structures.Conclusions:
ADM can induce migration and probably differentiation of blastema tissue cells; therefore, it is a suitable 3D-model to study cell behaviors in vitro.Keywords
Acellular Dermal Matrix Blastema Tissue Extracellular Matrix Decellularization
1. Background
Extracellular matrix (ECM) is a complex, dynamic and critical constituent of all tissues. It performs as a scaffold for tissue morphogenesis, provides cues for cell proliferation and differentiation, raises the maintenance of differentiated tissue and increases the repair after injury (1).
One approach to design exogenous ECMs for tissue engineering is to mimic the function of ECM molecules naturally found in tissue. These native ECMs act as a scaffold to gather cells in a tissue, control the tissue structure and regulate the cell phenotype (2).
Elimination of cells from a tissue or an organ leaves the complex mixture of structural and functional proteins that constitute the ECM (3). Biologic scaffold derived from decellularized tissues and organs are successfully applied in both pre-clinical animal studies and in human clinical uses (4, 5).
To study cell behaviors in a 3-dimensional matrix, in addition to the use acellular dermal matrix (ADM) as a scaffold, the type of cell source is required. In previous cell behavior studies on ADM, different kinds of cells such as keratinocytes (6), adipose derived stem cells (7, 8) and fibroblasts (9, 10) were utilized. In the current study, blastema tissue from pinna of New Zealand White rabbit was used as a cell source. Rabbit blastema tissue is a collection of undifferentiated cells with embryonic-like properties that can regenerate damaged tissues pinnas and are able to convert to different cell types presented before injury (11). Such tissues are accessible 2 days after puncture of the New Zealand White rabbit pinnas (12).
2. Objectives
The current study aimed to verify whether acellular dermal matrix (ADM) is a suitable 3D-matrix for blastema tissue cells originated from pinna of rabbit and study different behaviors of these cells on ADM.
3. Materials and Methods
Six-month-old male New Zealand white rabbits (Oryctolagus cuniculus) purchased from Razi vaccine and serum research institute (RVSRI, Mashhad Iran) were used to obtain both ADM and blastema tissue. Animal experiments were performed according to the Iranian council for the use and care of animals guidelines approved by the animal research ethical committee of Ferdowsi university of Mashhad, Mashhad, Iran.
3.1. Preparation of the ADM Scaffolds
3.1.1. Decellularization Process
An autopsy performed on rabbit’s skin samples; in this pursuit, primarily the rabbit’s furs were removed, and the skin was isolated from the underlying muscle. In this study, various physical and chemical methods were utilized for cellular skin isolation. The physical method was slow freezing at - 4°C, and snap freeze-thaw in liquid nitrogen. The chemical method was utilizing sodium dodecyl sulfate (SDS) as a detergent (3).
For slow freezing, samples were incubated for one week in normal saline and stored at -4°C. By removing the samples from freezer, they were allocated into small pieces, and placed in 2 mL cryotubes to perform snap freezing in liquid nitrogen for two minutes. Subsequently, during the rapid melting phase, the cryotubes were placed within the buccal containing running water. Afterwards, the samples were removed from the cryotubes and washed with phosphate buffered saline (PBS). The snap freezing and thawing phases were repeated five times.
The chemical procedures were done after doing the snap freezing and thawing processes, to achieve the best method for complete elimination of cell nuclei, while preserving the ECM components, various percentages of SDS in different time periods were examined.
3.1.2. Sterilization of the ADM Scaffolds for Culture
To eliminate the remained detergents and minimize potential contamination, decellularized scaffolds were placed primarily inside plates containing 70% ethanol for 30 minutes; after washing with distilled water, they were placed in sterile PBS plates for an hour (13).
3.2. Preparation of Blastema Rings
To prepare the blastema rings, after removing the dorsal and ventral surface hair on the rabbit’s ears, and inducing local anesthesia, 2-mm-diameter holes were punched on the rabbits ears. Punch areas were selected between the medial ear artery and the marginal ear veins where no major vessels were present; 48 hours after creating the first hole, another hole with 4 mm diameter was created around the first hole. It was during this phase that the blastema ring was removed (12). Sterilizing the rings was done with sterile normal saline in seven steps. Before transferring the samples under the laminar hood, the scaffolds were assembled into the blastema ring, and inserted into the Dulbecco modified eagle’s medium (DMEM) supplemented with 15% fetal bovine serum (FBS) and 100 µL penicillin/streptomycin. Specimens were incubated in a humidified atmosphere to confirm complete cellular removal and also attain the structure of the scaffold by selective decellularization, various tissue staining methods were utilized. Investigations on skin samples before and after decellularization were done via fluorescent 4’, 6-Diamidino-2-phenylindole (DAPI) staining techniques, which demonstrated complete removal of the nuclear material from the decellularized scaffolds.
To check the density of the existing collagens in the extracellular matrix of the dermis, picrosirius red staining method was employed and specimens were studied via the light microscope and also the polarizing microscope.
4. Results
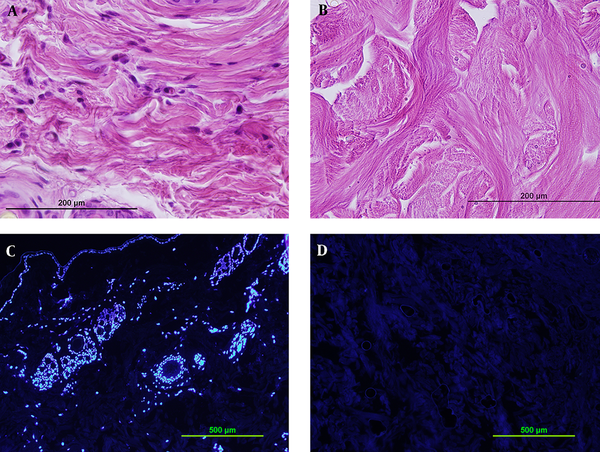
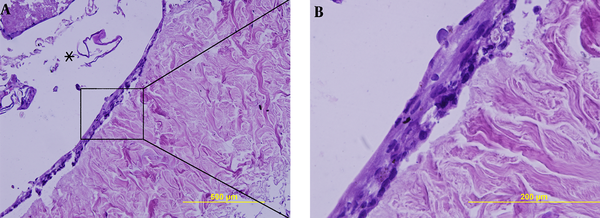
Using snap freeze thaw in liquid nitrogen as a physical method and treatment with 0.1 % SDS for 48 hours as a chemical method, resulted in the best elimination of cells while preserving the dermis structure. Complete decellularization was proved by hematoxylin and eosin (H & E) staining (Figure 1A, B) and DAPI fluorescent dye (Figure 1C, D).
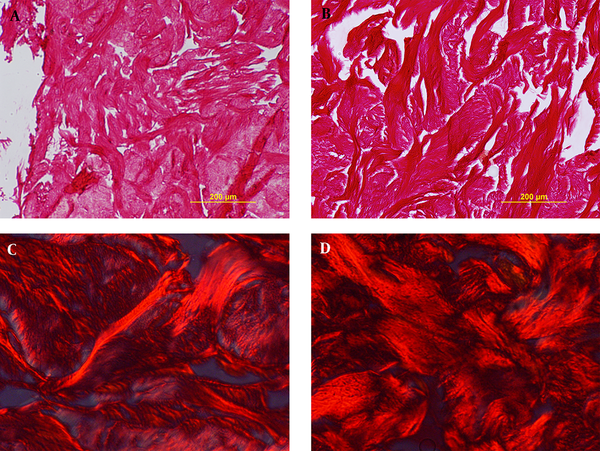
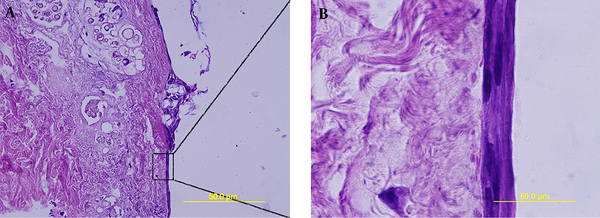
Decellularized samples were stained via picrosirus red staining; after studying the specimen by light microscope and also polarizing microscope, no significant difference was observed in the amount of collagen fibers, as depicted in the Figure 2A - D.
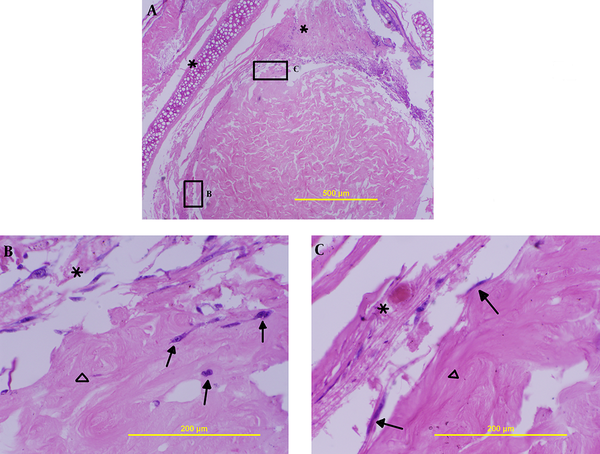
On the fifth day after culture, a few cells, as illustrated in Figure 3B, migrated into the scaffold, and some of them were located at the edge area of the dermal scaffold (Figure 3C).
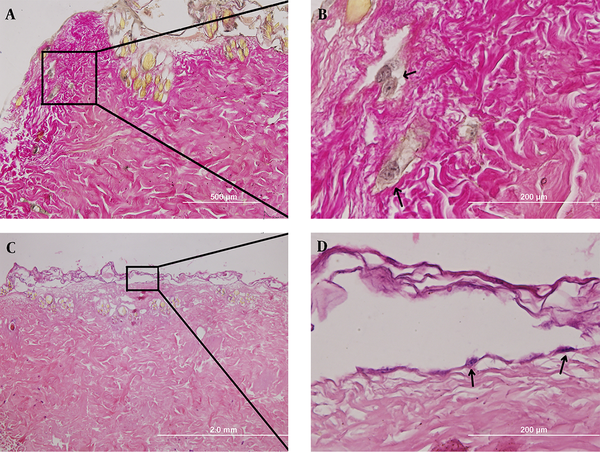
Ten days after culturing, cellular penetration continued into the scaffold (Figure 4A, B) as well as forming a separated, non-continuous cell layer located on the surface of ADM (Figure 4C, D ).
On the 15th day post culture, in addition to penetrating into the scaffold similar to the 5th day, some cells formed a continuous epidermal-like structure (Figure 5C) and some unrushed to the farthest marginal scaffold areas away from the blastema tissue (Figure 5C). The cells in these areas were located as a cell cluster (Figure 5C, D) or individuals. In addition to that, some sections formed a structure similar to blood vessels cross sections in which the cells gathered to form a circle (Figure 5D).
The remarkable points on the 20th day after implantation were the embarkation of multi-layered cell layer on the surface of the scaffold and an epidermis-like structure being created (Figure 6).
All events on the 25th day were similar to those of the 20th day; however, the cells became more stretched and fusiform (Figure 7).
Verifying decellularization by 0.1% SDS for 48 hours by H&E staining (A,B) and DAPI staining (C,D). Comparison between sections before decellularization (A,C) and after it (B,D) shows complete decellularization.
Verifying collagen density via picrosirus red staining with light microscope (A, B) and polarizing microscope (C,D). Sections show no difference before decellularization (A,C) and after it (B,D).
Interaction between blastema tissue and ADM on the 5th day (H&E staining); A) (100x) shows position of blastema ring around ADM, B) a few cells penetrated into the scaffold 400x, C) cells located superficially on the ADM 400; the stars indicate blastema rings, triangle shows ADM and arrows present cells.
The 10th day post culturing; A (100x) and B (400x) indicate cell penetration into the scaffold (picro-fuchsin staining), C (40x) and D (400x) show cell location on the surface of the scaffold (H&E staining).
The 15th day post culturing; A) the blastema ring position toward the ADM (longitudinal section, 40x), B) blastema tissue cells formed structures at farthest area away from the blastema ring (100x), C) continuous single cell layer located on the surface of ADM (400x), D) migrated cells created blood vessel-like structure (presented by arrow sign) in addition to forming cell colonies (indicated by filled circle). The star sign shows blastema ring.
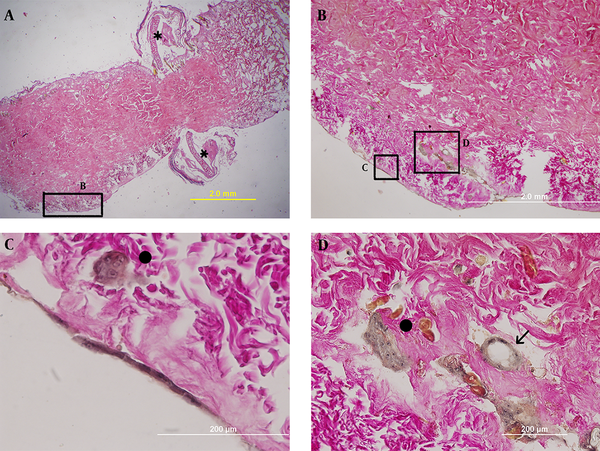
The 20th day post culturing; the single cell layer around ADM on the 15th day became multi- layered on this day. The star sign indicates blastema ring.
The 25th day post culturing; similar to the 20th day a multi- layered cell structure surrounded ADM. Unlike the 20th day, cells became more stretched on the day 25.
5. Discussion
The current study was designed to verify whether acellular dermal matrix is a suitable 3D-matrix for blastema tissue cells originated from pinna of rabbit and study different behaviors of these cells on ADM. The results of the in vitro blastema tissue- ADM interactions revealed various behaviors by blastema tissue cells on different days of culture. These cells first migrated to the ADM on the day five. Some migrated cells penetrated into the scaffold and some of them were located on the surface of ADM. Most migration and penetration by blastema tissue cells occurred on day 15. Penetrated cells created various structures such as blood vessel-like structures and different cell masses. On the other hand, on day 10 superficial cells formed a continuous cell layer which became multilayered on day 20.
5.1. Blastema Tissue Cell Migration
Migration of cells started since the fifth day after culturing. ADM may function as a scaffold for host cells, and as a biologically compatible framework into which epithelial cells and fibroblasts can adhere, migrate, repopulate and help the incorporation of the material into the newly formed tissue (5).
Fibronectins are essential for migration and differentiation of many cell types in embryogenesis (14). Fibronectins induce migration of cultured fibroblast (15). Schor suggests that the differences in the migratory behavior of Hela cells on various substrata in medium containing the same measure of fibronectin may be due to the preferential binding of fibronectin to collagen and the subsequent migration of the cells on the fibronectin-collagen complex (16). Considering that the dermal matrix is mainly composed of collagen, migration of blastema tissue cells would be expected.
5.2. Creation of Epidermal Like Structure
Placement of fibroblast cells as a superficial cell layer is also confirmed in other experiments (6, 17, 18). Maia claims that gingival fibroblasts and highly proliferative cells as B16F10 can only be superficially located on ADM (18). On the other hand, cell penetration into the scaffolds with high density of collagen is less than those of the ones with low density (19). Rodrigues suggests that ADM prepare good condition for seeded fibroblasts to adhere and spread on the matrix but migration into the matrix is low and cells penetrate into the scaffold through marginal zones or areas with low density of collagen (17). The placement of cells on the surface of the scaffold is probably due to the dense structure of the matrix, therefore this high density restricts the migration of cells into the scaffold and as a result, cells spread more on the surface. Cells observed in ADM penetrated more from marginal areas or areas with low density of collagen. Epidermal-like structure around ADM became multi-layered in the current study, unlike the results of gingival fibroblasts cultured on the scaffold, it may be probably caused by the different abilities of blastema tissue cells and fibroblasts.
5.3. Formation of Blood Vessel-Like Structure
Emergence of blood vessel-like structures was another astonishing event occurred on the 15th day. It is believed that during decellularization of mammal’s tissue, a natural three-dimensional structure is created in which the fine networks of blood vessels are preserved; therefore, after the occupation of scaffolding by new cells, vessel formation will happen faster (20). Laminin is also among the molecules creating effective vessel structures (1). Therefore, it can probably be said that in the dermal scaffold, the structure of vascular channels is somehow preserved and laminin extant in the vascular basement membrane is the reason for attracting cells to this area.
Acknowledgements
References
-
1.
Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14(5):526-32. [PubMed ID: 14580584].
-
2.
Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199(2):174-80. [PubMed ID: 15039999]. https://doi.org/10.1002/jcp.10471.
-
3.
Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27(19):3675-83. [PubMed ID: 16519932]. https://doi.org/10.1016/j.biomaterials.2006.02.014.
-
4.
Badylak S, Gilbert TW, Myers-Irvin J. The extracellular matrix as a biologic scaffold for tissue engineering. In: Blitterswijk C, editor. Tissue Engineering. London: Elsevier; 2008. p. 121-43. https://doi.org/10.1016/b978-0-12-370869-4.00005-7.
-
5.
Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995;21(4):243-8. [PubMed ID: 7662122].
-
6.
Gustafson CJ, Kratz G. Cultured autologous keratinocytes on a cell-free dermis in the treatment of full-thickness wounds. Burns. 1999;25(4):331-5. [PubMed ID: 10431981].
-
7.
Altman AM, Chiu ES, Bai X, Yan Y, Song YH, Newsome RE, et al. Human adipose-derived stem cells adhere to acellular dermal matrix. Aesthetic Plast Surg. 2008;32(4):698-9. [PubMed ID: 18414936]. https://doi.org/10.1007/s00266-008-9159-1.
-
8.
Nie C, Yang D, Morris SF. Local delivery of adipose-derived stem cells via acellular dermal matrix as a scaffold: a new promising strategy to accelerate wound healing. Med Hypotheses. 2009;72(6):679-82. [PubMed ID: 19243892]. https://doi.org/10.1016/j.mehy.2008.10.033.
-
9.
Guan L, Dan W, Lin H, Dan N, Wang K, Liao L, et al. [Studies on the growth of rabbit skin fibroblasts on the surfaces of acellular dermal matrix]. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009;26(5):1010-5. [PubMed ID: 19947479].
-
10.
Chen C, Li GF, Liu W, Yang NZ, Wang B, Zhang C, et al. [Construction of tissue-engineered skin by mix-seeding]. Zhonghua Zheng Xing Wai Ke Za Zhi. 2010;26(5):365-8. [PubMed ID: 21174794].
-
11.
Tsonis PA. Regeneration in vertebrates. Dev Biol. 2000;221(2):273-84. [PubMed ID: 10790325]. https://doi.org/10.1006/dbio.2000.9667.
-
12.
Naseri F, Shahri NM, Kheirabadi M, Babaie S, Shakib FS, Azarniya M. The ultra structural study of blastema in pinna tissues of rabbits with transmission electron microscope. J Biologic Sci. 2008;8(6):993-1000. https://doi.org/10.3923/jbs.2008.993.1000.
-
13.
Abousleiman RI, Reyes Y, McFetridge P, Sikavitsas V. The human umbilical vein: a novel scaffold for musculoskeletal soft tissue regeneration. Artif Organs. 2008;32(9):735-42. [PubMed ID: 18684203]. https://doi.org/10.1111/j.1525-1594.2008.00598.x.
-
14.
Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4 ed. New York: Garland Science; 2002.
-
15.
Ali IU, Hynes RO. Effects of LETS glycoprotein on cell motility. Cell. 1978;14(2):439-46. [PubMed ID: 667950].
-
16.
Schor SL. Cell proliferation and migration on collagen substrata in vitro. J Cell Sci. 1980;41:159-75. [PubMed ID: 7364880].
-
17.
Rodrigues AZ, Oliveira PT, Novaes AJ, Maia LP, Souza SL, Palioto DB. Evaluation of in vitro human gingival fibroblast seeding on acellular dermal matrix. Braz Dent J. 2010;21(3):179-89. [PubMed ID: 21203697].
-
18.
Maia LP, Novaes AJ, Souza SL, Grisi MF, Taba M, Palioto DB. In vitro evaluation of acellular dermal matrix as a three-dimensional scaffold for gingival fibroblasts seeding. J Periodontol. 2011;82(2):293-301. [PubMed ID: 20812778]. https://doi.org/10.1902/jop.2010.100121.
-
19.
Hillmann G, Gebert A, Geurtsen W. Matrix expression and proliferation of primary gingival fibroblasts in a three-dimensional cell culture model. J Cell Sci. 1999;112 ( Pt 17):2823-32. [PubMed ID: 10444377].
-
20.
Novosel EC, Kleinhans C, Kluger PJ. Vascularization is the key challenge in tissue engineering. Adv Drug Deliv Rev. 2011;63(4-5):300-11. [PubMed ID: 21396416]. https://doi.org/10.1016/j.addr.2011.03.004.