Abstract
Background:
Ovarian cancer is the most common fatal gynecologic malignancy in women. The BRCA2 gene has a role in regulation of cell cycle during proliferation, differentiation and DNA repair. Changes in the methylation of BRCA2 may be an effective mechanism for ovarian cancer.Objectives:
The aim of the present study was to evaluate the association between ovarian cancer and methylation status of BRCA2.Materials and Methods:
In this study, methylation changes of BRCA2 genes in 44 tissue samples from patients with ovarian cancer and 44 adjacent normal ovarian tissue samples were studied as the control group. After primer design and amplification of the BRCA2 gene sequence by the Polymerase Chain Reaction (PCR), gene methylation levels were evaluated using an enzymatic digestion method, Restriction Fragment Length Polymorphism (RFLP).Results:
According to the study of the methylation status of subjects, status changes were observed only in three cases. The results did not show a correlation between BRCA2 gene promoter methylation in ovarian cancer patients and healthy subjects.Conclusions:
According to the results obtained in this study, changes in the methylation status of BRCA2 cannot be the decisive factor to ascertain the development of ovarian cancer.Keywords
1. Background
Ovarian cancer is the most important cause of mortality from cancer of the female reproductive system and is the sixth most common cancer in women (1). Ovarian cancer is the accumulation of abnormal cells and an imbalance between proliferation and cell death that occurs in ovaries and is able to invade nearby tissues and distant areas (2). The most important symptom of ovarian cancer is pelvic solid, regular and constant mass in physical examination. Because of the location of the ovaries in pelvis deep space and presence of non-specific symptoms, physical examination and diagnosis is difficult during early stages (1, 3, 4). Around 85 to 90 percent of malignant tumors of the ovary are a type of epithelial cancer (5, 6). Followed by repeated ovulation, scare surface epithelium extends into the cortex of the ovaries and creates small epithelial cysts. These cysts can be involved in cancerous transformation and become epithelial tumors with different histological types (7-9). The incidence of ovarian cancer is different based on geographic region and ethnic group. Incidence in northwestern Europe, United States and Canada is high while it is low in Asia and Latin America (10). Age, alcohol consumption, smoking, family history, early menarche (age of less than 13 years), late menopause (the age range between 20 and 50) and infertility increase the incidence of this cancer. In contrast, pregnancy, breastfeeding, use of oral contraceptives tubal ligation and hysterectomy reduce the incidence of this type of cancer (11, 12). Having a family history of ovarian cancer in one of the family members is the most important factor for developing this disease. Diagnostic procedures such as pelvic examination, ultrasound evaluation CA-125, series of images of lower Gastrointestinal (GI) or barium enemas and biopsy are better and faster ways to identify patients with this disease. Knowing the stage of the disease by imaging helps the doctor determine the treatment plan. For the treatment of ovarian cancer different treatments and combinations of treatments such as surgery, chemotherapy and radiotherapy are used. Many tumor suppressor genes play a role in ovarian carcinogenesis. One of inhibitors of tumor involved in ovarian cancer is BRCA (Breast Cancer Susceptibility Gene). The BRCA2 is located on chromosome 13q12-13 and comprises of 27 coding exons and codes for 3418 amino acid proteins (13, 14). Furthermore, BRCA2 has a role in regulation of cell cycle during proliferation, differentiation and DNA repair (15). Genetic and epigenetic alterations in tumor suppressor genes include genetic mutations, abnormal methylation changes in the promoter region and loss of heterozygosity (16, 17). Among factors associated with ovarian cancer, it is abnormalities in the methylation promoter region of tumor suppressor genes BRCA1 and BRCA2 that leads to changes in gene expression and can be effective in ovarian cancer (18). Methylation is one of the common epigenetic events in mammalian genomes (19). Abnormal methylation alterations include increased methylation or hypermethylation and reduced methylation or hypomethylation. Hypermethylation in the promoter region of the gene is associated with reduced gene expression (20). The relationship between DNA methylation and cancer was expressed for the first time in 1983. In this study it was shown that the genome of cancer cells compared to their normal counterparts are hypomethylated (21). It seems that an improper methylation (aberrant) gene is an early event in carcinogenesis and different types of cancer, including lung and bladder are affected by changes in methylation of CPG islands.
2. Objectives
Thus, determining the pattern of DNA methylation may be useful for diagnosis and treatment (22). This change was reversible thus could be considered as a therapeutic target (19).
3. Materials and Methods
3.1. Genotyping
In this case-control study, the samples were all collected in form of paraffin blocks in pathology archives. As far as the research and statistical consulting were concerned, 44 ovarian cancer samples of premenopausal women under 50 years old were entitled as the case group and the other 44 samples, which were adjacent normal ovarian tissue from the same people were selected as the control group. After the sample collection, DNA was extracted by an extraction kit. In the next step, the methylation status of BRCA2 was studied using the Polymerase Chain Reaction (PCR) and MspI and HpaII enzymes. Polymerase Chain Reaction was performed using specific primers F: GAAGCGTGAGGGGACAGATT and R: GTAAGCTGACAAAAACCGC. The PCR reaction consisted of 300 - 100 ng of extracted DNA, 1 unit of each primer, 200 mM MgCl2, 1.5 m/M of each of dATP, dCTP, dTTP and dGTP. First stage: A - primary denaturation at 94°C for five minutes during one cycle, B - denaturation at 94°C for 40 seconds, C - annealing at 55°C for 35 seconds, D - extension at 7°C for 20 seconds, E - final extension at 72°C for five minutes for 35 cycles. Next, the PCR product was cut by restriction enzymes. One unit of each of Msp1 and HpaII at 37°C in a 1 - 16 hour period was used to evaluate the degree of methylation that these changes were studied by gel electrophoresis.
3.2. Statistical Analysis
Methylation statuses were compared using the SPSS-16 software and calculations were performed based on Pearson’s Chi-Square test. It turned out that the P value was less than 0.05.
4. Results
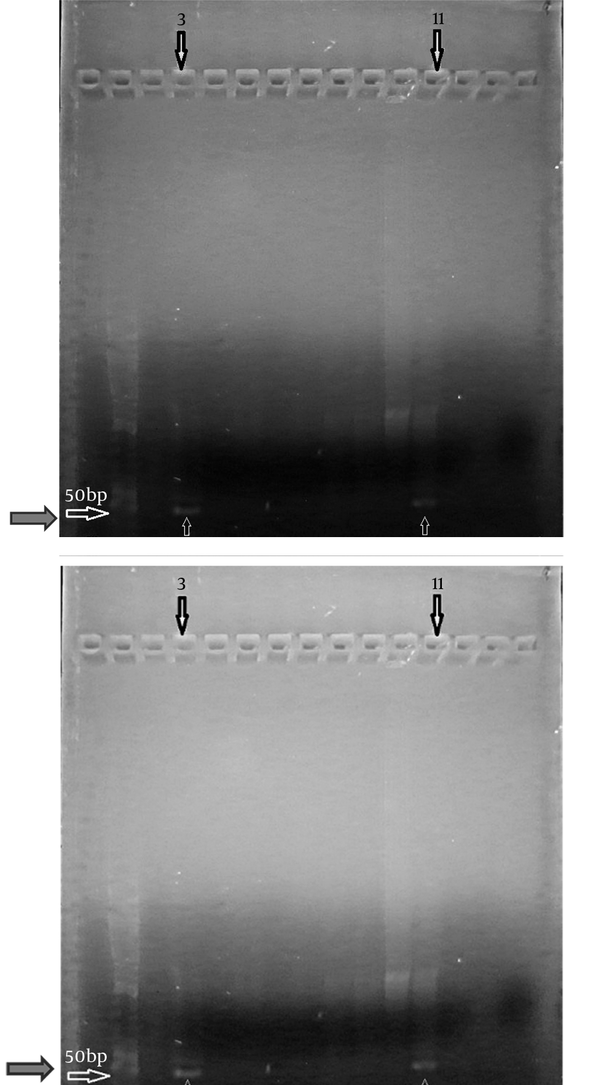
In this study we evaluated the methylation status of the BRCA2 promoter in cancer and control samples. The results revealed that 39 of 44 (88.63%) cancer cases were non-methylated in the promoter region and five of 44 (11.36%) were methylated (Table 1). Of the 44 control cases two (4.54%) cases were methylated in the promoter region and 42 (95.45%) cases were non-methylated. Methylation status change was observed only in three cases. In three of cases, non-methylated promoter had become methylated (Figure 1). Changes in methylation statuses were not created in other cases.
Methylation Status of BRCA2
Promoter Methylation Status of the Samples
5. Discussion
For reducing the risk of ovarian cancer, it is essential to understand and characterize the cases of the disease. We evaluated the promoter methylation status of BRCA2 on CpG islands. The BRCA2 gene is a tumor suppressor gene that may be useful in suppressing ovarian cancer. Previous studies demonstrated that BRCA1 and BRCA2 methylation is altered in breast cancer (23). The methylation status of BRCA1 in ovarian cancer has been previously examined, and we examined the BRCA2 methylation status in this study. Furthermore, CpG islands are routinely found in the promoter of genes. Normally, non-methylated CpG islands (regions of rich CG content) are seen in the promoters of expressed genes, whilst methylated promoters are usually associated with genes with low or decrease transcriptional rates. However, usual methylation status can be changed in neoplastic cells, possibly due to increased DNA MTase activity and/or through local protection mechanisms. Hypomethylation of regulatory DNA sequences can sometimes set up the transcription of proto-oncogenes (24-27), potentially giving them oncogenic operation. This can happen after the development of neoplastic progression. Promoters can become methylated in normal cells during the entire aging process. This latter alteration may give rise to aptitude to neoplasia (28). Methylation is the mechanism for gene deactivation that has been considered to happen in some BRCA2 tumors. However, it should also be noted that BRCA2 may also be inactivated post-translationally by improper phosphorylation or other post-translational modifications (29). This study investigated the promoter methylation status of BRCA2 in tumor samples by comparing them with benign tissue from an area adjacent to the tumor lesion. In our study from a total of 44 cases having ovarian cancer, it was observed that 39 patients had non-methylated promoters and five had promoter methylation. Of the total of 44 subjects of the control group, two cases had promoter methylation and 42 cases had non-methylated promoters. Methylation status change was observed only in three cases. In these three cases, the non-methylated promoter had become a methylated promoter. In the other groups, changes in methylation status were not created. In previous studies on the methylation status of BRCA2 in samples of ovarian cancer, Dhillon et al. studied 25 samples of ovarian cancer where one case of methylation change was observed in the promoter region of the BRCA2 gene (30). In other studies, Nurhan et al. studied 12 samples of ovarian cancer where five cases had methylation changes in the promoter region of the BRCA2 gene (31). The studies of Collins and colleagues in 1997, and Bosviel and colleagues in 2011 found no evidence of BRCA2 promoter methylation (32, 33). In our study, three cases had methylation changes in the promoter region of the BRCA2 gene. Based on our results and those of previous studies, it can be concluded that alteration in the methylation of BRCA2 may not be a risk factor for ovarian cancer development in this studied population, and thus it is not an appropriate biomarker for early diagnosis of ovarian cancer.
Acknowledgements
References
-
1.
Permuth-Wey J, Sellers TA. Epidemiology of ovarian cancer. Methods Mol Biol. 2009;472:413-37. [PubMed ID: 19107446]. https://doi.org/10.1007/978-1-60327-492-0_20.
-
2.
Robert LN, Rodrick RM. chapter 16. Genetic in medicine. 6 ed. 2001. p. 125-50.
-
3.
Asadollahi R, Hyde CA, Zhong XY. Epigenetics of ovarian cancer: from the lab to the clinic. Gynecol Oncol. 2010;118(1):81-7. [PubMed ID: 20421130]. https://doi.org/10.1016/j.ygyno.2010.03.015.
-
4.
Swisher EM, Gonzalez RM, Taniguchi T, Garcia RL, Walsh T, Goff BA, et al. Methylation and protein expression of DNA repair genes: association with chemotherapy exposure and survival in sporadic ovarian and peritoneal carcinomas. Mol Cancer. 2009;8:48. [PubMed ID: 19602291]. https://doi.org/10.1186/1476-4598-8-48.
-
5.
Berek J, Hacker N. Ovarian cancer in: Novak Gynecology. 12 ed. California: Williams and Wilkins; 1996. 1155 p.
-
6.
Disaia P, Creasman W. Epithelial ovarian cancer in: Gynecologic oncology. 5 ed. south Carolina: Mosby; 1997. p. 282-9.
-
7.
Lucchetti MC. Ovarian tumours: general approach and balance between risk of unnecessary surgery and oncological risk. Paediatrics and Child Health. 2009;19:S163-7. https://doi.org/10.1016/j.paed.2009.08.011.
-
8.
Danforth. Obsterics and gynecology. Lippincott Williams & Wilkins; 2008. p. 554-63.
-
9.
Kumar V. Robbins basic pathology. Saunders; 2007. p. 462-8.
-
10.
Holschneider CH, Berek JS. Ovarian cancer: epidemiology, biology, and prognostic factors. Semin Surg Oncol. 2000;19(1):3-10. [PubMed ID: 10883018].
-
11.
Quinn JE, Carser JE, James CR, Kennedy RD, Harkin DP. BRCA1 and implications for response to chemotherapy in ovarian cancer. Gynecol Oncol. 2009;113(1):134-42. [PubMed ID: 19168207]. https://doi.org/10.1016/j.ygyno.2008.12.015.
-
12.
Ghaemmaghami F, Modares Gilani M, Sadegi Fard NA. case-control study of risk factors for epithelial ovarian cancer. Med J. 2001;4:57-62.
-
13.
King MC, Marks JH, Mandell JB, New York Breast Cancer Study G. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643-6. [PubMed ID: 14576434]. https://doi.org/10.1126/science.1088759.
-
14.
Starita LM, Parvin JD. The multiple nuclear functions of BRCA1: transcription, ubiquitination and DNA repair. Curr Opin Cell Biol. 2003;15(3):345-50. [PubMed ID: 12787778].
-
15.
Hilton JL, Geisler JP, Rathe JA, Hattermann-Zogg MA, DeYoung B, Buller RE. Inactivation of BRCA1 and BRCA2 in ovarian cancer. J Natl Cancer Inst. 2002;94(18):1396-406. [PubMed ID: 12237285].
-
16.
Lodish H, Berk A, Zipursky L, Matsudaira P, Baltimore D. Molecular Cell Biology. ed 4. New York: W. H. Freeman; 2000. p. 551-5.
-
17.
Baldwin RL, Nemeth E, Tran H, Shvartsman H, Cass I, Narod S, et al. BRCA1 promoter region hypermethylation in ovarian carcinoma: a population-based study. Cancer Res. 2000;60(19):5329-33. [PubMed ID: 11034065].
-
18.
Markowitz S. DNA repair defects inactivate tumor suppressor genes and induce hereditary and sporadic colon cancers. J Clin Oncol. 2000;18(21 Suppl):75S-80S. [PubMed ID: 11060332].
-
19.
Mdas P, Singal R. DNA Methlation and cancer. Clinical Oncology J. 2004;22(22):632-42.
-
20.
Birgisdottir V, Stefansson OA, Bodvarsdottir SK, Hilmarsdottir H, Jonasson JG, Eyfjord JE. Epigenetic silencing and deletion of the BRCA1 gene in sporadic breast cancer. Breast Cancer Res. 2006;8(4):R38. [PubMed ID: 16846527]. https://doi.org/10.1186/bcr1522.
-
21.
Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6(8):597-610. [PubMed ID: 16136652]. https://doi.org/10.1038/nrg1655.
-
22.
Chiang JW, Karlan BY, Cass L, Baldwin RL. BRCA1 promoter methylation predicts adverse ovarian cancer prognosis. Gynecol Oncol. 2006;101(3):403-10. [PubMed ID: 16360812]. https://doi.org/10.1016/j.ygyno.2005.10.034.
-
23.
Antoniou A, Pharoah PD, Narod S, Risch HA, Eyfjord JE, Hopper JL, et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117-30. [PubMed ID: 12677558]. https://doi.org/10.1086/375033.
-
24.
Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Curr Opin Genet Dev. 2004;14(1):11-6. [PubMed ID: 15108799]. https://doi.org/10.1016/j.gde.2003.12.003.
-
25.
Paige AJ. Redefining tumour suppressor genes: exceptions to the two-hit hypothesis. Cell Mol Life Sci. 2003;60(10):2147-63. [PubMed ID: 14618262]. https://doi.org/10.1007/s00018-003-3027-6.
-
26.
Olek A, Oswald J, Walter J. A modified and improved method for bisulphite based cytosine methylation analysis. Nucleic Acids Res. 1996;24(24):5064-6. [PubMed ID: 9016686].
-
27.
An J, Wei Q, Liu Z, Lu KH, Cheng X, Mills GB, et al. Messenger RNA expression and methylation of candidate tumor-suppressor genes and risk of ovarian cancer-a case-control analysis. Int J Mol Epidemiol Genet. 2010;1(1):1-10. [PubMed ID: 20689651].
-
28.
Chobanian N, Dietrich C3. Ovarian cancer. Surg Clin North Am. 2008;88(2):285-99. vi. [PubMed ID: 18381114]. https://doi.org/10.1016/j.suc.2007.12.002.
-
29.
Welcsh PL, King MC. BRCA1 and BRCA2 and the genetics of breast and ovarian cancer. Hum Mol Genet. 2001;10(7):705-13. [PubMed ID: 11257103].
-
30.
Dhillon VS, Shahid M, Husain SA. CpG methylation of the FHIT, FANCF, cyclin-D2, BRCA2 and RUNX3 genes in Granulosa cell tumors (GCTs) of ovarian origin. Mol Cancer. 2004;3:33. [PubMed ID: 15574200]. https://doi.org/10.1186/1476-4598-3-33.
-
31.
Nurhan C, Serpil T, Engin O. Methylation status of CpG islands at sites −59 to +96 in exon 1 of the BRCA2 gene varies in mammary tissue among women with sporadic breast cancer. Genet J. 2008;87(2):155-8.
-
32.
Collins N, Wooster R, Stratton MR. Absence of methylation of CpG dinucleotides within the promoter of the breast cancer susceptibility gene BRCA2 in normal tissues and in breast and ovarian cancers. Br J Cancer. 1997;76(9):1150-6. [PubMed ID: 9365162].
-
33.
Bosviel R, Michard E, Lavediaux G, Kwiatkowski F, Bignon YJ, Bernard-Gallon DJ. Peripheral blood DNA methylation detected in the BRCA1 or BRCA2 promoter for sporadic ovarian cancer patients and controls. Clin Chim Acta. 2011;412(15-16):1472-5. [PubMed ID: 21557934]. https://doi.org/10.1016/j.cca.2011.04.027.