Abstract
Background:
Hemolytic anemia is associated with intravascular heme release and oxidative stresses that lead to endothelial dysfunctions. Hemopexin (HPX) is a plasma heme-binding β1-glycoprotein, which plays a pivotal role in heme transfer to hepatocytes and iron recycling. Recently, HPX administration in hemolytic patients attenuated hemolysis-driven oxidative damages and endothelial disorders that led to a rise in the strategy of HPX therapy. Human HPX production using recombinant DNA technology could provide a real alternative to plasma-derived HPX. The purpose of this study was to generate a stable Chinese hamster ovary (CHO) cell line expressing human rHPX.Methods:
Total RNA was extracted from HepG2 cells, and HPX gene was amplified by Real Time-Polymerase Chain Reaction (RT-PCR). Then, the HPX gene was cloned in pcDNA3.1 (+) shuttle vector. The recombinant pcDNA3.1-HPX was transformed to E. coli TOP10 strain. Gene cloning was verified by colony PCR, restriction digestion, and DNA sequencing. The CHO cells were chemically transfected with pcDNA3.1-HPX, and screening was performed by G418 sulfate effective concentration to develop stable single cell clones. The rHPX expression was verified by RT-PCR and Western blot.Results:
Cloning confirmation analyses showed that HPX gene was successfully cloned in pcDNA3.1 (+) vector. Screening of transfected cells with G418 sulfate enriched the population of single cell clones expressing human rHPX. The RT-PCR and Western blot analyses confirmed rHPX expression in CHO cell line both at transcriptional and translational levels.Conclusions:
Human rHPX protein was stably expressed in CHO cells. This study was a pioneering work for the future production of therapeutic rHPX.Keywords
Hemopexin Hemolysis Hemolytic Anemia CHO Cells Gene Expression
1. Background
Intravascular hemolysis with release of free hemoglobin and heme occurs in a wide range of hemolytic conditions such as thalassemia, sickle cell disease, autoimmune hemolytic anemia, etc. (1). Intravascular hemolysis is mainly associated with endothelial and cardiovascular dysfunctions leading to oxidative stress, vasomotor instability, proliferative vasculopathy, thrombosis (2), stimulation of adhesion molecules on the endothelium, high levels of circulating pro-inflammatory cytokines, and coagulopathy (3).
The adverse effects of free heme are physiologically attenuated by a plasma β1-glycoprotein (4) called Hemopexin (HPX) with an extraordinarily high affinity to heme (Kd < 1 pM) (5). Mainly expressed in hepatocytes (4), HPX is capable of scavenging free heme from blood circulation (6) and transporting it safely to hepatocytes via CD91 receptor. Thus, it plays a pivotal role in promoting iron homeostasis (7) and suppressing heme-driven oxidative damages (8).
Hemopexin is an acute phase plasma glycoprotein, which is synthesized in the liver, nervous system, skeletal muscle, retina, and kidney. As a multifunctional agent, it is implicated in important processes such as iron homeostasis, signaling pathways to promote cell survival, modulation of gene expression, nerve regeneration, control of the immune response, and pathogenesis of renal diseases (4).
The therapeutic employment and clinical importance of HPX as a predominant heme-binding protein have been reinforced since 2013 (9). It was demonstrated that HPX protects the endothelial tissue through the prevention of heme intercalation in endothelial cell membranes and the promotion of heme detoxification by hepatocytes through heme oxygenase-1 (HO-1) enzymatic activity (3).
On the other hand, blood transfusion is clinically recommended in severe hemolytic conditions. However, it further exacerbates intravascular hemolysis by intensifying heme release induction (2) and contributes to heme overload and heart failure in hemolytic patients (10). Recent studies revealed that HPX infusion in mouse models of hemolytic disorders pharmacologically alleviates heme-induced endothelial activation, inflammation, and tissue oxidative injury (3). Based on these findings, the strategy of HPX therapy was developed in order to design HPX-based medications with high binding affinity to heme (3).
Therefore, either plasma-purified or recombinant HPX (rHPX) administration could be associated with blood transfusion (11) in patients with anemia as a specific target therapy to enhance the efficiency of blood transfusion (3). This therapeutic strategy is expected to improve the health status of hemolytic patients by attenuating their endothelial and cardiovascular disorders (8).
Hemopexin purification from plasma is associated with HPX conformational and chemical modifications, which negatively effect the quality and purity of the recovered HPX (12).
Recently, human rHPX was expressed in both prokaryotic and eukaryotic hosts such as Escherichia coli (13, 14), Spodoptera frugiperda Sf9 insect cells (15), and Pichia pastoris (16). Moreover, commercially available human rHPX were reported to be expressed in Human Embryonic Kidney (HEK293) cells and mouse myeloma cell line (NS0). Nevertheless, no evidence was provided to confirm the product’s native HPX-like post-translational modifications, including human-like glycosylation and folding (12).
Furthermore, HPX is also a large structurally complex glycoprotein, requiring human-like post-translational modifications for its effective bioactivities (17). Thus, mammalian cell lines, particularly Chinese hamster ovary (CHO) cells (18), are generally harnessed as an efficient expression host due to the human-compatible glycosylation machinery to efficiently fold and secrete this glycoprotein (19).
Today, CHO cell expression system is exploited to produce therapeutic glycoproteins (20) due to their capability of adapting to various culture conditions, less risk of human virus contamination (21), generating human-like bioactive post-translational modifications to recombinant glycoproteins (22), less immunogenicity of the recombinant products (23), CHO genome instability (24), and higher yield of recombinant protein production (25).
Large-scale production of recombinant proteins depends on the stable integration of recombinant DNA in the host genome (26). This involves non-targeted DNA delivery and antibiotic-mediated selection to establish stable and high-producing single cell clones to express the gene of interest (27, 28).
2. Objectives
The aim of this study was to generate a stable CHO cell line that permanently expresses human rHPX. This is the first step towards the production of therapeutic human rHPX to be clinically administered in the future.
3. Methods
3.1. Cell Culture
HepG2 (IBRC C10096, Iran) and CHO cells (IBRC C10136, Iran) were cultured in RPMI 1640 (Gibco, Germany), supplemented with 10% heat-inactivated Fetal Bovine Serum (FBS) (Gibco, Germany) plus penicillin (100 U/mL) and streptomycin (100 μg/mL) (CinnaGen, Iran) in a humidified incubator at 37°C and 5% CO2. Cells were sub-cultured every 3 to 4 days by rinsing with phosphate buffered saline (PBS) (ICN Biomedicals, USA) and detaching with 0.25% trypsin-EDTA (Invitrogen, USA). Cell counting was performed with hemocytometer. The sub-cultures of these cells were used for RNA extraction.
3.2. Hemopexin Gene Isolation and Recombinant DNA Preparation
On normal culture condition, the expression of HPX as an acute phase protein in HepG2 cells is too low to be detected by PCR. Therefore, HepG2 cells were treated with 20 µL interleukin-6 (IL6) (30 ng/mL) for 24 hours. Then, total RNA was extracted using the RNX-Plus RNA Isolation Kit (CinnaGen, Iran). The cDNA synthesis was primed with oligo(dT) using AccuPowerTM cycle script RT PreMix Kit (Bioneer, Korea), according to the manufacturer’s instructions. Then, PCR was performed using specific HPX primers and Pfu DNA Polymerase (CinnaGen, Iran). Primers covering the complete coding region of HPX were designed based on the sequence present in the GenBank (accession number NM-000613.2). The forward primer contained HindIII restriction site and a kozak sequence, and the reverse primer contained XhoI restriction site downstream the coding region (Table 1). The HPX PCR program consisted of 35 cycles with a denaturation step of 30 seconds at 94°C, an annealing step of 30 seconds at 61°C and an extension step of 1 minute and 30 seconds at 72°C. The first denaturing step lasted for 5 minutes, and the final extension step lasted for 7 minutes. Amplification of β-actin was performed as an internal control using β-actin primers (Table 1).
Primer Sequences Designed for Hemopexin Gene Isolation and Real Time-Polymerase Chain Reaction Confirmation Tests
| Primer Sets | Amplicon Size, bp | Primer Sequence |
|---|---|---|
| HPX specific Fw.a | 1410 | 5'-CTG AAG CTT ACC ATG GCT AGG GTA CTG GGA G-3' |
| HPX specific Rs.a | 5'-GAA CTC GAG TCA GTG AGT GCA GCC CAG GAG-3' | |
| HPX Internal Fw. | 184 | 5'-TGT CAC CGT GGA GAA TGT CA-3' |
| HPX Internal Rs. | 5'-CGA AGC GCA GGA ATT GGT TA-3' | |
| β-actin Fw. | 200 | 5’-TTCTACAATGAGCTGCGTGTGG-3’ |
| β-actin Rs. | 5'-GTGTTGAAGGTCTCAAACATGAT-3' |
In order to clone the HPX transgene, pcDNA3.1(+) plasmid vector (Invitrogen, USA) was used. The HPX PCR product and pcDNA3.1(+) vector were digested by HindIII and XhoI restriction enzymes (Fermentase, USA). The digested PCR products and vector were purified with High Pure PCR Product Purification Kit (Roche, Germany). The purified DNA fragment was cloned in pcDNA3.1(+) expression vector using T4 DNA ligase (CinnaGen, Iran).
3.3. Bacterial Transformation and Cloning Confirmation
After successful ligation, competent bacterial cells were prepared using CaCl2 and heat shock conventional method. The engineered recombinant DNA (pcDNA3.1-HPX) was transformed in the competent E. coli bacteria TOP10 strain (CinnaGen, Iran). Screening was performed through colony PCR, pcDNA3.1-HPX electrophoresis, and restriction digestion. Furthermore, the accuracy of rHPX sequence and its ligation frame in pcDNA3.1-HPX was confirmed with DNA sequencing.
3.4. Transfection of Chinese Hamster Ovary Cells with pcDNA3.1-Hemopexin
For transfecting CHO cells with pcDNA3.1-HPX, 2 × 105 CHO cells were seeded in 6-well plates. Upon reaching 70% to 80% confluence, the CHO cells were transfected with pcDNA3.1-HPX using X-tremeGENE HP DNA transfection reagent (Roche, Germany), according to the protocol provided by the manufacturer (CHO-HPX). The rHPX transient expression was verified at different time intervals including 24, 48, and 72 hours post-transfection by RT-PCR and Western blot analyses.
3.5. Determination of Antibiotic (G418 Sulfate) Effective Dose
In order to determine the minimum G418 sulfate (Bio Basic Canada Inc., USA) effective concentration to kill non-resistant host cells, CHO cells were cultured in a 96-well plate and treated with several concentrations of G418 ranging from 50 - 700 µg/mL. This procedure was performed in triplicates. Finally, the minimum effective G418 concentration required for screening CHO-HPX cells was determined by trypan blue staining (StemCell Technologies Inc., USA).
3.6. Stable Expression of Recombinant
The pcDNA3.1-HPX was linearized using BglII restriction enzyme (Fermentase, USA) in order to facilitate the integration of pcDNA3.1-HPX in the CHO cell genome. Then, CHO cells were transfected with linearized pcDNA3.1-HPX using the foregoing transfection protocol and were maintained under selective pressure of G418 sulfate (200 µg/mL). Next, the culture medium was replaced with fresh G418-containing medium every 3 days. Once a population of G418-resistant CHO-HPX cells was obtained, the cells were passed in a 96-well plate in order to develop stable CHO-HPX single cell colony. The selected single cell colonies were expanded and stocked over different cellular passages. Finally, rHPX expression was verified at the 10th and 20th passage by RT-PCR and Western blot analyses. Beside CHO-HPX, a group of CHO cells transfected with empty pcDNA3.1 vector (CHO-V) was considered as the negative control in order to compare rHPX expression between the 2 cellular groups.
3.7. Real Time-Polymerase Chain Reaction analysis
Real time-polymerase chain reaction was performed in order to confirm the expression of rHPX at the transcriptional level. Total RNA was extracted from CHO-V and CHO-HPX cellular groups using RNX-Plus the RNA Isolation Kit, according to the instructions of the manufacturer. Then, cDNA synthesis and PCR with total HPX primers were performed as previously reported. Furthermore, HPX PCR was performed using HPX internal primers (Table 1) mediated by 2X Taq PreMix Master Mix (Parstous, Iran). β-actin was used as the internal control.
3.8. Western Blot Analysis
In order to analyze the expression of rHPX at the protein level, the secreted proteins in the CHO-V and CHO-HPX supernatants were separated by Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) on 12.5% polyacrylamide gel. After protein transfer to Polyvinylidene Difluoride (PVDF) membrane (Roche, Germany), employing semi-dry electro-blotter system (PeQLab, Germany), blocking of PVDF membrane was performed in 5% skim milk and PBS overnight. The membrane was then washed 3 times by PBST and incubated in diluted (1:50,000) primary rabbit anti-HPX antibody (Abcam, UK) at room temperature for 1 hour and 30 minutes. It was repeatedly washed prior to adding the diluted (1:5,000) secondary HRP-conjugated goat anti-rabbit antibody (Cell Signaling Technology, USA), and then incubated at room temperature for 1 hour. The final rHPX protein band was detected using ECL Western blotting substrate kit (Abcam, UK) in ChemiDocTM XRS + system (BioRad, USA). Besides, the CHO-V and CHO-HPX cellular lysates were prepared using Complete Lysis-M Kit (Roche, Germany) for the Western blot analysis of β-actin protein, which was used as the internal control.
4. Results
4.1. Isolation of Hemopexin Gene from HepG2 Cells
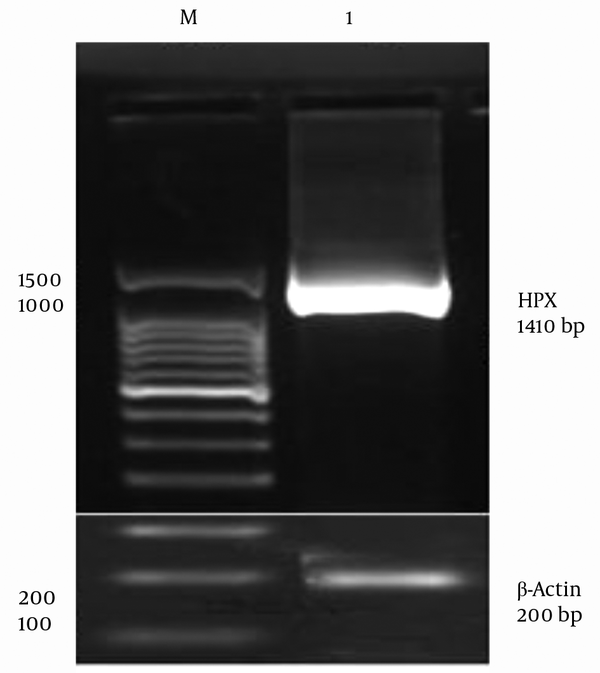
Since HPX is an acute phase protein, its expression is upregulated in the presence of inflammatory cytokines, such as IL6 (29), leukemia inhibitory factor (LIF), and Tumor necrosis factor α (TNFα) (30). Therefore, the HepG2 cells were stimulated with IL6 inflammatory cytokine in order to overexpress the HPX gene. Human HPX gene was further isolated using designed primers by RT-PCR. A specific band of 1410 bp was visualized on 1.5% agarose gel for Pfu DNA polymerase-mediated PCR reaction (Figure 1). The Pfu DNA polymerase is capable of proofreading the process of DNA polymerization whose PCR product is of higher sequence accuracy. Therefore, HPX gene isolation from HepG2 cell line was confirmed. β-actin was used as an internal control (Figure 1).
Polymerase Chain Reaction Amplification of Hemopexin Gene
4.2. Hemopexin Gene Cloning and Cloning Confirmation Tests
Restriction digestion of both PCR product and pcDNA3.1(+) and further DNA ligation led to the construction of recombinant pcDNA3.1-HPX. After colony formation of transformed TOP10 bacteria, 7 colonies were randomly selected to perform cloning confirmation tests. Colony PCR was performed to screen for bacterial colonies, which were accurately transformed with pcDNA3.1-HPX. The 1410 bp band confirmed the perfect HPX gene cloning (Figure 2B). Then, the pcDNA3.1-HPX recombinant vectors were extracted from the selected bacterial colonies using YTA plasmid DNA extraction mini kit (Yekta Tajhiz Azma, Iran). Slower movement of pcDNA3.1-HPX than that of empty vector on the 1% agarose gel confirmed the presence of the 1410-bp HPX gene in pcDNA3.1(+) vector (Figure 2A). Furthermore, restriction digestion of pcDNA3.1-HPX with HindIII and XhoI enzymes ensured the correctness of the recombinant vector construction (Figure 2C). Finally, the accuracy of HPX gene sequence in the recombinant vector was confirmed by DNA sequencing. The BLAST results in NCBI database confirmed 99% identities (data not shown).
Cloning of the Hemopexin Gene in the pcDNA3.1(+) Plasmid

4.3. Chinese Hamster Ovary Cells Express Recombinant Hemopexin Stably at Transcriptional and Translational Levels
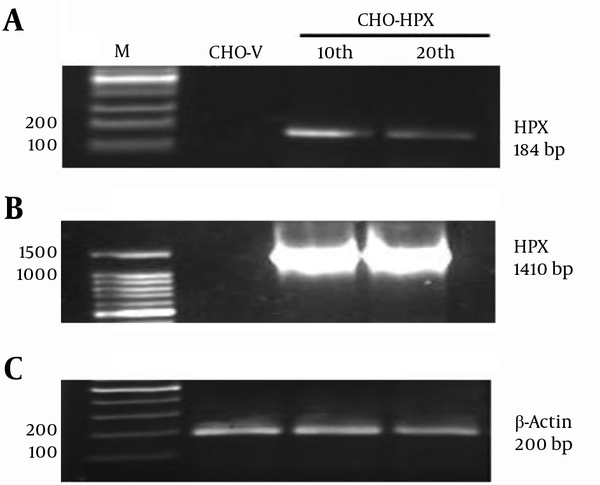
The rHPX mRNA expression in CHO-HPX cells was verified by RT-PCR. In order to screen for stable CHO-HPX single colonies and establish a stable CHO cell line, the CHO-HPX cells were maintained in 200 µg/mL G418 sulfate. Thus, their population was enriched in the presence of minimum antibiotic effective dose and further expanded over sequential cellular passages. Moreover, mRNA expression of rHPX was verified at the 10th and 20th passage. Real Time-Polymerase Chain Reaction resulted in a 184-bp band using internal primers (Figure 3A) and 1410-bp HPX band using total primers (Figure 3B), confirming the establishment of stable CHO cell line expressing human rHPX at the transcriptional level. Furthermore, β-actin was used as an internal control (Figure 3C).
Real Time-Polymerase Chain Reaction Analysis of Human rHPX Expression in CHO-HPX Cells
The expression of rHPX protein in CHO-HPX cells was verified by the Western blot method. The concentrated supernatants of CHO-HPX and CHO-V (negative control) cells were prepared as protein-containing samples. Western blot was performed using primary rabbit anti-Hemopexin antibody. Western blot analysis of CHO-HPX supernatants resulted in a 60-kDa protein band as expected for human rHPX, while no rHPX protein expression was detected in CHO-V cells (Figure 4A). Therefore, rHPX expression in CHO-HPX cells was confirmed at the translational level. The β-actin was used as an internal control (Figure 4B).
Western Blot Analysis of Human rHPX Expression in Chinese Hamster Ovary- Hemopexin Cells

5. Discussion
Today, hemolytic anemia is mainly treated with blood transfusion. This treatment is associated with mechanical shearing forces that accelerate red blood cell rupture (31), and lead to severe clinical complications, including intravascular hemolysis, tissue oxidative stress, and multi-organ dysfunctions (32).
The aim of the present study was to extend our availability to human HPX with the outlook of therapeutic employment for hemolytic patients. First, the HPX gene was isolated through RT-PCR. Second, the pcDNA3.1-HPX recombinant vector was constructed, and HPX gene cloning was confirmed with colony PCR, restriction digestion, and DNA sequencing. Third, CHO cells were transfected with linearized pcDNA3.1-HPX, and the CHO-HPX cellular population was enriched under the selective pressure of G418 sulfate. Finally, RT-PCR and Western blot analyses confirmed the stable expression of human rHPX at both transcriptional and translational levels.
Since human rHPX bears extensive human-specific glycosylation, mammalian expression systems, such as HEK293 or CHO cell lines, could be suitable hosts to provide sufficient post-translational modifications to afford heme binding and expected rHPX bioactivity (15). The CHO cell line is the premier expression host for therapeutic protein production due to its adaptability to various culture conditions, plasticity in terms of genetic alterations (18) and human-like glycosylation (24).
In a pioneering work during the early 1990s, Satoh et al. expressed human rHPX using different expression systems. Their initial attempts to express human HPX in an E. coli system produced a non-glycosylated protein with no heme-binding ability (15). Protein expression in prokaryotic system is associated with challenges such as limited eukaryotic post-translational machinery function, protein aggregation, and generation of inclusion bodies (33). Therefore, re-solubilization and refolding of recombinant polypeptide chains are required, leading to reduced quality and unexpected biological activity of the final proteins (34).
However, using baculovirus vector expression system, Satoh et al. were able to express human rHPX with 55 kDa molecular weight (MW) that was less than expected (60 kDa) (15). It should be noted that protein expression in insect cells is associated with some limitations. The decreased molecular mass of the rHPX is probably due to the N-glycosylation deficient system of insect cells (35). Besides, it is necessary to include a signal sequence, such as melittin peptide (15) along with the transgene in baculovirus vector, in order to render the rHPX protein secretory (35). Moreover, since baculovirus-contaminated insect cells are cultured in Serum-Free Media (SFM), the secreted proteins are highly exposed to proteolysis (35).
Bakker et al. exploited Pichia pastoris expression system to express human rHPX. Their research resulted in a 85-kDa rHPX (16) that was more than plasma HPX MW. This could be attributed to the hyper-glycosylation ability of P. pastoris expression system. Human HPX receives O-linked oligosaccharides composed of a variety of sugars including N-acetylgalactosamine, galactose, and sialic acid. In contrast, P. pastoris only performs high mannosylation. This limits the pharmaceutical use of secreted proteins due to the exceeding immunogenicity of the recombinant drug upon intravenous administration (36).
Although different studies reported various molecular weights for human rHPX due to different levels of glycosylation, the results of this research are consistent with human HPX being the molecule involved. This does not mean, however, that CHO expression system necessarily produced an identical isoform of HPX as compared with plasma-derived HPX. This needs further investigations with regards to rHPX structural and functional properties.
In contrast to the previous studies, the primers used in this study were designed in such a way that the forward primer contained the kozak sequence (5'-A/GNNATGG-3') in order to promote rHPX translation efficiency in CHO cells, which is considered as an advantage of this research.
Additionally, in this research, the rHPX PCR product was cloned in the pcDNA3.1(+) shuttle vector, which contains both the ampicillin and neomycin resistant genes along with strong CMV promoter (37). Thus, it could eliminate the sub-cloning step leading to cost-effectiveness of the procedure and saving of time. However, in Bakker’s study, the rHPX PCR product was first cloned in pCRII-TOPO cloning vector, and then, sub-cloned in pPICZaA expression vector (16) with AOX-1 promoter and zeocin resistant gene (38).
Furthermore, successful transfection is influenced by the type of transfection reagent and the duration of transfection. Satoh et al. used Lipofectin transfection reagent based on cationic liposomes (15), while in Bakker’s study, P. pastoris strain GS115 was transfected with hemopexin/pPICZaA construct using electroporation (16). Herein, the CHO cells were transfected using x-tremeGENE HP DNA transfection reagent that is highly compatible with mammalian cell line and suitable for both transient and stable transfection. It produces the least cytotoxicity or change in cellular morphology after transfection (39). Although recent studies have shown that the condensation of DNA in cationic liposomes and/or the overall positive charges of the cationic liposome/DNA complex enhance gene delivery, their mechanism of action still remains to be elucidated (40). Other factors, such as the health and viability of the cells, the quality of the nucleic acid used and the presence or absence of serum could also play a part.
Expression of functional, recombinant human glycoproteins often requires mammalian expression systems. In order to frequently express a recombinant protein, it will be best to generate a stable cell line instead of performing repeated transient transfections in mammalian cells (41). It is noteworthy that stable cell lines often lose their protein expression with time, due to the heterogeneity of the transfected cell population (41). Therefore, in this research, screening for CHO-HPX and picking up single colonies of G418-resistant cells led to obtaining an even more homogenous population of successfully-transfected CHO cells, which was further expanded.
It is expected for a large-scale stable expression of rHPX in an efficient mammalian cell line to provide an increased source of rHPX to be clinically employed upon blood transfusion in order to supplement the depleted level of HPX in hemolytic patients and reduce their heme-related complications.
To date, little has been done regarding rHPX expression, in particular the human type. In this study, a stable CHO cell line capable of expressing human rHPX was established, which raises many questions and perspectives for further investigations. Undoubtedly, future research is recommended in terms of increasing rHPX expression rate in CHO cells using vectors containing matrix attachment regions (MAR) or ubiquitous chromatin opening element (UCOE) (25), rHPX purification by chromatography, protein characterization, and in vivo studies in murine models to further improve the understanding of rHPX biological activities and therapeutic roles for future clinical settings.
Acknowledgements
References
-
1.
Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276-84. [PubMed ID: 23264591]. https://doi.org/10.1182/blood-2012-11-451229.
-
2.
Morris CR. Mechanisms of vasculopathy in sickle cell disease and thalassemia. Hematology Am Soc Hematol Educ Program. 2008:177-85. [PubMed ID: 19074078]. https://doi.org/10.1182/asheducation-2008.1.177.
-
3.
Vinchi F, De Franceschi L, Ghigo A, Townes T, Cimino J, Silengo L, et al. Hemopexin therapy improves cardiovascular function by preventing heme-induced endothelial toxicity in mouse models of hemolytic diseases. Circulation. 2013;127(12):1317-29. [PubMed ID: 23446829]. https://doi.org/10.1161/CIRCULATIONAHA.112.130179.
-
4.
Tolosano E, Fagoonee S, Morello N, Vinchi F, Fiorito V. Heme scavenging and the other facets of hemopexin. Antioxid Redox Signal. 2010;12(2):305-20. [PubMed ID: 19650691]. https://doi.org/10.1089/ars.2009.2787.
-
5.
Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106(7):2572-9. [PubMed ID: 15947085]. https://doi.org/10.1182/blood-2005-03-1185.
-
6.
Jeney V, Balla G, Balla J. Red blood cell, hemoglobin and heme in the progression of atherosclerosis. Front Physiol. 2014;5:379.
-
7.
Nielsen MJ, Moller HJ, Moestrup SK. Hemoglobin and heme scavenger receptors. Antioxid Redox Signal. 2010;12(2):261-73. [PubMed ID: 19659436]. https://doi.org/10.1089/ars.2009.2792.
-
8.
Schaer DJ, Vinchi F, Ingoglia G, Tolosano E, Buehler PW. Haptoglobin, hemopexin, and related defense pathways-basic science, clinical perspectives, and drug development. Front Physiol. 2014;5:415. [PubMed ID: 25389409]. https://doi.org/10.3389/fphys.2014.00415.
-
9.
Smith A, McCulloh RJ. Hemopexin and haptoglobin: allies against heme toxicity from hemoglobin not contenders. Front Physiol. 2015;6:187. [PubMed ID: 26175690]. https://doi.org/10.3389/fphys.2015.00187.
-
10.
Vinchi F, Tolosano E. Therapeutic approaches to limit hemolysis-driven endothelial dysfunction: scavenging free heme to preserve vasculature homeostasis. Oxid Med Cell Longev. 2013;2013:396527. [PubMed ID: 23781294]. https://doi.org/10.1155/2013/396527.
-
11.
Wahl S, Quirolo KC. Current issues in blood transfusion for sickle cell disease. Curr Opin Pediatr. 2009;21(1):15-21. [PubMed ID: 19242238]. https://doi.org/10.1097/MOP.0b013e328321882e.
-
12.
Mauk MR, Smith A, Mauk AG. An alternative view of the proposed alternative activities of hemopexin. Protein Sci. 2011;20(5):791-805. [PubMed ID: 21404362]. https://doi.org/10.1002/pro.616.
-
13.
Wallon UM, Overall CM. The hemopexin-like domain (C domain) of human gelatinase A (matrix metalloproteinase-2) requires Ca2+ for fibronectin and heparin binding. Binding properties of recombinant gelatinase A C domain to extracellular matrix and basement membrane components. J Biol Chem. 1997;272(11):7473-81. [PubMed ID: 9054449].
-
14.
Roeb E, Schleinkofer K, Kernebeck T, Potsch S, Jansen B, Behrmann I, et al. The matrix metalloproteinase 9 (mmp-9) hemopexin domain is a novel gelatin binding domain and acts as an antagonist. J Biol Chem. 2002;277(52):50326-32. [PubMed ID: 12384502]. https://doi.org/10.1074/jbc.M207446200.
-
15.
Satoh T, Satoh H, Iwahara S, Hrkal Z, Peyton DH, Muller-Eberhard U. Roles of heme iron-coordinating histidine residues of human hemopexin expressed in baculovirus-infected insect cells. Proc Natl Acad Sci U S A. 1994;91(18):8423-7. [PubMed ID: 8078898].
-
16.
Bakker WW, Borghuis T, Harmsen MC, van den Berg A, Kema IP, Niezen KE, et al. Protease activity of plasma hemopexin. Kidney Int. 2005;68(2):603-10. [PubMed ID: 16014037]. https://doi.org/10.1111/j.1523-1755.2005.00438.x.
-
17.
Walsh G, Jefferis R. Post-translational modifications in the context of therapeutic proteins. Nat Biotechnol. 2006;24(10):1241-52. [PubMed ID: 17033665]. https://doi.org/10.1038/nbt1252.
-
18.
Jayapal KP, Wlaschin KF, Hu W, Yap MGS. Recombinant protein therapeutics from CHO cells-20 years and counting. Chem Engin Prog. 2007;103(10):40.
-
19.
Schirrmann T, Al-Halabi L, Dubel S, Hust M. Production systems for recombinant antibodies. Front Biosci. 2008;13:4576-94. [PubMed ID: 18508530].
-
20.
Wiberg FC, Rasmussen SK, Frandsen TP, Rasmussen LK, Tengbjerg K, Coljee VW, et al. Production of target-specific recombinant human polyclonal antibodies in mammalian cells. Biotechnol Bioeng. 2006;94(2):396-405. [PubMed ID: 16596663]. https://doi.org/10.1002/bit.20865.
-
21.
Boeger H, Bushnell DA, Davis R, Griesenbeck J, Lorch Y, Strattan JS, et al. Structural basis of eukaryotic gene transcription. FEBS Lett. 2005;579(4):899-903. [PubMed ID: 15680971]. https://doi.org/10.1016/j.febslet.2004.11.027.
-
22.
Kim JY, Kim YG, Lee GM. CHO cells in biotechnology for production of recombinant proteins: current state and further potential. Appl Microbiol Biotechnol. 2012;93(3):917-30. [PubMed ID: 22159888]. https://doi.org/10.1007/s00253-011-3758-5.
-
23.
Ghaderi D, Zhang M, Hurtado-Ziola N, Varki A. Production platforms for biotherapeutic glycoproteins. Occurrence, impact, and challenges of non-human sialylation. Biotechnol Genet Eng Rev. 2012;28:147-75. [PubMed ID: 22616486].
-
24.
Xu X, Nagarajan H, Lewis NE, Pan S, Cai Z, Liu X, et al. The genomic sequence of the Chinese hamster ovary (CHO)-K1 cell line. Nat Biotechnol. 2011;29(8):735-41. [PubMed ID: 21804562]. https://doi.org/10.1038/nbt.1932.
-
25.
Lai T, Yang Y, Ng SK. Advances in Mammalian cell line development technologies for recombinant protein production. Pharmaceuticals (Basel). 2013;6(5):579-603. [PubMed ID: 24276168]. https://doi.org/10.3390/ph6050579.
-
26.
Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393-8. [PubMed ID: 15529164]. https://doi.org/10.1038/nbt1026.
-
27.
Kaufman RJ. Selection and coamplification of heterologous genes in mammalian cells. Methods Enzymol. 1990;185:537-66. [PubMed ID: 1974323].
-
28.
Pilbrough W, Munro TP, Gray P. Intraclonal protein expression heterogeneity in recombinant CHO cells. PLoS One. 2009;4(12). e8432. [PubMed ID: 20037651]. https://doi.org/10.1371/journal.pone.0008432.
-
29.
Poli V, Cortese R. Interleukin 6 induces a liver-specific nuclear protein that binds to the promoter of acute-phase genes. Proc Natl Acad Sci U S A. 1989;86(21):8202-6. [PubMed ID: 2479021].
-
30.
Kapojos JJ, van den Berg A, van Goor H, te Loo MW, Poelstra K, Borghuis T, et al. Production of hemopexin by TNF-alpha stimulated human mesangial cells. Kidney Int. 2003;63(5):1681-6. [PubMed ID: 12675843]. https://doi.org/10.1046/j.1523-1755.2003.00907.x.
-
31.
Schaer DJ, Buehler PW. Cell-free hemoglobin and its scavenger proteins: new disease models leading the way to targeted therapies. Cold Spring Harb Perspect Med. 2013;3(6). [PubMed ID: 23645855]. https://doi.org/10.1101/cshperspect.a013433.
-
32.
Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, et al. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest. 2012;122(4):1444-58. [PubMed ID: 22446185]. https://doi.org/10.1172/JCI59770.
-
33.
Khow O, Suntrarachun S. Strategies for production of active eukaryotic proteins in bacterial expression system. Asian Pac J Trop Biomed. 2012;2(2):159-62. [PubMed ID: 23569889]. https://doi.org/10.1016/S2221-1691(11)60213-X.
-
34.
Eiberle MK, Jungbauer A. Technical refolding of proteins: Do we have freedom to operate? Biotechnol J. 2010;5(6):547-59. [PubMed ID: 20518058]. https://doi.org/10.1002/biot.201000001.
-
35.
Gerstein AS. Molecular biology problem solver: a laboratory guide. John Wiley & Sons; 2004.
-
36.
Macauley-Patrick S, Fazenda ML, McNeil B, Harvey LM. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22(4):249-70. [PubMed ID: 15704221]. https://doi.org/10.1002/yea.1208.
-
37.
Paridar M, Amirizadeh N, Habibi Roudkenar M, Amiri F, Abolghasemi H, Jalili MA. Expression of Recombinant Coagulation Factor IX in Human Amniotic Membrane-derived Mesenchymal Stem Cells: A New Strategy to Gene Therapy of Hemophilia B. Iran J Blood Cancer. 2014;6(3):133-41.
-
38.
Li P, Anumanthan A, Gao XG, Ilangovan K, Suzara VV, Duzgunes N, et al. Expression of recombinant proteins in Pichia pastoris. Appl Biochem Biotechnol. 2007;142(2):105-24. [PubMed ID: 18025573].
-
39.
Fischer S, Charara N, Gerber A, Wolfel J, Schiedner G, Voedisch B, et al. Transient recombinant protein expression in a human amniocyte cell line: the CAP-T(R) cell system. Biotechnol Bioeng. 2012;109(9):2250-61. [PubMed ID: 22488157]. https://doi.org/10.1002/bit.24514.
-
40.
Son KK, Patel DH, Tkach D, Park A. Cationic liposome and plasmid DNA complexes formed in serum-free medium under optimum transfection condition are negatively charged. Biochim Biophys Acta. 2000;1466(1-2):11-5. [PubMed ID: 10825426].
-
41.
A. Longo P, Kavran JM, Kim MS, Leahy DJ. Generating mammalian stable cell lines by electroporation. Methods Enzymol. 2013;529:209-26. [PubMed ID: 24011048]. https://doi.org/10.1016/B978-0-12-418687-3.00017-3.