Abstract
Background:
Continuous and indiscriminate use of chemical drugs causes an important phenomenon of resistance to microorganisms. Accordingly, the effect of medications is minimized or offset, increasing drug use and the need to study mixtures with more latest and powerful formulations. On the other hand, it has been reported that many plants essential oils have a significant inhibitory effect on pathogenic microorganisms.Objectives:
This study aimed to evaluate the antimicrobial activity of some curative herbs against some clinical bacteria of humans and sheep.Methods:
The leaves of chicory (Cichorium intybus L.), Hypericum perforatum L., Lavandula angustifolia, Thymus vulgaris L., and Taxus baccata L. were collected and determined in the botanical laboratory of the University of Zabol. Forty grams of dried leaves was used in 400 mL of ethanol (96%) to prepare the ethanolic extract. DPPH was used to determine the activity of reactive oxygen species (ROS) trapping. The antimicrobial effects were studied by the disk diffusion (6 mm) method in Müller-Hinton agar medium according to the method by Bauer et al.Results:
The minimum inhibitory concentrations (MICs) of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in human clinical Staphylococcus aureus were 6.25, 12.5, 3.1, 25, and 6.25 ppm, respectively, but, in sheep, clinical S. aureus, were 12.5, 6.25, 3.1, 12.5, and 25 ppm, respectively. The minimum bactericidal concentrations (MBCs) of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in human clinical S. aureus were 12.5, 25, 6.25, 50, and 12.5 ppm, respectively, but, in sheep clinical S. aureus, were 25, 12.5, 6.25, 25, and 50 ppm, respectively. The most effective extract in inhibiting the growth of S. aureus was the H. perforatum L. extract with an 8.9-mm diameter growth inhibition zone.Conclusions:
Regarding the side effects of artificial medications and antimicrobials, as well as the significant influence of healing herb extracts used in this study, it was found that H. perforatum was the most effective plant against S. aureus. It should be noted that plant extracts were more effective in human clinical S. aureus than in sheep clinical S. aureus.Keywords
Taxus baccata Thymus vulgaris Hypericum perforatum Escherichia coli Lavandula angustifolia Staphylococcus aureus
1. Background
Continuous and indiscriminate use of chemical drugs causes an important phenomenon of resistance to microorganisms. Accordingly, the influence of medicines is minimized or offset, increasing drug use and the tendency to use new compounds. Also, another problem of using these medications is increased side effects, leading to diseases that are more dangerous than the original disease (1, 2).
Many plant essential oils have been reported to have significant inhibitory effects on pathogenic microorganisms, and it has been shown that most herb essential oils obtained from medicinal herbs have antifungal, antiparasitic, antibacterial, and antimicrobial properties; therefore, plant essential oils have been greatly utilized in some fields such as pharmacology, herbal pharmacology, pharmaceutical/clinical microbiology, and phytopathology, as well as maintenance of food, fruits, and vegetables (3, 4). These herbal medicines are popular among people (5).
Plants have played significant roles in maintaining human health and promoting the quality of life for thousands of years. In recent years, herbal products (secondary metabolites) have been used to treat most human and animal diseases due to their easy availability, ease of use, and fewer side effects compared to chemical products (6-8). On the other hand, plant-derived secondary metabolites, such as phenol and total flavonoids, have a strong potential to scavenge free radicals that are present in all different parts of the plant, such as leaves, fruits, seeds, roots, and skin. Therefore, regarding the high predominance of chronic and erosive diseases, it is reasonable to use plants to provide the antioxidants needed by the body, especially plants that are high in phenol and flavonoids. Therefore, to provide the natural antioxidants needed by the body, the consumption of plants with high phenolic compounds is recommended (9).
Phenolic compounds and flavonoids have several biological properties, such as trapping free radicals and antioxidant and anti-inflammatory properties. These compounds prevent or delay oxidative damage to fats and other important molecules and prevent cancer and coronary heart disease (10-12). Phenolic compounds are among the compounds that are present in all plants, including fruits, vegetables, grains, etc. These compounds are among the secondary metabolites of plants. Naturally, there are over 8000 different phenolic compounds with effects such as involvement in cell wall construction, involvement in plant defense mechanisms, and involvement in fruit properties (such as color, aroma, taste, and taste in plants). Phenolic compounds are also considered as indicators for physiological stages during fruit growth (10).
Studies have shown that the source of phenols and flavonoids in different parts of the world depends on the diet of people in the region. For instance, in countries such as Japan and China, the consumption of green tea provides these compounds needed by the body, while in Western countries, these substances are supplied by consuming apples and onions, and in Eastern countries, by consuming fermented vegetables and foods (13).
In Iran, no specific source is widely used for antioxidant intake; in other words, various sources, such as raw and cooked vegetables, leaves of various plants and trees (in the form of infusions, essences, essential oils, extracts, jams, syrups, pickles, ingredients of detergents [including cedar], and even in the form of curds), are used in this regard.
Secondary metabolites of medicinal herbs have been studied for their antimicrobial effects. It has been estimated that at least one-third of all therapeutic outputs are originated from plants or are modified after extraction from plants. Besides preventing the growth of bacteria and mold contaminating food, these materials are used to improve the shelf life of processed fruits and vegetables (14, 15).
2. Objectives
Given that various curative herbs have different influences on microorganisms, and the use of different antibiotics is sadly common among people (16), in the present study, we tried to study some of these medicinal plants, including Cichorium intybus L. (17, 18), Hypericum perforatum L. (19, 20), Lavandula angustifolia (21, 22), Thymus vulgaris L. (23), and Taxus baccata (24), in human and sheep clinical Staphylococcus aureus.
3. Methods
3.1. Plant Materials
Cichorium intybus L., H. perforatum L., L. angustifolia, and T. vulgaris L. were collected from Shahrekord (Figure 1). The leaves of the yew tree (T. baccata L.; Figure 1) were collected from Behshahr. The plant species were identified in the botanical laboratory of the University of Zabol.
3.2. Ethanolic Extract Preparation Method
Forty grams of dried leaves (in the shade and the vicinity of air) of each plant were used. They were soaked in 400 mL of ethanol (96%) and then shaken in a shaker (SKIR-601L, UniEquip, Germany) for 48 hours at room temperature. After the desired time, the extracts were filtered. The solvent was evaporated at 40°C by a rotary device. Further, 100 mg of plant extract powder was weighed and dissolved in dimethyl sulfoxide (DMSO) solvent (1 mL). The plant extracts were saved at a temperature of 4°C (4).
3.3. Bacterial and Fungal Strains
Clinical strains of bacteria (such as S. aureus) were prepared from male patients (human) and sheep in Zabol City.
The appearance of plants used Thymus vulgaris L. (A), Chicory (B), Hypericum perforatum L. (C), Lavandula angustifolia (D), and yew (E). (Reference; Section A (25) And Sections B, C, and D (26), Section E (27)).

3.4. Determination of Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Essential Oils in Escherichia coli
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) were determined for plants’ essential oils (28, 29). The minimum inhibitory concentration was defined as the lowest concentration of plant extracts to stop the growth of bacteria at the end of 24 hours of incubation. The minimum bactericidal concentration was defined as the lowest concentration of plant extracts in which 99.9% of bacteria did not grow (28, 29). All antimicrobial tests were repeated 3 times.
3.5. Antimicrobial Activity
The antimicrobial activities of 5 types of plant extract against human microbes were conducted by the disk diffusion (6 mm) method (30). The determination of microbial susceptibility was conducted according to the method by Bauer et al. (31).
The disks were exposed to UV light for 2 hours to be sterilized. After preparing the microbial suspension, the plates were inoculated with sterile swabs impregnated with microbial suspension for 5 minutes. After complete contact with the culture medium, 10 μL of plant extracts were poured onto the disks with a sterile sampler. Then, the plates were kept in an incubator for 18 to 24 hours at 37°C. Next, the diameter of the non-growth zones was measured with a caliper. To avoid any errors in the obtained results, this experiment was repeated 3 times (30, 32).
3.6. Data Analysis
The experiment was done 3 times. The Statistix software package version 10 (Analytical Software, Florida, USA) was used for statistical analysis. Mean comparisons were performed using the least significant difference (LSD) at a 1% probability level. Excel 2010 was used to draw the figures.
4. Results
4.1. Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts
The MIC of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in human clinical S. aureus were 6.25, 12.5, 3.1, 25, and 6.25 ppm, respectively, while MBC in the mentioned extracts were 12.5, 25, 6.2, 50, and 12.5 ppm, respectively (Table 1). The MIC of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in sheep clinical S. aureus were 12.5, 6.25, 3.1, 12.5, and 25 ppm, respectively, while MBC in the mentioned extracts were 25, 12.5, 6.2, 25, and 50 ppm, respectively (Table 2).
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts in Human Clinical Staphylococcus aureus
| Bacteria Strains | Taxus baccata L. MIC/MBC | Nepeta binaludensis Jamzad MIC/MBC | Hypericum perforatum L. MIC/MBC | Thymus vulgaris L. MIC/MBC | Cichorium intybus L. MIC/MBC |
|---|---|---|---|---|---|
| 1 | Not growth | 25 - 50 | 12.5 - 25 | 12.5 - 25 | 25 - 50 |
| 2 | 6.25 - 12.5 | 25 - 50 | 3.1 - 6.25 | 12.5 - 25 | 25 - 50 |
| 3 | 6.25 - 12.5 | 25 - 50 | 3.1 - 6.25 | 12.5 - 25 | 25 - 50 |
| 4 | 50 - 100 | 25 - 50 | 6.25 - 12.5 | 12.5 - 25 | 6.25 - 12.5 |
| 5 | Not growth | 25 - 50 | 6.25 - 12.5 | 12.5 - 25 | 25 - 50 |
| 6 | 25 - 50 | 50 - 100 | 6.25 - 12.5 | 12.5 - 25 | 25 - 50 |
| 7 | 6.25 - 12.5 | 50 - 100 | Not growth | 12.5 - 25 | 6.25 - 12.5 |
| 8 | 25 - 50 | 50 - 100 | Not growth | 12.5 - 25 | 25 - 50 |
| 9 | Not growth | Not growth | Not growth | 25 - 50 | 12.5 - 25 |
Minimum Inhibitory Concentration and Minimum Bactericidal Concentration of Plant Extracts in Sheep Clinical Staphylococcus aureus
| Bacteria Strains | Taxus baccata L. MIC/MBC | Nepeta binaludensis Jamzad MIC/MBC | Hypericum perforatum L. MIC/MBC | Thymus vulgaris L. MIC/MBC | Cichorium intybus L. MIC/MBC |
|---|---|---|---|---|---|
| 1 | 25 - 50 | 50 - 100 | Not growth | 25 - 50 | 12.5 - 25 |
| 2 | 50 - 100 | 50 - 100 | Not growth | 12.5 - 25 | 25 - 50 |
| 3 | 50 - 100 | 12.5 - 25 | Not growth | 12.5 - 25 | 25 - 50 |
| 4 | 25 - 50 | 50 - 100 | 3.1 - 6.25 | 12.5 - 25 | 50 - 100 |
| 5 | 25 - 50 | 50 - 100 | 3.1 - 6.25 | 6.25 - 12.5 | 50 - 100 |
| 6 | 50 - 100 | 25 - 50 | Not growth | Not growth | 50 - 100 |
| 7 | 50 - 100 | 25 - 50 | Not growth | 12.5 - 25 | 50 - 100 |
| 8 | 50 - 100 | 12.5 - 25 | Not growth | Not growth | 25 - 50 |
| 9 | 50 - 100 | 12.5 - 25 | 3.1 - 6.25 | Not growth | 25 - 50 |
4.2. Diameter of Inhibition Zone of Plant Extracts in Human and Sheep Clinical Staphylococcus aureus
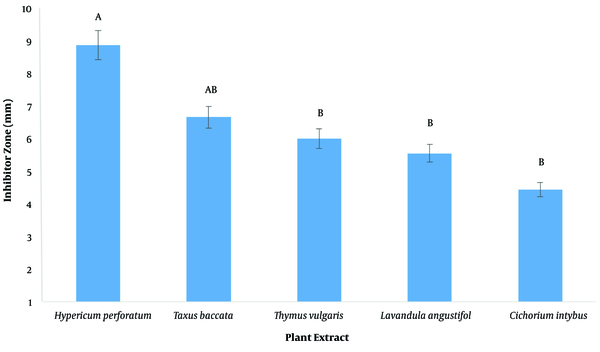
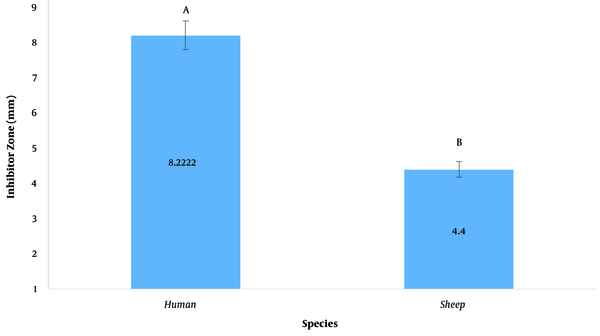
The inhibitory diameter of plant extracts against human and sheep S. aureus at a dilution of 100 ppm was investigated, and it was found that different extracts had different effects on inhibiting bacterial growth (P < 0.01; Figure 2; Table 3). The LSD post hoc test showed that among plant extracts, the most effective extract in inhibiting the growth of S. aureus was the H. perforatum L. extract with an 8.9-mm diameter growth inhibition zone. In addition, the yew tree with a 6.6-mm diameter of growth inhibition halo was ranked second (Figure 3). It should be noted that plant extracts were more effective in human clinical S. aureus than in sheep clinical S. aureus (Figure 4).
Diameter of plant growth inhibition zone in sheep and human clinical Staphylococcus aureus (A [Cichorium intybus L.], B [Hypericum perforatum], C [Taxus baccata], D [Lavandula angustifolia], E [Thymus vulgaris L.], F [Cichorium intybus], G [Hypericum perforatum], H [Taxus baccata], I [Lavandula angustifolia], J [Thymus vulgaris L.])
![Diameter of plant growth inhibition zone in sheep and human clinical Staphylococcus aureus (A [Cichorium intybus L.], B [Hypericum perforatum], C [Taxus baccata], D [Lavandula angustifolia], E [Thymus vulgaris L.], F [Cichorium intybus], G [Hypericum perforatum], H [Taxus baccata], I [Lavandula angustifolia], J [Thymus vulgaris L.]) Diameter of plant growth inhibition zone in sheep and human clinical Staphylococcus aureus (A [Cichorium intybus L.], B [Hypericum perforatum], C [Taxus baccata], D [Lavandula angustifolia], E [Thymus vulgaris L.], F [Cichorium intybus], G [Hypericum perforatum], H [Taxus baccata], I [Lavandula angustifolia], J [Thymus vulgaris L.])](https://services.brieflands.com/cdn/serve/315dd/dd5a29c4de348513e90f09935e68786af293fe7b/gct-9-2-118752-g002-preview.png)
Variance Analysis of Growth Inhibition Zone Diameter of Different Plants Extracts Against Different Bacterial Agents at a Dilution of 100 ppm a
| Source | DF | SS | MS | F |
|---|---|---|---|---|
| Species | 1 | 328.71 | 328.711 | 23.05** |
| Bacteria | 4 | 196.62 | 49.156 | 3.45* |
| Species*bacteria | 4 | 53.07 | 13.267 | 0.93ns |
| Error | 80 | 1140.89 | 14.261 | |
| Total | 89 | 1719.29 |
Evaluation of diameter of growth inhibition zone, plant extract in growth inhibition of Staphylococcus aureus. Similar letters indicate no significant difference.
Growth inhibition zone diameter of plant extracts in sheep and human clinical Staphylococcus aureus. Similar letters indicate no significant difference.
5. Discussion
The MICs of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in human clinical S. aureus were 6.25, 12.5, 3.1, 25, and 6.25 ppm, respectively but, in sheep clinical S. aureus, were 12.5, 6.25, 3.1, 12.5, and 25 ppm, respectively. The MBCs of chicory, thyme, H. perforatum, French lavender, and yarrow extracts in human clinical S. aureus were 12.5, 25, 6.25, 50, and 12.5 ppm, respectively but, in sheep clinical S. aureus, were 25, 12.5, 6.25, 25, and 50 ppm, respectively. The most effective extract in inhibiting the growth of S. aureus was the H. perforatum L. extract with an 8.9-mm diameter growth inhibition zone.
The antimicrobial activity of bacteriocin-like compounds produced by lactic acid bacteria isolated from sheep milk, yogurt, and local butter against food-borne bacteria was found; in the punctuation method, at least one of the indicator bacteria showed inhibitory properties. However, in in the well method, this number was reduced to 39 isolates. All these interesting physicochemical properties allow the cell-free extract (supernatant) of some of these isolates to be considered as biological preservatives in food (33). In the present study, out of 45 tested strains, 38 human and 36 sheep clinical strains were inhibited by all extracts.
In a study, the chemical composition and antibacterial activity of Iranian Lavandula × hybrid were investigated, and it was concluded that the diameter of the growth inhibition zone was obtained in a range from 9.36 mm against S. aureus to 23.3 mm against Escherichia coli. They also reported that there was a significant relationship between the composition of essential oil and the level of antibacterial effect expressed as inhibition areas (34). In the present study, the majority of plant extracts were effective against S. aureus.
The effect of basil essential oil was investigated on the microbial and sensory characteristics of Iranian traditional white cheese during ripening; the results showed that the concentration of 150 mg/kg of essential oil on day 90 and the concentration of 250 mg/kg on days 30, 60, and 90 had an effect on the number of S. aureus and showed significant inhibition (35). In the present study, it was found that the plant extracts used in the minimum suffering, between 3.1 to 25 ppm, inhibited the growth of S. aureus.
The effects of eucalyptus (Eucalyptus globulus L.) leaf powder and its essential oil were investigated on growth performance and immune response of broiler chickens; the results showed that chickens receiving ELP and EEO had higher antibodies against sheep erythrocytes (SRBC) compared to the initial control response. However, no difference was observed in the secondary antibody response against SRBC (36).
The antibacterial effects of nisin on S. aureus in refrigerated mutton were found, and it was concluded that different amounts of nisin had a significant effect on the growth of the studied bacterium, but over time, the inhibitory properties of nisin against the growth of S. aureus were reduced (37). In general, it can be concluded that the antimicrobial properties and also the use of plants to improve the immune system of animals vary depending on the plant species and type of microbes.
The synthesis of stable colloidal silver nanoparticles with antibacterial properties using the T. baccata extract was investigated; the results showed the MIC for E. coli and S. aureus obtained at a concentration of 25 μg/mL (38). The present study found that the MICs of ethanolic extracts of chicory, thyme, hops, and yew in human clinical S. aureus were 6.25, 12.5, 3.1, 25, and 6.25, respectively. The mentioned extracts in sheep clinical S. aureus were 12.5, 6.25, 3.1, 12.5, and 25 ppm, respectively. In general, it can be concluded that the MIC against bacteria varies, depending on the type of plant and microbe.
Different levels of the cheese extract were studied on performance, carcass characteristics, and some safety and blood parameters of broilers. The results showed a significant difference between the experimental treatments regarding safety parameters. At 32 days of age, the highest anti-SRBC antibody was observed in 0.3% treatment, and, in 39 days, the highest property was observed in 0.2% and 0.3%. In general, they concluded that the cheese extract in the diet could affect the bird’s immune system (39).
The effects of turmeric, cinnamon powder, and probiotic and antibiotic supplements in diets were investigated on broiler performance, blood biochemistry, and immune. The results showed that the use of various additives had no effect on antibody titer against sheep erythrocytes (40). Considering the positive effect of plant extracts used in the present study in sheep clinical S. aureus and especially the greater effect of herbaceous herb, it is suggested that additional tests be used in the diet to possibly improve the immune system of sheep.
Although the occurrence of antimicrobial activity is often very obvious, its mechanism of action is not fully understood. There is evidence that essential oils exert their antibacterial effect by altering the structure and function of cell membranes. Studies on the mechanism of action of essential oils have shown that these compounds increase membrane permeability. The essential oil components penetrate the membrane, causing the membrane to swell and affect (reduce) its activity, eventually leading to cell death. Essential oil components also have different antibacterial effects. Even hydroxyl groups in the molecule of essential oils (such as carvacrol, thymol, paracetamol, and menthol) are very important for their antibacterial properties (1, 41).
This study proved that the 5 studied species have significant antibacterial activity. These observations also revealed that licorice extract has the strongest antibacterial properties against S. aureus among the 5 tested species (42, 43).
Many herbal remedies have a synergistic effect on 1 or more target areas. Recently, in order to overcome the obstacles and limitations of using essential oils as antimicrobials in the food industry, cases such as oxidation process, evaporation, dissolution problems, reaction with other substances, change of aroma, and organoleptic properties of food and encapsulation techniques have been studied. Therefore, in future research, it is suggested to study these ideas.
5.1. Conclusions
Considering the side effects of chemical drugs and antibiotics, as well as the considerable effect of medicinal plant extracts used in this study, it was found that H. perforatum was the most effective plant against S. aureus.
Chemical compounds passed the test; thus, they are valid in short- and even medium-term use, but in long-term use, they may be dangerous. However, medicinal plants, many of which have been approved for their edible properties and have adapted to the human body over time, have far fewer risks than chemicals. However, the duration of treatment with herbal substances is longer than chemicals; thus, in the treatments that need to be urgent, the same chemical drugs are currently offered. Herbs are very useful for many treatments that do not need immediate treatment.
References
-
1.
Valizadeh M, ur Rehman F, Hassanzadeh MA, Beigomi M, Fazeli-Nasab B. Investigating the Antimicrobial Effects of Glycyrrhiza glabra and Salvia officinalis Ethanolic Extract Against Helicobacter pylori. Int J Infect. 2021;8(4). https://doi.org/10.5812/iji.114563.
-
2.
Rohani R, Biabangard A, Beigomi M. Evaluation of antimicrobial effects of Rosemary and Withania somnifera methanol extract prepared by ultrasound waveform on Escherichia coli biofilm isolated from urinary tract infection. Micro Environer. 2021;1(1):17-25. https://doi.org/10.54458/mev.v1i01.6670.
-
3.
Rota C, Carraminana JJ, Burillo J, Herrera A. In vitro antimicrobial activity of essential oils from aromatic plants against selected foodborne pathogens. J Food Prot. 2004;67(6):1252-6. [PubMed ID: 15222560]. https://doi.org/10.4315/0362-028x-67.6.1252.
-
4.
Ahmadi H, Jahantigh M. Analysis of the antimicrobial activity of Ashurak extracts prepared with different solvents on Klebsiella pneumoniae and Shigella dysentery isolated from poultry faeces. Micro Environer. 2021;1(1):54-62. https://doi.org/10.54458/mev.v1i01.6673.
-
5.
Beigomi Z, Behzadmehr R, Shahraki-Mojahed L. Antimicrobial Effects of Ethanol, Methanol and Ethyl Acetate Teucrium polium and Citrullus colocynthis extract on Pseudomonas aeruginosa. Micro Environer. 2021;1(1):26-32. https://doi.org/10.54458/mev.v1i01.6671.
-
6.
Gertsch J. The Metabolic Plant Feedback Hypothesis: How Plant Secondary Metabolites Nonspecifically Impact Human Health. Planta Med. 2016;82(11-12):920-9. [PubMed ID: 27286339]. https://doi.org/10.1055/s-0042-108340.
-
7.
Bernardini S, Tiezzi A, Laghezza Masci V, Ovidi E. Natural products for human health: an historical overview of the drug discovery approaches. Nat Prod Res. 2018;32(16):1926-50. [PubMed ID: 28748726]. https://doi.org/10.1080/14786419.2017.1356838.
-
8.
Gurib-Fakim A. Medicinal plants: traditions of yesterday and drugs of tomorrow. Mol Aspects Med. 2006;27(1):1-93. [PubMed ID: 16105678]. https://doi.org/10.1016/j.mam.2005.07.008.
-
9.
Karabulut F. Emerging trends for Harnessing plant metabolome and microbiome for sustainable food Production. Micro Environer. 2021;1(1):33-53. https://doi.org/10.54458/mev.v1i01.6672.
-
10.
Goncalves A, Silva E, Brito C, Martins S, Pinto L, Dinis LT, et al. Olive tree physiology and chemical composition of fruits are modulated by different deficit irrigation strategies. J Sci Food Agric. 2020;100(2):682-94. [PubMed ID: 31583702]. https://doi.org/10.1002/jsfa.10064.
-
11.
Chylinska M, Szymanska-Chargot M, Derylo K, Tchorzewska D, Zdunek A. Changing of biochemical parameters and cell wall polysaccharides distribution during physiological development of tomato fruit. Plant Physiol Biochem. 2017;119:328-37. [PubMed ID: 28942290]. https://doi.org/10.1016/j.plaphy.2017.09.010.
-
12.
Williams RS, Benkeblia N. Biochemical and physiological changes of star apple fruit ( Chrysophyllum cainito) during different “on plant” maturation and ripening stages. Sci Hortic. 2018;236:36-42. https://doi.org/10.1016/j.scienta.2018.03.007.
-
13.
Wach A, Pyrzyńska K, Biesaga M. Quercetin content in some food and herbal samples. Food Chem. 2007;100(2):699-704. https://doi.org/10.1016/j.foodchem.2005.10.028.
-
14.
Savithramma N, Rao ML, Suhrulatha D. Screening of medicinal plants for secondary metabolites. Middle East J Sci Res. 2011;8(3):579-84.
-
15.
Soni U, Brar S, Gauttam VK. Effect of seasonal variation on secondary metabolites of medicinal plants. Int J Pharm Sci Res. 2015;6(9):3654-62.
-
16.
Mulat M, Pandita A, Khan F. Medicinal Plant Compounds for Combating the Multi-drug Resistant Pathogenic Bacteria: A Review. Curr Pharm Biotechnol. 2019;20(3):183-96. [PubMed ID: 30854956]. https://doi.org/10.2174/1872210513666190308133429.
-
17.
Hadizadeh H, Mohebodini M, Esmaeilpoor B, Chamani E. [Studies on callus induction and regeneration of medicinal plant chicory (Cichorium intybus L.) from leaf and petiole explants]. Int J Hortic Sci. 2016;29(4):621-30. Persian.
-
18.
Othman M, Helmi L, Hosni A. Effect of Growth Regulator NAA and IBA Applications on Total Phenolic and Flavonoid Compounds Extracted from In Vitro Produced Callus of Chicory Plant (Cichorium Intybus L.). Arab Univ J Agric Sci. 2019;27(3):1929-36. https://doi.org/10.21608/ajs.2019.15333.1067.
-
19.
Babaei Z, Solouki M, Fazeli-Nasab B. [Investigating The Effect of Biological and non-Biological Elicitor on Expression of Hyp-1 Gene in Hypericum perforatum]. Mod Genet. 2019;13(4):543-9. Persian.
-
20.
Ng QX, Venkatanarayanan N, Ho CY. Clinical use of Hypericum perforatum (St John's wort) in depression: A meta-analysis. J Affect Disord. 2017;210:211-21. [PubMed ID: 28064110]. https://doi.org/10.1016/j.jad.2016.12.048.
-
21.
Yaghoobi K, Kaka GR, Sh D, Ashayeri H. [Therapeutic effects of Lavandula angustifolia]. J Gorgan Univ Med Sci. 2016;17(4):1-9. Persian.
-
22.
Radulescu C, Stihi C, Ilie M, Lazurcă D, Gruia R, Olaru OT, et al. Characterization of Phenolics in Lavandula angustifolia. Anal Lett. 2017;50(17):2839-50. https://doi.org/10.1080/00032719.2016.1264409.
-
23.
Chen Y, Guo Q, Liu L, Liao L, Zhu Z. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J Med Plant Res. 2011;5(9):1749-55.
-
24.
Vanisree M, Lee C, Lo S, Nalawade SM, Lin CY, Tsay H. Studies on the production of some important secondary metabolites from medicinal plants by plant tissue cultures. Bot Bull Acad Sin. 2004;45(1):1-22.
-
25.
Iranbehlimo. Thymus vulgaris. 2021. Available from: http://iranbehlimo.com.
-
26.
Nadjafi F, Koocheki A, Rezvani Moghaddam P, Honermeier B, Asili J. Autecology, ethnomedicinal and phytochemical studies of Nepeta binaludensis Jamzad, a highly endangered medicinal plant of Iran. Planta Med. 2007;73(9). https://doi.org/10.1055/s-2007-987389.
-
27.
Bazram. Yew seed. 2021. Available from: www.bazram.com/taxus-baccata-seed.
-
28.
Chikezie IO. Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using a novel dilution tube method. Afr J Microbiol Res. 2017;11(23):977-80. https://doi.org/10.5897/ajmr2017.8545.
-
29.
Lambert RJ, Pearson J. Susceptibility testing: accurate and reproducible minimum inhibitory concentration (MIC) and non-inhibitory concentration (NIC) values. J Appl Microbiol. 2000;88(5):784-90. [PubMed ID: 10792538]. https://doi.org/10.1046/j.1365-2672.2000.01017.x.
-
30.
Abubakar L, Mwangi C, Uku J, Ndirangu S. Antimicrobial activity of various extracts of the sea urchin Tripneustes gratilla (Echinoidea). Afr J Pharmacol Ther. 2012;1(1):19-23.
-
31.
Bauer AW, Kirby WM, Sherris JC, Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966;45(4):493-6. [PubMed ID: 5325707].
-
32.
Noor J, Razia K, Anwar S, Mohammad S, Siraj A. Comparison of antibacterial activity of parent plant of Tylophora indica Merr. with its in vitro raised plant and leaf callus. Afr J Biotechnol. 2013;12(31):4891-6. https://doi.org/10.5897/ajb2013.12488.
-
33.
Edalatian Dovom MR, Yavarmanesh M, Ghiamati Yazdi F, Khomeiri M, Nayyeri N. [Evaluation of antimicrobial activities of lactic flora isolated from production stages of Maskeh against food indicator bacteria]. Iran Food Sci Technol Res J. 2016;12(4):438-52. Persian.
-
34.
Bajalan I, Rouzbahani R, Pirbalouti AG, Maggi F. Chemical Composition and Antibacterial Activity of Iranian Lavandula x hybrida. Chem Biodivers. 2017;14(7). [PubMed ID: 28306205]. https://doi.org/10.1002/cbdv.201700064.
-
35.
Bakhshi F, Mirzaei H, Asefi N. [Effect of Basil Essential Oil on the Microbial and Sensory Characteristics of Iranian Traditional White Cheese During Ripening]. J Vet Res. 2020;75(1):47-56. Persian.
-
36.
Farhadi D, Karimi A, Sadeghi G, Sheikhahmadi A, Habibian M, Raei A, et al. Effects of using eucalyptus (Eucalyptusglobulus L.) leaf powder and its essential oil on growth performance and immune response of broiler chickens. Iran J Vet Res. 2017;18(1):60-2. [PubMed ID: 28588635]. [PubMed Central ID: PMC5454581].
-
37.
Ghashooni Zadeh R, Hosseini SE, Mahasti P, Shabani S. [The Antimicrobial Effect of nisin, Against Staphylococcus aureus in Minced Sheep during Refrigerated Storage]. J Food Microbiol. 2015;2(1):69-77. Persian.
-
38.
Abbasi Kajani A, Zarkesh-Esfahani SH, Bordbar A. [Synthesis of stable colloidal silver nanoparticles with antibacterial properties by using Taxus baccata extract]. Biol J Microorganisms. 2016;5(19):79-92. Persian.
-
39.
Mansourzadeh S, Salari S, Bojarpour M, Sari M, Ghorbanpor Najafabadi M. [Effects of various levels of Mallvasylvesteris extract on performance, carcass characteristics and some immune and blood parameters of broiler chickens]. Anim Sci J. 2015;28(106):3-12. Persian.
-
40.
Nemati MH, Hosseini SA, Ghasemloo V, Meimanipour A. [Effects of Turmeric, cinnamon powder, probiotic and antibiotic supplements in diets on broiler performance, blood biochemistry and immune response]. Anim Sci J. 2017;29(113):111-22. Persian. https://doi.org/10.22092/asj.2017.109468.
-
41.
Fazeli-Nasab B, Yazdanpour Z. [Antimicrobial effects of extract of Citrullus colocynthis and Teucrium polium on some Bacteria]. New Findings in Veterinary Microbiology. 2020;3(1):1-10. Persian.
-
42.
Malayeri FA, Yazdanpour Z, Bandani H, Fazeli Nasab B, Saeidi S. [Antimicrobial and anti-biofilm effects of Thyme essential oils and Peppermint on Acinetobacter baumannii and Staphylococcus aureus resistant to different antibiotics]. New Findings in Veterinary Microbiology. 2020;2(2):41-51. Persian.
-
43.
Saeidi S, Fazeli Nasab B. [Evaluation of antibacterial and antifungal activity of various extracts of the Rhazya stricta, Capparis spinosa, cretica Cressa]. New Findings in Veterinary Microbiology. 2019;2(1):57-66. Persian.