Abstract
Background:
LncRNAs have been shown to be implicated in the initiation and progression of gastric cancer (GC). Here, the transcription levels of the AK023391 and FOXO3a genes, as well as their relationship with clinical traits, were examined in GC.Methods:
This cross-sectional study was performed on 50 formalin-fixed paraffin-embedded (FFPE) GC tumor and 50 normal tissues. The RNA levels of the FOXO3a and AK023391 genes encoding lncRNAs were evaluated by real-time RT-PCR.Results:
A significant difference was observed in the mRNA level of AK023391 between GC tumor and normal tissues (Mean difference = 2.683, P = 0.0371). The transcription level of FOXO3A showed no remarkably significant difference between GC and normal tissues (Mean difference = 0.9177, P = 0.2199). In addition, AK023391 and FOXO3a mRNA expression levels showed no significant associations with the clinicopathologic features of GC. Also, we found no significant correlation between the expressions of AK023391 and FOXO3a in GC tissues (R = 0.014; P = 0.34).Conclusions:
Our finding suggested that there might be a significant relationship between the expression level of AK023391 and GC. Our results also showed that there was no correlation between the mRNA levels of the AK023391 and FOXO3a genes in GC tissues.Keywords
Gastric Cancer AK023391 FOXO3a Clinicopathological feature LncRNA
1. Background
Gastric cancer (GC) is the third principal cause of cancer-related death (1) and has a poor prognosis due to the lack of specific symptoms, leading to late diagnosis, as well as low survival rates (2, 3). Common chemotherapy drugs for the treatment of GC include paclitaxel (PTX), adriamycin (ADR), vincristine (VCR), 5-fluorouracil (5-FU), and platinum; however, chemoresistance is a major obstacle in treating GC (4). Therefore, understanding GC pathogenesis and finding new diagnostic and therapeutic biomarkers are necessary for the managing and targeted-therapy of GC.
Non-coding RNAs are classified into two groups: microRNAs (miRNAs, as a group of small ncRNAs, ~18 nt) and long non-coding RNAs (lncRNAs, > 200 nt) (5, 6). The recent group has been shown to play important roles in regulating gene transcription, post- transcriptional modifications, epigenetic regulation, and finally, protein translation (7). Various studies have shown that LncRNAs are involved in a variety of biological processes like chromatin regeneration, carcinogenesis, and cell differentiation (8). Furthermore, lncRNAs have been reported to be associated with tumor invasion, cellular proliferation, and metastasis in a variety of cancers (9-11). Studies have shown that several lncRNAs, such as HOXA11-AS, LINC00673, and XIST, facilitate GC progression by regulating β-catenin, LSD1, and miR-101 (12-14). On the other hand, linc00261 was shown to inhibit GC progression (15). Another lncRNA, AK023391, has also been reported to be involved in tumorigenesis and metastasis by activating the PI3K/Akt signaling pathway in GC (16). Also, investigating the profile of lncRNA expression revealed the upregulation of AK023391 in GC tissues and cell lines, indicating its important function in GC. Despite the fact that FISH analysis (using a tissue microarray) revealed no link between AK023391 expression and the clinicopathological features of 77 individuals with GC, Kaplan–Meier analysis revealed that elevated AK023391 expression was associated with poor survival in patients with GC. Furthermore, multivariate analysis indicated that AK023391 expression was an independent predictor of GC patients’ overall survival (OS) (16).
Forkhead box O3A (FOXO3a), also known as FOXO3 or forehead in rhabdomyosarcoma-like 1 (FKHRL1), is a member of the FOXO subfamily, which was initially discovered in the human placental cosmid. The FOXO3a gene is found on chromosome 6q21 (17) and regulates a wide range of cellular activities via controlling the expression and activity of effector genes. The activities and functions of FOXO3a are dependent on its subcellular location (18). The phosphorylation of FOXO3a leads to its translocation from the nucleus to the cytoplasm where it interacts with the 14-3-3 protein, preventing it from returning to the nucleus (19).
Forkhead box O3A is an important transcription factor that plays an essential role in tumor progression, angiogenesis, and cancer metastasis. This transcription factor is also important in GC progression by targeting the signaling pathway of PI3K/ protein kinase B (20). In addition, functional studies indicate that the upregulation of FOXO3a in GC tissues is associated with a poor prognosis in GC patients with stage II / III disease (21). More research is needed to identify an AK023391 LncRNA level correlation with FOXO3a in GC tissues.
2. Objectives
In the current study, the mRNA levels of the FOXO3a and AK023391 genes were investigated in GC tumor tissues. Also, the relationships of FOXO3a and AK023391 expressions with the clinicopathological features of GC were investigated.
3. Methods
3.1. Patients
In the current case-control study, 50 formalin-fixed paraffin-embedded (FFPE) GC tumor tissues and 50 normal FFPE tissues were obtained from Aramesh Lab, Tehran, Iran. Histopathological examination of the tissue specimens by a pathologist confirmed the diagnosis. All the participants were Iranians. Detailed clinicopathological parameters were gathered, including age, sex, Helicobacter pylori infection, and tumor grade, stage, and size. Non-Iranian patients, as well as the patients undergoing chemotherapy or radiotherapy were eliminated from the study. American Joint Committee guidelines were used to ascertain tumor stage (22). The Ethics Committee of the Islamic Azad University of Tehran Medical Sciences, Faculty of Pharmacy and Pharmaceutical Sciences, Tehran, Iran, approved the present study (IR.IAU.PS.REC.1399.034).
3.2. Selection of Genes
For selecting target genes, previously identified lncRNAs and their molecular epidemiologic data were carefully reviewed. Regarding certain criteria, AK02339, a lncRNA, and its target, FOXO3a, were finally selected because of their proposed association with cancer.
3.3. Gene Expression
A RNeasy DSP FFPE RNA extraction kit (Qiagen Co, Germany) was employed to extract total RNA from tumor samples. Using a PCR cycler (Rotor-Gene Q MDx; Qiagen GmbH), qPCR was performed using cDNA fragments as templates to amplify the lncRNA AK023391 and FOXO3A genes using SYBR® Premix Ex Taq™ (Takara Bio, Inc.), following the manufacturer’s protocol. The experimental protocol was performed as follows: i) Thermocycling conditions included an initial activation phase at 94°C for 30 seconds, 35 cycles at 94°C for five seconds, and 35 cycles at 60°C for 35 seconds, and ii) Melting curve analysis. Primer sequences were designed by GeneRunner software for both genes. Primer-BLAST (NCBI) was employed to evaluate the specificities of the primers designed (Table 1). The B2M gene was used as a normalizer endogenous gene, and the 2-ΔΔCt method was applied to analyze qPCR data.
The Primer Sequences Used for Real-time PCR
| Primer Name | Sequence (5’ to 3’) | Primer Size | GC% | Tm °C |
|---|---|---|---|---|
| FOXO3a | ||||
| Forward | TCTACGAGTGGATGGTGCGTT | 21 | 52.37 | 61 |
| Revers | CGACTATGCAGTGACAGGTTGTG | 23 | 52.18 | 61 |
| AK023391 | ||||
| Forward | ACCCCCATCCTAAACCCTGTAAAAC | 25 | 62.53 | 60 |
| Revers | TGTGGATTTGCTCATACTGCCCTG | 24 | 63.21 | 60 |
| B2M | ||||
| Forward | TGCTGTCTCCATGTTTGATGTATTCT | 25 | 40 | 56.98 |
| Revers | TCTCTGCTCCCCACCTCTAAGT | 22 | 54 | 57.93 |
3.4. Statistical Analysis
Data analysis was done in SPSS 21 (IBM Corp., USA), and graphs were plotted in GraphPad Prism. The unpaired t-test was used to determine statistically significant differences in gene expression, and P < 0.05 was regarded as the significance level. The association between lncRNA and FOXO3a genes’ expression was assessed via spearman correlation.
4. Results
4.1. General Descriptive Statistics
In this study, 50 patients with the final diagnosis of GC were studied, of whom 41 (82 %) were males; 10 (20 %) were smokers, and the mean age of the patients was 60.18 ± 13.65 years. Alcohol consumption was negative in all patients, but 28 (56%) of them were positive for the H. pylori infection. Regarding the 50 healthy individuals (i.e., control), the mean age was 36.72 ± 14.86 years. The patients from whom the GC tumor tissues were harvested had received no treatment. Detailed demographic characteristics have been shown in Table 2.
Demographic Variables of the Patients Studied
| Variables | Patient, No. (%) | Controls, No. (%) |
|---|---|---|
| Gender | ||
| Male | 41 (82) | 39 (78) |
| Female | 9 (18) | 11 (22) |
| Smoking | ||
| No | 40 (80) | 38 (76) |
| Yes | 10 (20) | 12 (24) |
| Disease stage | ||
| I | 9 (18) | |
| II | 2 (4) | |
| III | 21 (42) | |
| IV | 18 (36) | |
| Disease grade | ||
| I | 7 (14) | |
| II | 18 (36) | |
| III | 25 (50) | |
| Tumor size | ||
| > 5 | 21 (42) | |
| < 5 | 29 (58) | |
| H. pylori infection | ||
| Positive | 28 (56) | |
| Negative | 22 (44) |
4.2. Gene Expression of LncRNA AK023391 and FOXO3A
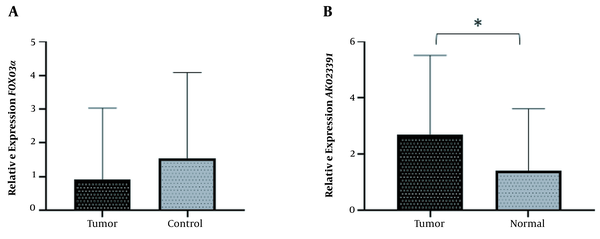
In order to investigate the role of AK023391 lncRNA and FOXO3a in GC, the expression levels of these genes were evaluated in GC tissues. No significant difference was observed in the transcription level of FOXO3a between GC and normal tissues (Mean difference = 0.9177, P = 0.2199) (Figure 1A). The expression of AK023391 was significantly up-regulated in GC tissues compared with normal tissues (1.284-fold, P = 0.0371) (Figure 1B).
A comparison of relative gene expression between GC tumor and normal tissues. (A) FOXO3a relative expression, (B) AK023391 relative expression. *P < 0.05
4.3. Association of AK023391 LncRNA and FOXO3A Expression with Clinical Characteristics
The relative expression of AK023391 demonstrated no statistically significant difference between the grad I vs. II (P = 0.2234), I vs. III (P = 0.1715), and II vs. III (P = 0.9426) disease (Figure 2A). No significant differences were identified in the transcription level of AK023391 between various disease stages [I&II vs. III (P = 0.8040), stage I&II vs. IV (P = 0.5725), and stage III vs. IV (P = 0.6544)] (Figure 2B). Moreover, no significant associations were observed between the transcription level of AK023391 and clinicopathological variables, including tumor size (< 5 & > 5 cm) and H. pylori infection (positive or negative) in GC patients (P = 0.9028 and P = 0.7205, respectively) (Figure 2C and D).
Association of the mRNA levels of the FOXO3a and AK023391 genes with clinicopathological features. (A) The transcription level of AK023391 in different grades of GC tissues, (B) The transcription level of AK023391 in different tumor stages, (C) The transcription level of AK023391 in tumors with different sizes (≤ 5 and > 5 cm), (D) The transcription level of AK023391 in patients with positive and negative H. pylori infection, (E) The relative expression of FOXO3a in different grades of GC tissues, (F) The transcription level of FOXO3a in tumors at different stages, (G) The transcription level of FOXO3a in tumors with different sizes (≤ 5 and > 5 cm), (H) The transcription level of FOXO3a in patients with positive or negative H. pylori infection.
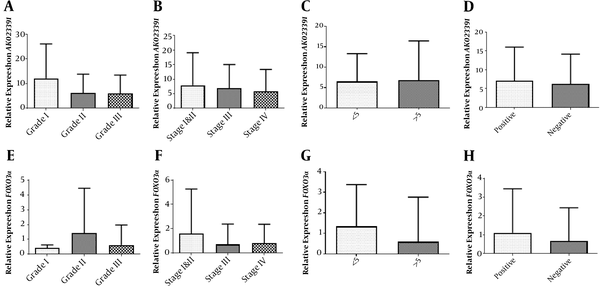
In addition, statistical analysis revealed no significant association between FOXO3A expression and clinicopathological features or a significant difference in its expression comparing disease stage I vs. II (P = 0.5187), I vs. III (P = 0.7730), and II vs. III (P = 0.2395) (Figure 2E). There were no significant associations between the transcription level of FOXO3a and disease stage [I&II vs. III (P = 0.3663), I&II vs. IV (P = 0.4268), and III vs. IV (P = 0.8565), Figure 2F]. Also, the transcription level of FOXO3a was not associated with clinicopathological variables, including tumor size (< 5 & > 5 cm) and H. pylori infection (positive or negative) in GC patients (P = 0.2327 and P = 0.4939, respectively, Figure 2G and H).
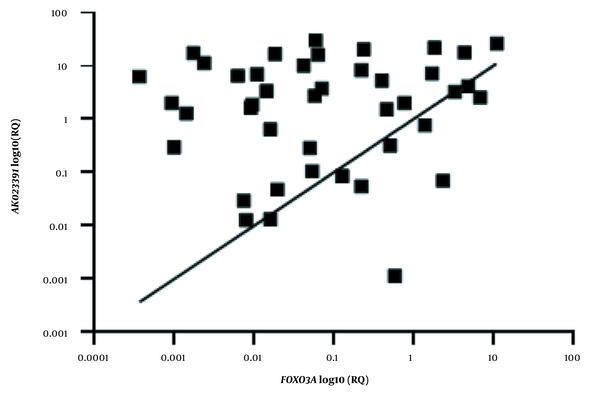
Linear regression analysis was performed to evaluate the correlation between the expression levels of the AK023391 lncRNA and FOXO3a genes in GC tumor tissues. The extent of mRNA expression is presented as log10 transformed values. The R and P values are presented for each analysis. RQ, relative quantification.
4.4. The Transcription Levels of AK023391 LncRNA and FOXO3a
Our results revealed that there was no significant correlation between the transcription levels of AK023391 and FOXO3a in GC tissues (R = 0.014; P = 0.34, Figure 3).
5. Discussion
It has been previously shown that lncRNAs contribute to GC development through several signaling pathways such as AKT and MAPK (23). Furthermore, interactions between lncRNAs and their target genes seem to affect pathogenic mechanisms in GC (24). Indeed, lncRNAs have a wide range of functions, including tumor growth suppression and oncogenesis regulation, and can act through a number of pathways (25). Our results indicated that AK023391 expression was significantly up-regulated in GC tissues compared with normal tissues. However, no significant association was found between AK023391 expression and clinicopathologic features. Similarly, Huang et al. reported that the expression level of AK023391 lncRNA was upregulated in GC tissues, which was positively associated with poor survival in GC patients (16). Likewise, AK023391 lncRNA has been reported to play a major role in cancer cell proliferation, development, and migration, as well as in tumor development and invasion by activating the PI3K/Akt signaling pathway (16). Previous studies have shown that AK023391 activates the PI3K/Akt pathway, resulting in GC tumorigenesis and invasion, accompanied by p53 downregulation, BCL-6, cyclinB1/G2, and c-myb elevated expressions, FOXO3a inactivation, and NF-κB induction; all of which contribute to GC progression. Thus, the AKT pathway is involved in GC tumorigenesis and metastasis (16, 26, 27).
However, in the present study, no significant correlation was observed between the expression levels of AK023391 lncRNA and FOXO3a. The results also showed no significant difference in FOXO3A expression between GC and normal tissues. In addition, FOXO3a expression was not associated with disease grade, disease stage, tumor size, and H. pylori infection. As a member of forkhead (FOX) transcription factors, FOXO3a regulates a variety of cellular functions such as cell death, proliferation, cell cycle progression, DNA damage, and tumor progression (17). Functional studies have shown that FOXO3a acts as a tumor suppressor in various cancers. Previous studies have shown that the down-regulation of FOXO3a was linked with a poor prognosis in glioma (28) and ovarian cancer (29) while its up-regulation correlated with a poor prognosis in triple-negative breast cancer (30), glioblastoma (31), hepatocellular carcinoma (32), and GC (22). By upregulating cathepsin L, FOXO3a overexpression facilitated GC tumor cells’ migration and invasion. The migration and invasion of GC cells were inhibited by knocking down FOXO3a, accompanied by cathepsin L downregulation (33). Furthermore, RUNX3 collaborates the complex of FoxO3a/FKHRL1 to induce apoptosis in GC cells by activating Bim promoter (34). Previous studies have also shown that AK023391 lncRNA and FOXO3a play different roles in different cancers. Further studies on the current topic are recommended.
References
-
1.
Thrift AP, El-Serag HB. Burden of Gastric Cancer. Clin Gastroenterol Hepatol. 2020;18(3):534-42. [PubMed ID: 31362118]. https://doi.org/10.1016/j.cgh.2019.07.045.
-
2.
Orditura M, Galizia G, Sforza V, Gambardella V, Fabozzi A, Laterza MM, et al. Treatment of gastric cancer. World J Gastroenterol. 2014;20(7):1635-49. [PubMed ID: 24587643]. [PubMed Central ID: PMC3930964]. https://doi.org/10.3748/wjg.v20.i7.1635.
-
3.
Rona KA, Schwameis K, Zehetner J, Samakar K, Green K, Samaan J, et al. Gastric cancer in the young: An advanced disease with poor prognostic features. J Surg Oncol. 2017;115(4):371-5. [PubMed ID: 28008624]. https://doi.org/10.1002/jso.24533.
-
4.
Jiang L, Gong X, Liao W, Lv N, Yan R. Molecular targeted treatment and drug delivery system for gastric cancer. J Cancer Res Clin Oncol. 2021;147(4):973-86. https://doi.org/10.1007/s00432-021-03520-x.
-
5.
Nandwani A, Rathore S, Datta M. LncRNAs in cancer: Regulatory and therapeutic implications. Cancer Lett. 2021;501:162-71. [PubMed ID: 33359709]. https://doi.org/10.1016/j.canlet.2020.11.048.
-
6.
Chaleshi V, Tajali R, Savabkar S, Zali N, Mojarad EN, Haghazali M, et al. Lack of Association between NOD2 rs3135500 and IL12B rs1368439 microRNA Binding Site SNPs and Colorectal Cancer Susceptibility in an Iranian Population. Microrna. 2016;5(2):152-6. [PubMed ID: 27426943]. https://doi.org/10.2174/2211536605666160715151535.
-
7.
Aftabi Y, Ansarin K, Shanehbandi D, Khalili M, Seyedrezazadeh E, Rahbarnia L, et al. Long non-coding RNAs as potential biomarkers in the prognosis and diagnosis of lung cancer: A review and target analysis. IUBMB Life. 2021;73(2):307-27. [PubMed ID: 33369006]. https://doi.org/10.1002/iub.2430.
-
8.
Jiang MC, Ni JJ, Cui WY, Wang BY, Zhuo W. Emerging roles of lncRNA in cancer and therapeutic opportunities. Am J Cancer Res. 2019;9(7):1354-66. [PubMed ID: 31392074]. [PubMed Central ID: PMC6682721].
-
9.
Huang W, Cui X, Chen J, Feng Y, Song E, Li J, et al. Long non-coding RNA NKILA inhibits migration and invasion of tongue squamous cell carcinoma cells via suppressing epithelial-mesenchymal transition. Oncotarget. 2016;7(38):62520-32. [PubMed ID: 27613832]. [PubMed Central ID: PMC5308743]. https://doi.org/10.18632/oncotarget.11528.
-
10.
Wang SH, Zhang WJ, Wu XC, Zhang MD, Weng MZ, Zhou D, et al. Long non-coding RNA Malat1 promotes gallbladder cancer development by acting as a molecular sponge to regulate miR-206. Oncotarget. 2016;7(25):37857-67. [PubMed ID: 27191262]. [PubMed Central ID: PMC5122355]. https://doi.org/10.18632/oncotarget.9347.
-
11.
Chen X, Li D, Gao Y, Tang W, Iw L, Cao Y, et al. Long Intergenic Noncoding RNA 00152 Promotes Glioma Cell Proliferation and Invasion by Interacting with MiR-16. Cell Physiol Biochem. 2018;46(3):1055-64. [PubMed ID: 29669323]. https://doi.org/10.1159/000488836.
-
12.
Chen DL, Ju HQ, Lu YX, Chen LZ, Zeng ZL, Zhang DS, et al. Long non-coding RNA XIST regulates gastric cancer progression by acting as a molecular sponge of miR-101 to modulate EZH2 expression. J Exp Clin Cancer Res. 2016;35(1):142. [PubMed ID: 27620004]. [PubMed Central ID: PMC5020507]. https://doi.org/10.1186/s13046-016-0420-1.
-
13.
Huang M, Hou J, Wang Y, Xie M, Wei C, Nie F, et al. Long Noncoding RNA LINC00673 Is Activated by SP1 and Exerts Oncogenic Properties by Interacting with LSD1 and EZH2 in Gastric Cancer. Mol Ther. 2017;25(4):1014-26. [PubMed ID: 28214253]. [PubMed Central ID: PMC5383578]. https://doi.org/10.1016/j.ymthe.2017.01.017.
-
14.
Liu D, Li Y, Luo G, Xiao X, Tao D, Wu X, et al. LncRNA SPRY4-IT1 sponges miR-101-3p to promote proliferation and metastasis of bladder cancer cells through up-regulating EZH2. Cancer Lett. 2017;388:281-91. [PubMed ID: 27998761]. https://doi.org/10.1016/j.canlet.2016.12.005.
-
15.
Yu Y, Li L, Zheng Z, Chen S, Chen E, Hu Y. Long non-coding RNA linc00261 suppresses gastric cancer progression via promoting Slug degradation. J Cell Mol Med. 2017;21(5):955-67. [PubMed ID: 27878953]. [PubMed Central ID: PMC5387161]. https://doi.org/10.1111/jcmm.13035.
-
16.
Huang Y, Zhang J, Hou L, Wang G, Liu H, Zhang R, et al. LncRNA AK023391 promotes tumorigenesis and invasion of gastric cancer through activation of the PI3K/Akt signaling pathway. J Exp Clin Cancer Res. 2017;36(1):194. [PubMed ID: 29282102]. [PubMed Central ID: PMC5745957]. https://doi.org/10.1186/s13046-017-0666-2.
-
17.
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W, Hao X, et al. Critical role of FOXO3a in carcinogenesis. Mol Cancer. 2018;17(1):104. [PubMed ID: 30045773]. [PubMed Central ID: PMC6060507]. https://doi.org/10.1186/s12943-018-0856-3.
-
18.
Zanella F, Rosado A, Garcia B, Carnero A, Link W. Chemical genetic analysis of FOXO nuclear-cytoplasmic shuttling by using image-based cell screening. Chembiochem. 2008;9(14):2229-37. [PubMed ID: 18756565]. https://doi.org/10.1002/cbic.200800255.
-
19.
Nielsen MD, Luo X, Biteau B, Syverson K, Jasper H. 14-3-3 Epsilon antagonizes FoxO to control growth, apoptosis and longevity in Drosophila. Aging Cell. 2008;7(5):688-99. [PubMed ID: 18665908]. [PubMed Central ID: PMC3851013]. https://doi.org/10.1111/j.1474-9726.2008.00420.x.
-
20.
Burgering BM. A brief introduction to FOXOlogy. Oncogene. 2008;27(16):2258-62. [PubMed ID: 18391968]. https://doi.org/10.1038/onc.2008.29.
-
21.
Yu S, Yu Y, Sun Y, Wang X, Luo R, Zhao N, et al. Activation of FOXO3a suggests good prognosis of patients with radically resected gastric cancer. Int J Clin Exp Pathol. 2015;8(3):2963-70. [PubMed ID: 26045805]. [PubMed Central ID: PMC4440114].
-
22.
Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17(12):3077-9. [PubMed ID: 20882416]. https://doi.org/10.1245/s10434-010-1362-z.
-
23.
Dai Q, Zhang T, Li C. LncRNA MALAT1 Regulates the Cell Proliferation and Cisplatin Resistance in Gastric Cancer via PI3K/AKT Pathway. Cancer Manag Res. 2020;12:1929-39. [PubMed ID: 32214850]. [PubMed Central ID: PMC7078812]. https://doi.org/10.2147/CMAR.S243796.
-
24.
Chaleshi V, Irani S, Alebouyeh M, Mirfakhraie R, Aghdaei HA. Association of lncRNA-p53 regulatory network (lincRNA-p21, lincRNA-ROR and MALAT1) and p53 with the clinicopathological features of colorectal primary lesions and tumors. Oncol Lett. 2020;19(6):3937-49. [PubMed ID: 32391102]. [PubMed Central ID: PMC7204634]. https://doi.org/10.3892/ol.2020.11518.
-
25.
Gu Y, Chen T, Li G, Yu X, Lu Y, Wang H, et al. LncRNAs: emerging biomarkers in gastric cancer. Future Oncol. 2015;11(17):2427-41. [PubMed ID: 26289363]. https://doi.org/10.2217/fon.15.175.
-
26.
Wang ZQ, Cai Q, Hu L, He CY, Li JF, Quan ZW, et al. Long noncoding RNA UCA1 induced by SP1 promotes cell proliferation via recruiting EZH2 and activating AKT pathway in gastric cancer. Cell Death Dis. 2017;8(6). e2839. [PubMed ID: 28569779]. [PubMed Central ID: PMC5520878]. https://doi.org/10.1038/cddis.2017.143.
-
27.
Yan J, Dang Y, Liu S, Zhang Y, Zhang G. LncRNA HOTAIR promotes cisplatin resistance in gastric cancer by targeting miR-126 to activate the PI3K/AKT/MRP1 genes. Tumour Biol. 2016. [PubMed ID: 27900563]. https://doi.org/10.1007/s13277-016-5448-5.
-
28.
Shi J, Zhang L, Shen A, Zhang J, Wang Y, Zhao Y, et al. Clinical and biological significance of forkhead class box O 3a expression in glioma: mediation of glioma malignancy by transcriptional regulation of p27kip1. J Neurooncol. 2010;98(1):57-69. [PubMed ID: 19911116]. https://doi.org/10.1007/s11060-009-0045-8.
-
29.
Fei M, Zhao Y, Wang Y, Lu M, Cheng C, Huang X, et al. Low expression of Foxo3a is associated with poor prognosis in ovarian cancer patients. Cancer Invest. 2009;27(1):52-9. [PubMed ID: 19160093]. https://doi.org/10.1080/07357900802146204.
-
30.
Rehman A, Kim Y, Kim H, Sim J, Ahn H, Chung MS, et al. FOXO3a expression is associated with lymph node metastasis and poor disease-free survival in triple-negative breast cancer. J Clin Pathol. 2018;71(9):806-13. [PubMed ID: 29588373]. https://doi.org/10.1136/jclinpath-2018-205052.
-
31.
Qian Z, Ren L, Wu D, Yang X, Zhou Z, Nie Q, et al. Overexpression of FoxO3a is associated with glioblastoma progression and predicts poor patient prognosis. Int J Cancer. 2017;140(12):2792-804. [PubMed ID: 28295288]. https://doi.org/10.1002/ijc.30690.
-
32.
Ahn H, Kim H, Abdul R, Kim Y, Sim J, Choi D, et al. Overexpression of Forkhead Box O3a and Its Association With Aggressive Phenotypes and Poor Prognosis in Human Hepatocellular Carcinoma. Am J Clin Pathol. 2018;149(2):117-27. [PubMed ID: 29365018]. https://doi.org/10.1093/ajcp/aqx132.
-
33.
Yu S, Yu Y, Zhang W, Yuan W, Zhao N, Li Q, et al. FOXO3a promotes gastric cancer cell migration and invasion through the induction of cathepsin L. Oncotarget. 2016;7(23):34773-84. [PubMed ID: 27127880]. [PubMed Central ID: PMC5085188]. https://doi.org/10.18632/oncotarget.8977.
-
34.
Yamamura Y, Lee WL, Inoue K, Ida H, Ito Y. RUNX3 cooperates with FoxO3a to induce apoptosis in gastric cancer cells. J Biol Chem. 2006;281(8):5267-76. [PubMed ID: 16373335]. https://doi.org/10.1074/jbc.M512151200.