Abstract
Introduction:
Delayed or avoided medical care due to coronavirus disease 2019 (COVID-19) related concerns may increase morbidity and mortality associated with both acute and chronic health conditions. Thymoma is uncommon in pregnancy, although it could be accompanied with unfavorable outcomes. We report a puerperal woman presented with dyspnea and cardiac arrest with a recent diagnosis of thymoma that led to maternal mortality.Case Presentation:
A 38-year-old woman with occasional dyspnea during pregnancy without medical referral was admitted to the hospital with severe dyspnea and orthopnea three days after cesarean section. Spiral computed tomography (CT) angiography showed a 64 × 84-centimeter mass with soft tissue density in the left perivascular that was originated from the anterior mediastinum; in biopsy, thymoma was suggested. She died shortly after due to severe dyspnea and cardiac arrest.Conclusions:
Prenatal care during COVID-19 pandemic should not be postponed. Indeed, any symptom similar to the physiologic changes in pregnancy needs to be evaluated for optimal clinical management.Keywords
1. Introduction
It is estimated that many people have delayed or avoided seeking for medical care because of concerns about coronavirus disease 2019 (COVID-19) that may lead to higher morbidity and mortality cases associated with both acute and chronic health conditions (1).
Thymoma is the most common primary anterior mediastinum neoplasm accounting for about one percent of all adult malignancies (2). According to the World Health Organization (WHO) criteria, thymoma is classified based on histologic cells (round/epithelioid cells as type A or spindle/oval cells as type B) and is divided into A, AB, B1, and B2 subtypes (3).
Patients usually present with mass-associated respiratory symptoms, superior vena cava syndrome, or paraneoplastic syndrome, including myasthenia gravis, pure red cell aplasia, or acquired hypogammaglobulinemia, and connective tissue disorders (4).
Thymoma in pregnancy has unfavorable outcomes with over 90% mortality during pregnancy or in puerperium (5). It may not be diagnosed easily due to the overlap with pregnancy-related physiologic changes that may lead to delay or missed diagnosis. There are few published reports assessing thymoma in the postpartum period (6, 7). Herein, we report a case of maternal mortality due to dyspnea and cardiac arrest in puerperium with a recent diagnosis of thymoma.
2. Case Presentation
A 38-year-old woman gravida 3 para 2 with two previous cesarean section at 38 weeks of gestational age attended the emergency room of a university hospital affiliated to Iran University of Medical Sciences in February 2020 with labor pain. The vital sign on arrival showed pulse rate (PR) (8) of 90, respiratory rate (RR) of 22, blood pressure (BP) equal to 127/88 mm/Hg, and normal temperature. The O2 saturation was 96% without mask. Evaluation of the upper airway showed no signs suggestive of difficult laryngoscopy, and no hoarseness of voice. Jugular venous pressure was high, and the neck veins were engorged, that was perused as physiologic volume overload of pregnancy. The results of other physical examinations were unremarkable. She mentioned no previous illness except hypothyroidism in pregnancy that was managed with levothyroxine and denied any history of paraneoplastic diseases. Indeed, she experienced short episodes of dyspnea during pregnancy without any follow up. Her prenatal care was not appropriate due to her fear of being infected with COVID-19.
She was transferred to the operation room, and cesarean section with spinal anesthesia was performed, and a healthy baby girl was born with an Apgar score of 9 and 10 in first and fifth minutes. We recommended her to visit a cardiologist, but she did not accept and was discharged with personal consent. She was readmitted after three days for being tachypneic and dyspneic with RR of 30, orthopnea, and PR of 120, and decreasing O2 saturation to 82%. Lung auscultation showed sound decreasing in apical region of the left lung. Chest x-ray revealed compression of the heart due to probable cardiac tamponade and obstruction of the superior vena cava and trachea.
She was admitted to the intensive care unit (ICU) with O2 mask, and laboratory test was requested. Her liver function test was abnormal with serum glutamic oxaloacetic transaminase (SGOT) equal to 196 IU/L, serum glutamic pyruvic transaminase (SGPT) equal to 251 IU/L, lactate dehydrogenase (LDH) equal to 1822 U/L, alkaline phosphatase (AlkP) equal to 503 IU/L. The results of the other abnormal laboratory tests were thyroid stimulating hormone (TSH) equal to 4.5, erythrocyte sedimentation rate (ESR) equal to 69 mm/hr, creatine phosphokinase (CPK) equal to 5484 IU/L, and creatine kinase-MB (CK-MB) equal to 119 U/L with abnormal venous blood gases (VBG) suggesting mixed acidosis. However, the d-dimer test was negative. To rule out COVID-19, reverse transcription polymerase chain reaction (RT-PCR) was requested that was negative.
In addition, an immediate consultation with a cardiologist was performed with the suspicion of pulmonary thromboembolism. He requested an electrocardiogram that showed sinus tachycardia. Normal lower limb Doppler ultrasound ruled out deep vein thrombosis (DVT). In echocardiography, a solid nature tumor was detected. Then, a spiral computed tomography (CT) angiography of the thorax was requested, which showed a 64 × 84-centimeter soft tissue mass compressing her left lung that was originated from anterior mediastinum (Figure 1).
Computed tomographic scan shows anterior mediastinal mass; measures 64 × 84 cm (white arrows).
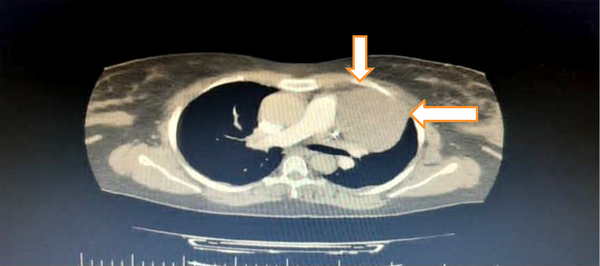
Therefore, a closed biopsy was suggested, and no evidence of thromboembolism was detected. Ultrasound guided lung mass biopsy revealed bland looking glandular and cribriform neoplasm. Furthermore, immunohistochemistry (IHC) staining analysis was negative for Napsin A, mammaglobin, estrogen receptor (ER), and CD117. The lining of the glandular and cribriform structures also intermingled spindle cells and mostly positive P40, and the diagnosis of thymoma type AB was made. The abdominal CT scan was normal without any metastasis. Due to consultation with a thoracic surgeon, surgery for removal of the mass was ordered after stabilizing the patient. That night she was intubated due to a decrease in O2 saturation to 70% and severe dyspnea. Unfortunately, she became asystole and died after an unsuccessful cardiopulmonary resuscitation.
3. Discussion
This patient had occasional dyspnea during pregnancy but she did not see a cardiologist due to fear of COVID-19 pandemic. Therefore, the latency in diagnosis and further management led to adverse outcomes.
Thymoma is a generally slow-growing and rare epithelial tumor arising from the thymus gland with no sex predilection. The etiology in most cases is unknown. Although there are rare case reports of thymoma diagnosed during pregnancy, a specific link has not been identified between them (7).
Thymoma has organotypic histology with dimorphic lymphocytes and epithelial cells (3). Type AB overexpresses p53, galectin-3, and Ki67 that might coordinately manipulate the process of development, progression, and malignant transformation (9). The tumor is mostly negative for estrogen and progesterone receptor immunoreactivity, and IHC for both ER and progesterone receptor were negative (7). In our case, Napsin A, mammaglobin, ER, and CD117 were negative and most cells were positive for P40.
Thymoma can be diagnosed with widely different presentations. Tuan and Minh Duc described the symptoms as myasthenia gravis, including generalized muscle weakness, ptosis, dysphagia, and dyspnea (10), or venous intraluminal extension (11). Also, the dominant chief complaint of our case was respiratory symptoms. Thymoma occurs more commonly in association with autoimmune conditions, such as myasthenia gravis, aplastic anemia, systemic lupus erythematous, and multiple endocrine neoplasia syndrome, as well as hypogammaglobulinemia (12). However, in our case, no previous medical disease was reported.
One differential diagnosis of postpartum dyspnea is pulmonary thromboembolism that was ruled out in our study with normal CT angiography, Doppler ultrasound, and a negative D-dimer. Preeclampsia is the other differential diagnosis due to pulmonary edema (8). In our patient, despite high liver enzymes, no metastasis was observed in the abdominal CT scan, and she had normal BP during pregnancy and after delivery.
This neoplasm has almost poor prognosis, and most reported cases are due to metastasis and recurrent disease (13, 14). In one case, the death was due to post cesarean section cardiac arrest (15), which was similar to our study. This is caused by rapid growth and even metastasis during pregnancy. It may be due to nonspecific suppression of the immune system that makes the tumor a tolerated entity (16).
3.1. Conclusions
Diagnosis and management of thymoma in pregnancy remain challenging and may deteriorate with pregnancy termination. Prenatal care during pregnancy should not be abandoned due to COVID-19 pandemic, and any symptoms similar to the physiologic changes in pregnancy should be evaluated for optimal clinical management.
References
-
1.
Czeisler MÉ, Marynak K, Clarke KE, Salah Z, Shakya I, Thierry JM, et al. Delay or Avoidance of Medical Care Because of COVID-19–Related Concerns — United States, June 2020. Morb Mortal Wkly Rep. 2020;69(35). https://doi.org/10.15585/mmwr.mm6935e3.
-
2.
Marom EM. Imaging thymoma. J Thorac Oncol. 2010;5(10 Suppl 4):S296-303. [PubMed ID: 20859123]. https://doi.org/10.1097/JTO.0b013e3181f209ca.
-
3.
Kornstein MJ. Pathology of the thymus and mediastinum. Philadelphia (PA): WB Saunders; 1995.
-
4.
Liang X, Lovell MA, Capocelli KE, Albano EA, Birch S, Keating AK, et al. Thymoma in children: report of 2 cases and review of the literature. Pediatr Dev Pathol. 2010;13(3):202-8. [PubMed ID: 20055684]. https://doi.org/10.2350/09-07-0672-OA.1.
-
5.
Peleg D, Zabari A, Shalev E. Relapsing thymic carcinoma during pregnancy. Acta Obstet Gynecol Scand. 1992;71(5):398-400. [PubMed ID: 1326220]. https://doi.org/10.3109/00016349209021082.
-
6.
Huang CC, Lee CC. Thymoma and myasthenia gravis in pregnancy: report of a case. J Formos Med Assoc. 1991;90(2):206-8. [PubMed ID: 1678418].
-
7.
Hechtman JF, Chepovetsky JA, Strauchen JA, Burstein DE, Beasley MB. Thymomas diagnosed during pregnancy: two cases in young women without paraneoplastic or autoimmune disease. Ann Diagn Pathol. 2012;16(5):392-6. [PubMed ID: 21652248]. https://doi.org/10.1016/j.anndiagpath.2011.03.003.
-
8.
Prueksaritanond S, Ali AM, Aronu GN, Hussain N, Ganjoo A, Mirrakhimov AE, et al. An uncommon cause of shortness of breath in a young puerpera. Case Rep Obstet Gynecol. 2013;2013:710620. [PubMed ID: 23585977]. [PubMed Central ID: PMC3622346]. https://doi.org/10.1155/2013/710620.
-
9.
Wang Z, Li H, Cao H, Zheng J. Clinicopathological features of type AB thymoma with liver metastases. Int J Clin Exp Pathol. 2014;7(12):8700-5. [PubMed ID: 25674235]. [PubMed Central ID: PMC4314039].
-
10.
Tuan PA, Minh Duc N. Ectopic thymoma in the middle mediastinum: A case report and literature review. Clin Ter. 2021;172(2):94-8. [PubMed ID: 33763676]. https://doi.org/10.7417/CT.2021.2291.
-
11.
Tsutsui S, Ashizawa K, Tagawa T, Nagayasu T, Hayashi T, Uetani M. Invasive thymoma with venous intraluminal extension: CT and MRI findings. Clin Imaging. 2012;36(6):854-7. [PubMed ID: 23154023]. https://doi.org/10.1016/j.clinimag.2012.01.033.
-
12.
Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC. World Health Organization classification of tumours. Pathology and genetics of tumours of the lung, pleura, thymus and heart. Lyon, France: IARC press; 2004.
-
13.
Jodczyk J, Popow J. [Thymoma granulomatosum in pregnancv]. Patol Pol. 1961;12:467-72. Polish. [PubMed ID: 14451906].
-
14.
Massart JJ, Bishop WA. Thymoma occurring during pregnancy: a case report. Obstet Gynecol. 1968;32(4):490-3.
-
15.
Goldman KP. Malignant thymoma in pregnancy. Br J Dis Chest. 1974;68:279-83. https://doi.org/10.1016/0007-0971(74)90052-7.
-
16.
Ray PK, Saha S. Tumor growth versus fetal development--similarities and confusions. Adv Immun Cancer Ther. 1986;2:155-87. [PubMed ID: 3321946].
