Abstract
Background:
The reduction of shoulder pain and postoperative nausea and vomiting (PONV), causing great discomfort for patients after gynecological laparoscopy, requires preventive or treatment strategies.Objectives:
The present study aimed to determine the efficacy of intraperitoneal (IP) dexamethasone in the reduction of shoulder pain and PONV after gynecological laparoscopy.Methods:
In this double-blind, randomized clinical trial, 130 consecutive patients undergoing gynecological laparoscopy were randomly assigned to two groups of 65 patients within May 2015 to May 2016. One group received 16 mg IP dexamethasone before the end of the surgery, and the other group received the placebo (i.e., the IP infusion of distilled water). Patients' age and body mass index (BMI), and surgery duration were recorded in this study. The severity of shoulder pain was evaluated by the visual analog scale (VAS) at recovery and 2, 6, 12, and 24 h after the surgery. Moreover, the need for opioid use and PONV were recorded within the first 24 h after the surgery. The study outcomes were compared between the two study groups and among the different intervals using SPSS software (version 21).Results:
The groups had similar demographics (i.e., age and BMI) and mean surgery duration (P > 0.05). The mean values of VAS scores of the intervention group were lower than those of the placebo group at five intervals (P = 0.001). The frequency of opioid use was significantly lower in the dexamethasone group (P = 0.010). In addition, 20% and 60% of the patients in the dexamethasone and placebo groups had PONV, respectively (P < 0.001).Conclusions:
The IP dexamethasone is effective in the reduction of shoulder pain and nausea/vomiting after gynecological laparoscopy and can significantly reduce opioid requirement within the first 24 h after surgery; however, IP dexamethasone does not increase surgery duration. Therefore, it is recommended to use this technique during gynecological laparoscopy.Keywords
1. Background
Gynecological surgical procedures are widely performed worldwide for different indications, comprising about one-third of all surgical procedures in women (1). Although laparoscopy is the preferred approach due to the lower complications, such as better wound healing, lower infection rates, less blood loss, better recovery, and shorter hospital stay (2), this type of surgery has its own complications (3). One of the important sources of pain after laparoscopic gynecological surgery is postoperative shoulder pain, observed in up to 85% of patients, lasting up to 72 h after surgery and causing great discomfort for patients (4). Postoperative nausea and vomiting (PONV) is another important complication after surgical procedures (5), observed in 20 - 40% of patients after gynecological laparoscopy (6).
Evidence suggests that shoulder pain after laparoscopy mainly occurs due to the irritation of the afferent nerve fibers of the diaphragm phrenic nerve, induced by the residual CO2, blood, or other materials (e.g., amniotic fluid) below the diaphragm (7). Different techniques have been suggested for reducing shoulder pain after gynecological laparoscopy; nevertheless, none have been confirmed as the gold standard method for the prevention of shoulder pain (8). Some studies have suggested the efficacy of evacuation of the residual gas from the abdominal cavity (9); nonetheless, other studies have suggested that complete CO2 deflation is not possible and recommended postoperative positioning of patients in 20 degrees Trendelenburg for the reduction of postoperative shoulder pain in gynecological laparoscopic procedures (10).
Other studies have suggested the use of pulmonary recruitment maneuver and intraperitoneal (IP) drainage for reducing the severity of shoulder pain and analgesic requirement after gynecological laparoscopy (11, 12). Other techniques used to reduce shoulder pain after gynecological laparoscopy include the infiltration of local anesthetics, which have been proven efficient when infiltrated into the peritoneal cavity but not in subdiaphragmatic IP infiltration (13). Nevertheless, none of these methods have been accepted as the gold standard method for the prevention of shoulder pain, as each of them has its own limitations and adverse effects (8).
The IP infiltration of dexamethasone is suggested as an efficient technique for the reduction of shoulder pain after gynecological laparoscopy, compared to placebo, with minimal/no adverse effects that can also reduce opioid requirement after surgery (14). Furthermore, both intravenous (IV) and IP dexamethasone during surgery have been shown effective in the reduction of pain and PONV after gynecological laparoscopy (15).
2. Objectives
Due to the limited number of studies in this regard, the present study aimed to determine the efficacy of IP dexamethasone in the reduction of shoulder pain, opioid use, and PONV in the first 24 h after gynecological laparoscopy.
3. Methods
3.1. Study Design
This double-blind, randomized clinical trial (RCT) was conducted on women within the age range of 15 - 75 years with the American Society of Anesthesiologist class I and II, undergoing elective gynecological laparoscopy at Shohadaye Tajrish Hospital, Tehran, Iran, as eligible participants within May 2015 to May 2016. Then, the patients were fully explained about the study objectives and methods and signed written informed consent. Any patient with a history of reaction to dexamethasone or recent use of steroids was not included in this study. The sample size of the study was calculated at 65 in each group, according to previous studies (14).
The recruited participants were randomly assigned to two groups using the randomization method at the end of the surgical procedure. Moreover, 16 mg IP dexamethasone in one group and placebo in the other group were infused. Two vials of dexamethasone (8 mg in 2 cc) were purchased from Chemidarou (Iran) and diluted to 10 cc with distilled water and placebo, containing 10 cc distilled water was prepared by a nurse and infused in the same way. The surgeon used the labeled syringes and was not aware of the allocations. Neither the patients nor the researcher, who examined the patients’ outcomes, were aware of the allocation.
For the assessment of the primary outcome of the study (i.e., shoulder pain) after the surgery (at recovery room) and 2, 6, 12, and 24 h after the surgery, the researcher referred to the patients and asked them about the presence of shoulder pain. If reported, the researcher asked the patient to indicate the severity of the shoulder pain based on a 10-point Likert scale on a visual analog scale (VAS) ruler where 0 and 10 indicating no pain and the worst pain, respectively. In case of severe pain (i.e., VAS score > 6), 50 mg pethidine was administered.
The frequency of pethidine requirement and PONV was considered the secondary outcome of this study. The PONV was evaluated in the first 24 hours after surgery. The type of surgery was also recorded in the study checklist. Any patient who required conversion of the surgery to open laparotomy or refused to continue the study was excluded from the study. The following formula was used for the calculation of sample size:
3.2. Statistical Analysis
The collected data were analyzed using SPSS statistical software (version 21.0; IBM Corp. 2012. Armonk, NY, USA). For reporting the results, first, the categorical variables were described by frequency (percentage) and quantitative variables by mean ± standard deviation. As the results of the one-sample Kolmogorov-Smirnov test showed normal distribution of data, the comparison between the groups was performed using the independent samples t-test and comparison among the intervals using repeated measures analysis of variance (ANOVA). The categorical variables were compared using the chi-square test. P-values of less than 0.05 were considered statistically significant.
4. Results
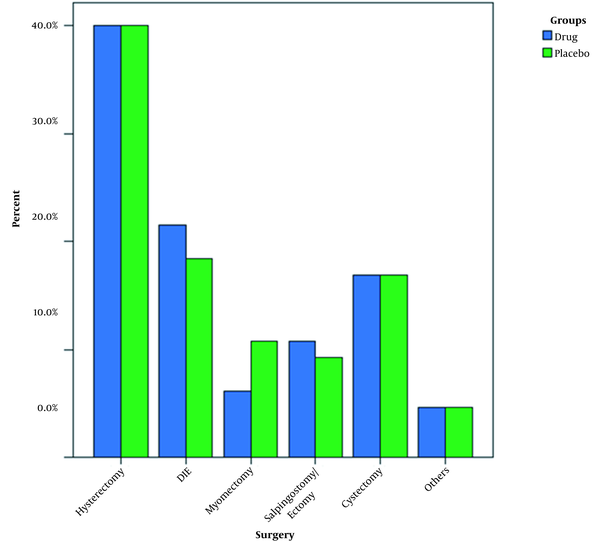
A total of 65 patients completed the study in each group. As shown in Table 1, the mean age and body mass index (BMI) of the study groups were not different (P > 0.05; Table 1). In addition, the mean values of surgery duration were not different between the groups (P > 0.05; Table 1). Hysterectomy was the most prevalent surgery, performed in 40% of the patients in both groups. The frequency of surgery type was not different between the groups (P > 0.05; Figure 1). The PONV was positive in 20% (n = 13) and 60% (n = 39) of the patients in the intervention and control groups, respectively, with a statistically significant difference (P < 0.001).
| Intervention Group | Control Group | P-Valueb | |
|---|---|---|---|
| Age (y) | 37.73 ± 10.99 | 36.45 ± 11.94 | > 0.05 |
| Body mass index (kg/m2) | 28.55 ± 4.88 | 27.04 ± 4.39 | > 0.05 |
| Surgery duration (min) | 136.85 ± 58.61 | 135.54 ± 55.79 | > 0.05 |
Frequency of surgery type in two study groups
In the intervention group, one patient required analgesic once (1.5%), 60 patients (92.3%) twice, and 4 patients (6.2%) three times; however, in the control group, 49 (75.4%) and 16 (24.6%) patients required analgesic twice and three times, respectively, with a statistically significant difference (P = 0.010).
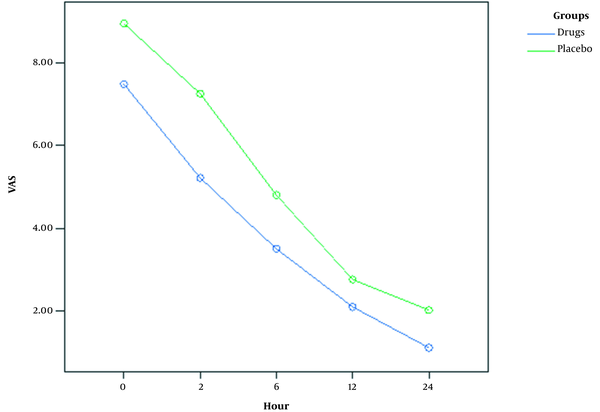
Table 2 tabulates the mean VAS scores of the study groups at each interval. The results of one-way ANOVA showed a statistically significant difference between the groups in mean VAS scores (P = 0.001). Furthermore, Figure 2 depicts the trend of changes in the shoulder pain scores of the two study groups. As illustrated, both groups had a reducing trend with lower pain scores at all intervals in the intervention group.
| Intervention Group | Control Group | P-Valueb | |
|---|---|---|---|
| After surgery | 7.47 ± 1.30 | 8.93 ± 1.08 | 0.001 |
| 2 h after surgery | 5.21 ± 1.57 | 7.24 ± 1.42 | 0.001 |
| 6 h after surgery | 3.51 ± 1.22 | 4.80 ± 1.33 | 0.001 |
| 12 h after surgery | 2.11 ± 1.16 | 2.76 ± 1.15 | 0.001 |
| 24 h after surgery | 1.12 ± 0.96 | 2.03 ± 1.04 | 0.001 |
Severity of shoulder pain at five intervals in two study groups
5. Discussion
The results of this study showed that women in the intervention group, receiving 16 mg IP dexamethasone, had a lower level of pain severity at the evaluated intervals and lower frequency of opioid requirement and PONV, compared to those reported for the placebo (i.e., distilled water) group. The aforementioned results, in line with the results of previous studies (14, 15), suggest IP dexamethasone as an appropriate technique used during gynecology laparoscopy for the reduction of postoperative shoulder pain, opioid requirement, and PONV.
In a study performed by Asgari et al., the investigation of the severity of shoulder pain in the first 24 h after gynecology laparoscopy showed the analgesic effect of 16 mg IP dexamethasone on reducing postoperative shoulder pain (14), which is consistent with the results of the present study. However, in the study conducted by Asgari et al., the severity of shoulder pain in the control group (receiving 16 cc IP normal saline) increased up to 8 h after surgery and then decreased; nevertheless, a consistently decreasing trend in the severity of shoulder pain was observed in the intervention group (14). Nonetheless, in the current study, both groups showed a decreasing trend in the severity of shoulder pain. This difference could be due to the different rates of pethidine use by the patients and different types of surgical procedures.
Furthermore, the mean duration of surgery in the study by Asgari et al. (14) was about 50 min in both groups; nevertheless, in the present study, the mean duration of surgery was higher than 135 min, indicating the different techniques and types of surgical procedures in the studies. Nevertheless, the mean duration of surgery and frequency of surgery types were not different between the study groups in the present study and were not a source of bias in the results of the current study. Furthermore, both groups of the present study had similar demographic characteristics, including mean age and BMI. The lower severity level of shoulder pain at all five intervals of the present study in the intervention group was an important finding in the present study, demonstrating the efficient analgesic property of IP dexamethasone, as the patients, evaluator, and surgeon were unaware of the allocations.
The analgesic efficacy of dexamethasone has been attributed to the anti-inflammatory properties of glucocorticoids, resulting in the suppression of bradykinin, release of neuropeptides from nerve endings, reduced prostaglandin production, and inhibition of cyclooxygenase isoform-2 synthesis and other inflammatory mediators, such as tumor necrosis factor alpha, interleukin-17B, and interleukin-6 (16). Moreover, the results of the present study are consistent with the results of previous studies indicating the analgesic effect of dexamethasone on other surgical procedures (17-20), also shown to enhance recovery, reduce the hospital stay of patients, and improve patients’ outcomes (17, 21).
The results of the current study also showed a significantly lower frequency of pethidine use in the intervention group, which confirms the results of previous studies (14, 15). Ismail et al. also reported that IP and IV dexamethasone resulted in reduced meperidine use in the first 24 h after gynecologic laparoscopy (15), which is consistent with the results of the present study. In addition, according to the results of the study performed by Asgari et al., IP dexamethasone did not decrease the pethidine use in the first hour after surgery; however, the mean dose of pethidine used by the intervention group was significantly lower than that of the control group (14).
Similar to the above-mentioned results, it was also observed that pethidine as an opioid is frequently used for the reduction of patients’ pain after surgery, especially shoulder pain, which is believed to be caused by afferent impulses; therefore, it is not easily controlled by nonsteroidal anti-inflammatory drugs (22). However, due to the adverse effects of opioids on patients, such as constipation, nausea/vomiting, delirium, and bladder dysfunction, resulting in decreased quality of life and morbidity, especially in older adults, it is necessary to reduce its use as much as possible (23). In this study, it was shown that IP dexamethasone was an effective strategy to reduce pethidine requirement for patients undergoing gynecologic laparoscopy.
The PONV was also evaluated as the secondary outcome of this study. The results showed a significantly lower rate of PONV in the intervention group (20%), compared to that reported for the control group (60%). The higher severity level of pain and higher level of pethidine use in the control group could be the sources of higher PONV in this group. In the study conducted by Ismail et al., IP and IV dexamethasone is effective in reducing patients’ pain and PONV in women undergoing gynecologic laparoscopy (15), which confirms the results of the present study. However, in the study performed by Ismail et al., only 7.5% of women receiving IP dexamethasone experienced nausea, and 5% of women had vomiting within the first 24 h after surgery (15), which is much lower than the rates of the intervention group in the present study.
The mechanism of antiemetic property of dexamethasone could be explained by the involvement of physiological transmission pathway of glucocorticoid receptors in vomiting (including serotonin neurotransmitter, neurokinin 1 and neurokinin 2 tachykinin protein receptors, and alpha-adrenergic receptors), central inhibition of prostaglandin synthesis, and regulation of the hypothalamic-pituitary-adrenal axis (24). As a complex postoperative complication, further attention should be paid to controlling PONV, and using prophylactic agents is considered superior to treatment (25). Dexamethasone is a cheap and available steroid with a half-life of 36-72 h and has been suggested as an effective prophylactic antiemetic in other surgical procedures, such as laparoscopic cholecystectomy (21). The antiemetic property of dexamethasone has been suggested to outweigh its adverse effect on wound healing; therefore, it is suggested as an effective and appropriate antiemetic to be pre-operatively used (26). The results of the present study showed that IP dexamethasone had favorable effects on the reduction of PONV in gynecologic laparoscopic procedures.
One of the limitations of the present study was the selection of the participants from one center and inclusion into the study using the nonrandomized method, which increases the risk of bias in the study results and limits their generalizability. Furthermore, there was no possibility to evaluate the causal relationships between the study variables due to the nature of the study design. Moreover, the patients were only followed for 24 h, and the studied outcomes and adverse effects after this period were not evaluated.
5.1. Conclusions
In conclusion, the results of this RCT showed that IP dexamethasone is effective in the reduction of shoulder pain and nausea/vomiting after gynecological laparoscopy and can significantly reduce opioid requirement within the first 24 h after the surgery; nevertheless, IP dexamethasone does not increase surgery duration. Therefore, it is recommended to use this technique during gynecological laparoscopy.
References
-
1.
Oliphant SS, Jones KA, Wang L, Bunker CH, Lowder JL. Trends over time with commonly performed obstetric and gynecologic inpatient procedures. Obstet Gynecol. 2010;116(4):926-31. [PubMed ID: 20859157]. [PubMed Central ID: PMC3253706]. https://doi.org/10.1097/AOG.0b013e3181f38599.
-
2.
Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: A qualified systematic review. World J Methodol. 2015;5(4):238-54. [PubMed ID: 26713285]. [PubMed Central ID: PMC4686422]. https://doi.org/10.5662/wjm.v5.i4.238.
-
3.
Fuentes MN, Rodriguez-Oliver A, Naveiro Rilo JC, Paredes AG, Aguilar Romero MT, Parra JF. Complications of laparoscopic gynecologic surgery. JSLS. 2014;18(3). [PubMed ID: 25392659]. [PubMed Central ID: PMC4208895]. https://doi.org/10.4293/JSLS.2014.00058.
-
4.
Hsien CF, Wang CL, Long CY, Chen YH, Lee WY, Chen SC, et al. Factors associated with types and intensity of postoperative pain following gynecological laparoscopic surgery: A cross-sectional study. Biomed Res Int. 2017;2017:2470397. [PubMed ID: 29312993]. [PubMed Central ID: PMC5615988]. https://doi.org/10.1155/2017/2470397.
-
5.
Amirshahi M, Behnamfar N, Badakhsh M, Rafiemanesh H, Keikhaie KR, Sheyback M, et al. Prevalence of postoperative nausea and vomiting: A systematic review and meta-analysis. Saudi J Anaesth. 2020;14(1):48-56. [PubMed ID: 31998020]. [PubMed Central ID: PMC6970369]. https://doi.org/10.4103/sja.SJA_401_19.
-
6.
Bhakta P, Ghosh BR, Singh U, Govind PS, Gupta A, Kapoor KS, et al. Incidence of postoperative nausea and vomiting following gynecological laparoscopy: A comparison of standard anesthetic technique and propofol infusion. Acta Anaesthesiologica Taiwanica. 2016;54(4):108-13. https://doi.org/10.1016/j.aat.2016.10.002.
-
7.
Fernández-López I, Peña-Otero D, Ángeles Atín-Arratibel MDL, Eguillor-Mutiloa M. Influence of the phrenic nerve in shoulder pain: A systematic review. International Journal of Osteopathic Medicine. 2020;36:36-48. https://doi.org/10.1016/j.ijosm.2020.03.003.
-
8.
Sao CH, Chan-Tiopianco M, Chung KC, Chen YJ, Horng HC, Lee WL, et al. Pain after laparoscopic surgery: Focus on shoulder-tip pain after gynecological laparoscopic surgery. J Chin Med Assoc. 2019;82(11):819-26. [PubMed ID: 31517775]. https://doi.org/10.1097/JCMA.0000000000000190.
-
9.
Chaichian S, Moazzami B, Haghgoo A, Sheibani K. A new approach to an old concept for reducing shoulder pain caused by gynecological laparoscopy. J Reprod Infertil. 2018;19(1):56-60. [PubMed ID: 29850448]. [PubMed Central ID: PMC5960053].
-
10.
Zeeni C, Chamsy D, Khalil A, Abu Musa A, Al Hassanieh M, Shebbo F, et al. Effect of postoperative Trendelenburg position on shoulder pain after gynecological laparoscopic procedures: a randomized clinical trial. BMC Anesthesiol. 2020;20(1):27. [PubMed ID: 31996139]. [PubMed Central ID: PMC6988196]. https://doi.org/10.1186/s12871-020-0946-9.
-
11.
Taş B, Donatsky AM, Gögenur I. Techniques to reduce shoulder pain after laparoscopic surgery for benign gynaecological disease: a systematic review. Gynecological Surgery. 2013;10(3):169-75. https://doi.org/10.1007/s10397-013-0791-7.
-
12.
van Dijk JEW, Dedden SJ, Geomini P, Meijer P, van Hanegem N, Bongers MY. POstL aparoscopic reduction of pain by combining intraperitoneal normal saline and the pulmonary recruitment maneuver (POLAR BEAR trial). RCT to estimate reduction in pain after laparoscopic surgery when using a combination therapy of intraperitoneal normal saline and the pulmonary recruitment maneuver. BMC Womens Health. 2017;17(1):42. [PubMed ID: 28610572]. [PubMed Central ID: PMC5470318]. https://doi.org/10.1186/s12905-017-0397-8.
-
13.
Kaloo P, Armstrong S, Kaloo C, Jordan V. Interventions to reduce shoulder pain following gynaecological laparoscopic procedures. Cochrane Database Syst Rev. 2019;1. CD011101. [PubMed ID: 30699235]. [PubMed Central ID: PMC6353625]. https://doi.org/10.1002/14651858.CD011101.pub2.
-
14.
Asgari Z, Mozafar-Jalali S, Faridi-Tazehkand N, Sabet S. Intraperitoneal dexamethasone as a new method for relieving postoperative shoulder pain after gynecologic laparoscopy. Int J Fertil Steril. 2012;6(1):59-64. [PubMed ID: 25505513]. [PubMed Central ID: PMC4260641].
-
15.
Ismail EA, Abo Elfadl GM, Bahloul M. Comparison of intraperitoneal versus intravenous dexamethasone on postoperative nausea and vomiting after gynecological laparoscopy: a randomized clinical trial. Korean J Anesthesiol. 2019;72(1):47-52. [PubMed ID: 30223315]. [PubMed Central ID: PMC6369338]. https://doi.org/10.4097/kja.d.18.00132.
-
16.
Becker DE. Basic and clinical pharmacology of glucocorticosteroids. Anesth Prog. 2013;60(1):25-31. quiz 32. [PubMed ID: 23506281]. [PubMed Central ID: PMC3601727]. https://doi.org/10.2344/0003-3006-60.1.25.
-
17.
Backes JR, Bentley JC, Politi JR, Chambers BT. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: A prospective, randomized controlled trial. J Arthroplasty. 2013;28(8):11-7. [PubMed ID: 23937923]. https://doi.org/10.1016/j.arth.2013.05.041.
-
18.
Buland K, Zahoor MU, Asghar A, Khan S, Zaid AY. Efficacy of single dose perioperative intravenous steroid (dexamethasone) for postoperative pain relief in tonsillectomy patients. J Coll Physicians Surg Pak. 2012;22(6):349-52. [PubMed ID: 22630091].
-
19.
Gómez-Hernández J, Orozco-Alatorre AL, Domínguez-Contreras M, Oceguera-Villanueva A, Gómez-Romo S, Villaseñor ASA, et al. Preoperative dexamethasone reduces postoperative pain, nausea and vomiting following mastectomy for breast cancer. BMC cancer. 2010;10(1):692. https://doi.org/10.1186/1471-2407-10-692.
-
20.
Mohtadi A, Nesioonpour S, Salari A, Akhondzadeh R, Masood Rad B, Aslani SM. The effect of single-dose administration of dexamethasone on postoperative pain in patients undergoing laparoscopic cholecystectomy. Anesth Pain Med. 2014;4(3). e17872. [PubMed ID: 25237639]. [PubMed Central ID: PMC4165022]. https://doi.org/10.5812/aapm.17872.
-
21.
Tavakolpour S, Kheiry F, Mirsafaei HS, Akhlaghdoust M. The possible role of interleukin-35 and its therapeutic potential in pemphigus. Int Immunopharmacol. 2017;42:11-7. https://doi.org/10.1016/j.intimp.2016.11.005.
-
22.
Kolettas A, Lazaridis G, Baka S, Mpoukovinas I, Karavasilis V, Kioumis I, et al. Postoperative pain management. J Thorac Dis. 2015;7(Suppl 1):S62-72. [PubMed ID: 25774311]. [PubMed Central ID: PMC4332101]. https://doi.org/10.3978/j.issn.2072-1439.2015.01.15.
-
23.
Rogers E, Mehta S, Shengelia R, Reid MC. Four strategies for managing opioid-induced side effects in older adults. Clin Geriatr. 2013;21(4). [PubMed ID: 25949094]. [PubMed Central ID: PMC4418642].
-
24.
Chu CC, Hsing CH, Shieh JP, Chien CC, Ho CM, Wang JJ. The cellular mechanisms of the antiemetic action of dexamethasone and related glucocorticoids against vomiting. Eur J Pharmacol. 2014;722:48-54. [PubMed ID: 24184695]. https://doi.org/10.1016/j.ejphar.2013.10.008.
-
25.
Shaikh SI, Nagarekha D, Hegade G, Marutheesh M. Postoperative nausea and vomiting: A simple yet complex problem. Anesth Essays Res. 2016;10(3):388-96. [PubMed ID: 27746521]. [PubMed Central ID: PMC5062207]. https://doi.org/10.4103/0259-1162.179310.
-
26.
Hoorsan H, Alavi Majd H, Chaichian S, Mehdizadehkashi A, Hoorsan R, Akhlaqghdoust M, et al. Maternal anthropometric characteristics and adverse pregnancy outcomes in Iranian women: A confirmation analysis. Arch Iran Med. 2018;21(2):61-6. [PubMed ID: 29664656].