Abstract
Background:
Aerobic exercise and relaxing music induce parasympathetic activity over the heart. The combined effect of the two interventions was shown to reduce heart rate (HR), rate of perceived exertion (RPE), and anxiety levels.Objectives:
The purpose of the study was to evaluate the effect of aerobic exercise along with music vs. only aerobic exercise, on the autonomic function of the heart during recovery in collegiate overweight and obese individuals.Methods:
Thirty-two collegiate overweight and obese individuals were recruited based on inclusion and exclusion criteria. Random allocation of participants was done to the aerobic exercise group (n = 16) and aerobic exercise plus music group (n = 16). Each group performed 30 minutes/session, six times/week for four weeks. Pre and post measures of body composition, exercise HR, RPE, square root of mean squared difference between adjacent R-R intervals (RMSSD), interval differences between of adjacent R-R intervals > 50 ms derived from difference between consecutive RR intervals (NN50), low-frequency (LF), high frequency (HF), and LF/HF measures of heart rate variability (HRV) during recovery were recorded.Results:
RMSSD (P = 0.003) and LF (P = 0.009) scores showed a significant difference at baseline. A significant time effect was found to be in HR (P < 0.001), RPE (< 0.001), NN50 (P = 0.001), HF (P = 0.016) and LF/HF score (P < 0.001) of HRV indicating difference between pre and post measures, while no difference was found in RMSSD and LF score. A significant group effect was found to be in HR (P = 0.016) and LF/HF score (P = 0.008), indicating the difference between the two groups.Conclusions:
Regular aerobic exercise, in conjunction with relaxing music, appears to confer a beneficial effect on the autonomic modulation during the post-exercise recovery period.Keywords
Autonomic Activity Heart Rate Variability Heart Rate Rated Perceived Exertion Fat Mass
1. Background
Obesity is one of the most critical health problems worldwide. The prevalence of overweightness was around 1.9 billion in 18 years and older adults worldwide in 2016. Of these, over 650 million were obese (1). Its incidence is increasing mostly due to a sedentary lifestyle. The rising incidence of obesity has a direct correlation with hypertension, dyslipidemia, type 2 diabetes mellitus, and cardiovascular disease (CVD) (2). Overweight and obesity are considered significant predictors for the development of CVD (3, 4). Overweight and obese individuals also have a higher prevalence of depression, anxiety, and other psychological illness (5, 6).
Heart rate variability (HRV) assesses the autonomic activity over the heart by analyzing beat-to-beat variation in the R-R interval (7). The contribution of sympathetic and parasympathetic control over the heart is represented by time-domain and frequency-domain HRV. Obese people show increased sympathetic cardiovascular outflow over parasympathetic, thus reduced HRV (8-10). A study found that obesity was strongly associated with increased sympathetic outflow and reduced parasympathetic activity, indicating higher risk of cardiovascular mortality and morbidity (11).
Heart rate (HR) rises during exercise which is attributed to the combination of parasympathetic withdrawal and sympathetic activation (12). After cessation of exercise, HR returns to the pre-exercise levels. During HR recovery, there is reactivation of parasympathetic activity and decreased sympathetic outflow (13). The parasympathetic activity and sympathetic outflow reaches pre-exercise levels in around 15 to 60 min after moderate-intensity exercise (14-16). Increased vagal activity is associated with a reduction in the risk of death (17).
Aerobic activity is a primary management of overweightness and obesity with broad variations. Exercises are tailored individually based on age, body mass index (BMI), and associated comorbidities. Exercises help in fat loss, improve cardiovascular fitness, increase energy expenditure, and evoke sensations of well-being. Previous studies have shown that weight reduction effectively restores reflex (18) and tonic (9) autonomic control and reduces sympathetic hyperactivity (19). Physical activity and weight reduction are standard prescriptions to mitigate abdominal adipose deposition and obesity. Aerobic activity has shown to increase post-exercise HR recovery and autonomic modulation of heart in obese individuals (20).
Music therapy involves the skillful use of musical elements to improve a person’s mental and physical health. Music exhibits psychological benefits and promotes emotional health. Music has an anxiolytic effect in coping with stress and boosting psychological and physiologic well-being. Music helps in decreasing the rate of perceived exertion (RPE) during low to moderate-intensity exercise (21, 22), while no such change is found at higher intensity (22). Some studies have shown HR to decrease by calming music (23), soft slow music (24), and self-selected music (25, 26), whereas others suggest no difference (27-29). Listening to music enhances parasympathetic dominance with a reduction in sympathetic tone (30). Previous studies have shown long-term and short-term beneficial effects of musical auditory stimulation on autonomic function (26, 31-33). Relaxing music increases parasympathetic activity by inducing positive emotions (34, 35). A study reported that two weeks of relaxing music along with aerobic exercise showed a reduction in the RPE, HR, and anxiety levels in collegiate athletes (36). To the best of our knowledge, the combined effect of these two on the autonomic function of the heart is still unknown.
2. Objectives
The purpose of the study was to evaluate the combined effect of aerobic exercise with music vs. aerobic exercise alone on body composition, exercise HR, RPE, and autonomic function of the heart in collegiate overweight and obese individuals.
3. Methods
3.1. Participants
Thirty-two (19 male and 13 female) collegiate individuals were recruited from Jamia Millia Islamia and other nearby universities. Subject inclusion criteria were: between ages of 21 and 29 years, BMI > 27 kg/m2 and physically inactive (less than 20 min/day of vigorous-intensity physical activity on at least 3 days/week, or less than 30 min/day of moderate-intensity physical activity on at least 5 days/week) (37). Participants were excluded if they had musculoskeletal or neurological disorders affecting the gait, cardiovascular or respiratory abnormality, hypertension, diabetes, were on medications like anti-inflammatory agents, were smoking, or taking alcohol.
Participants gave their written informed consent for participation after being explained to about the nature and procedure of the study. All doubts from the participants were clear before starting the procedure. Participants underwent a general physical examination to test for any ailments of the cardiorespiratory and locomotor systems. Ethical clearance was taken from the Institutional Ethical Committee, Jamia Millia Islamia, New Delhi, India, before the commencement of the study.
3.2. Study Design
The study was a parallel group with two arms, a randomized controlled trial with single blinding (blinding of outcome assessor). Participants who met inclusion and exclusion criteria were randomly allocated either to an aerobic exercise group or aerobic exercise plus music group in a 1:1 allocation ratio using the coin method. Subjects reported to the laboratory on two different occasions. On first visit, anthropometric and body composition was assessed followed by assessment of the target heart rate, THR = [(HRmax - RHR) × 40 - 50% intensity] + RHR, where HRmax is heart rate maximum, calculated by using formula, HRmax = 206.9 - 0.69 × age (y) and RHR is resting heart rate. Two to three familiarization sessions were provided to each participant with the treadmill running exercise protocol.
Before the second visit, participants were instructed to get appropriate night rest, not perform any strenuous exercise and abstain from caffeine consumption before and on testing days. They were called for the second visit to the lab at the same time of day (between 10:00 AM to 1:00 PM). The participants then performed an incremental treadmill running test at a target heart rate. The incremental running test starts at an initial speed of 10 km/h with no inclination or gradient for 1 minute, after which the speed was increased every 30 seconds by 0.5 km/h until subjects achieved either their target heart rate or volitional exhaustion. Heart rate (Polar Electro, RS 400, Kempele, Finland) and RPE (Borg scale 6 - 20) were recorded. The subjects were asked to get into supine lying within 10 seconds. Electrocardiogram recordings were then collected for 20 minutes of recovery. Participants were asked to maintain breathing at 15 breaths per min using a metronome during the last 10 minutes of recovery. The subjects were instructed to relax and not to move or sleep during measurements. The HRV analysis was done on the final 5 minutes of data from the record. Following the lab visit, each subject performed four weeks of supervised aerobic exercise or aerobic exercise plus music. The participants were asked to report back after four weeks between 24 and 48 hours of their last exercise session for post measurements. Wherein all measurements were taken in the same order of the pre-training measurements (Figure 1).
Flow chart of the study protocol

3.3. Exercise Training
Aerobic exercise was performed on an electronically controlled treadmill (Landice L7, NJ, USA) and consisted of 30 minutes/session, six times per week for four weeks. Participants were asked to perform treadmill running at a speed that maintained their target heart rate between 40% - 50% heart rate reserve for the full 30-minute session (38). The protocol was designed using the FIIT recommendation for overweight and obese individuals given by the American College of Sports Medicine guidelines (39). Subjects were progressed every week to adjust the training heart rate. They were not allowed to engage in any other activities while exercising (e.g., talking, eating) to minimize distractions from arousal sensations.
3.4. Music Selection
Several pieces of instrumental Indian classical music was provided to each individual. Music was selected by participants themselves honoring personal choice, but they were instructed to choose relaxing music. This music was referred to as “favorite music” to the participants (40). The tempo of the music was kept between 80 - 110 beats per minute. Rhythms were relatively subtle, simple, and constant. Melodies were strong and secure, and harmonies were consonant. The music was transferred to a portable listening device and played by using headphones. Intensity (volume) was self-selected by the subject according to their comfort.
3.5. Anthropometric and Body Composition Assessment
Weight was measured via a digital weighing machine, while height was measured using a stadiometer. Body mass index (BMI) was calculated as weight (kg)/square of height (m). Body composition (fat percentage and fat mass) was assessed using bioelectrical impedance analysis (MaltronBioScan 916, Reyleigh Essex, UK).
3.6. Data Acquisition and Analysis of HRV
The electrocardiograph (ECG) lead was placed after preparing the skin with an alcohol swab. Beat to beat HR was recorded using CM5 lead configuration. The sample rate of ECG data was kept at 1 kHz, band pass filtered between 0.3 Hz and 100 Hz, and stored on a computer by data acquisition software (Lab Chart pro AD Instrument, Australia) using PowerLab 8/30 (AD Instrument, Australia). All stored data were offline and used later for analysis. The offline signal processed for 5 minutes epochs segment of ECG. The HR peaks were automatically detected via an established QRS detection algorithm and used to generate an R-R interval time event series. R-R intervals were selected by setting a threshold at 0.5 mV and ectopics were excluded from analysis through the “beat classifier” function of the software. Power spectral analysis was performed using fast Fourier transformation size 1024 with the Welch window and 50% overlap.
Time-domain variables of HRV consisted of RMSSD (square root of the mean squared difference between adjacent R-R intervals) which denotes vagal activity and NN50 (interval differences between adjacent R-R intervals > 50 ms derived from the difference between consecutive RR intervals) which also indicate parasympathetic activity. Fast Fourier transformation obtained frequency domain HRV measures revealed three types of bandwidths defined as: very low frequency region (VLF < 0.04 Hz) resulting from non-harmonic fractal oscillations of unknown origin, low-frequency region (LF = 0.04 - 0.15 Hz) related to baroreflex activity and thermoregulatory components mediated by both the sympathetic and parasympathetic arms of the autonomic nervous system, and high-frequency region (HF = 0.15 - 0.4 Hz) caused by arrhythmic respiratory oscillations indicative of parasympathetic modulation of the heart. The VLF component was not used in this study other than in the calculation of normalized LF and HF. The LF-HF ratio (LF/HF) is an indicator of sympathovagal balance (41). All data acquisition and post-acquisition analyses were carried out following standards put forth by the Task Force of the European Society of Cardiology and North American Society of Pacing and Electrophysiology (7).
3.7. Sample Size
The number of subjects was determined using Software G*Power 3.1.9.2, from the study done by Jamali et al. (36) showing the effect of aerobic vs. aerobic plus music in collegiate athletes taken post measures of exercise HR. A total of 16 subjects (including 10% of drop-outs) per group were shown to be necessary based on the effect size of 1.64, an alpha level of 0.05, and power (1-beta) of 0.98.
3.8. Statistical Analysis
Data analysis was done using SPSS version 21.0. Normality distribution of variables was verified using the Shapiro-Wilk test. Fat percentage, HR, RPE, NN50, and LF/HF scores showing non-normal distribution were log transformed for further analysis. Demographic characteristics and baseline criterion measures were assessed using an independent t-test between the aerobic group and aerobic plus music group. Mixed analysis of variance was used for demographic characteristics, HR, RPE, and HRV measures to find out group effect, time effect, and group × time interaction. Also, a mixed analysis of covariance was used for fat mass percentage, RMSSD, and LF score considering “pre” values as a covariate. The confidence interval was kept at 95% and the level of significance at P ≤ 0.05.
4. Results
Mean ± SD of demographic characteristics and baseline criterion of variables for both groups has been reported in Table 1. No significant difference found in any demographic characteristics such as age, weight, height, BMI, and fat mass (P > 0.05); whereas fat percentage showed a significant difference between the groups (P = 0.02) at baseline. The RMSSD (P = 0.003) and LF (P = 0.009) scores showed a significant difference at baseline (Table 1).
Comparison of Demographic Characteristics and Baseline Criterion Measures Between the Aerobic Group and Aerobic + Music Groupa
| Variable | Aerobic Group (N = 16) | Aerobic + music group (N = 16) | t value | P Value |
|---|---|---|---|---|
| Age | 25.44 ± 1.96 | 25.56 ± 2.03 | 0.177 | 0.861 |
| Height, m | 1.66 ± 0.05 | 1.65 ± 0.04 | 0.749 | 0.460 |
| Weight, kg | 89.66 ± 8.30 | 88.60 ± 10.59 | 0.318 | 0.753 |
| BMI, kg/m2 | 32.26 ± 2.59 | 32.35 ± 3.26 | 0.086 | 0.932 |
| Fat mass, kg | 57.47 ± 6.60 | 59.47 ± 7.96 | 0.772 | 0.446 |
| Fat percentage, % | 64.08 ± 4.02 | 67.07 ± 2.84 | 2.457 | 0.02b |
| HR, bpm | 123 ± 2.50 | 122.75 ± 2.86 | 0.27 | 0.789 |
| RPE | 12.06 ± 1.65 | 12.88 ± 1.02 | 1.755 | 0.089 |
| RMSSD, nu | 30.39 ± 8.55 | 38.33 ± 3.71 | 3.187 | 0.003b |
| NN50 | 2.50 ± 1.15 | 2.38 ± 1.36 | 0.36 | 0.721 |
| LF | 75.04 ± 7.96 | 64.52 ± 14.33 | 2.796 | 0.009b |
| HF | 152.39 ± 23.39 | 162.5 ± 11.84 | 1.541 | 0.134 |
| LF/HF | 1.00 ± 0.135 | 0.94 ± 0.149 | 1.485 | 0.148 |
Mean ± SD of outcome variables before and after four weeks of intervention for both groups has been reported in Table 2. There was a significant reduction in the weight (P = 0.001), BMI (P < 0.001), and fat mass (P < 0.001) between pre and post measures (time effect) in both the groups while no significant time effect was found for fat percentage. The between-group comparison showed no significant group effect and group × time interaction in weight, BMI, fat mass, and fat percentage.
There was a significant time effect found for HR (P < 0.001) and RPE (P < 0.001). Measurement of HR revealed a significant group effect (P = 0.016) and group × time interaction (P = 0.004) indicating a significant difference between the two groups, whereas RPE showed no significant group effect as well as group×time interaction (Table 2).
Results of Mixed ANOVA or ANCOVA of Body Composition, HR, RPE, Time and Frequency Domain of Heart Rate Variabilitya
| Variables | Aerobic Group (N = 16) | Aerobic + Music Group (N = 16) | Time (T) Effect | Group (G) Effect | G × T Effect | ||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ηp2; P Value | ηp2; P Value | ηp2; P Value | |
| Weight, kg | 89.66 ± 8.30 | 89.40 ± 8.42 | 88.60 ± 10.59 | 88.01 ± 10.64 | 0.336; 0.001b | 0.004; 0.719 | 0.064; 0.162 |
| BMI, kg/m2 | 32.26 ± 2.59 | 32.17 ± 2.64 | 32.35 ± 3.26 | 32.14 ± 3.26 | 0.34; < 0.001b | 0.000; 0.977 | 0.07; 0.144 |
| Fat mass, kg | 57.47 ± 6.60 | 56.88 ± 6.87 | 59.47 ± 7.96 | 58.53 ± 7.82 | 0.465; < 0.001b | 0.016; 0.487 | 0.042; 0.258 |
| Fat percentage, % | 64.08 ± 4.02 | 63.60 ± 4.31 | 67.07 ± 2.84 | 66.46 ± 2.95 | 0.087; 0.107 | 0.033; 0.325 | 0.033; 0.325 |
| HR, bpm | 123 ± 2.50 | 115.25 ± 3.23 | 122.75 ± 2.86 | 110.06 ± 5.59 | 0.837; < 0.001b | 0.183; 0.016b | 0.251; 0.004b |
| RPE | 12.06 ± 1.65 | 7.94 ± 1.43 | 12.88 ± 1.02 | 8 ± 0.81 | 0.886; < 0.001b | 0.05; 0.217 | 0.026; 0.375 |
| RMSSD, nu | 30.89 ± 8.55 | 34.36 ± 8.58 | 38.33 ± 3.71 | 41.23 ± 5.61 | 0.109; 0.069 | 0.004; 0.746 | 0.004; 0.746 |
| NN50 | 2.50 ± 1.15 | 3.38 ± 1.5 | 2.38 ± 1.36 | 3.94 ± 2.14 | 0.34; 0.001b | 0.000; 0.994 | 0.014; 0.522 |
| LF, nu | 75.04 ± 7.96 | 75.42 ± 11.02 | 64.52 ± 12.77 | 69.79 ± 14.33 | 0.04; 0.282 | 0.033; 0.331 | 0. 033; 0.331 |
| HF, nu | 152.39 ± 23.39 | 161.04 ± 26.25 | 162.5 ± 11.84 | 170.87 ± 8.98 | 0.178; 0.016b | 0.088; 0.1 | 0.000; 0.967 |
| LF/HF | 1.0 ± 0.135 | 0.48 ± 0.12 | 0.94 ± 0.14 | 0.41 ± 0.09 | 0.83; < 0.001b | 0.214; 0.008b | 0.015; 0.503 |
Time effect was significant for NN50 (P = 0.001), HF (P = 0.016), and LF/HF scores (P < 0.001) of HRV, while no significant time effect was found in RMSSD and LF scores. Group effect was significant only in the LF/HF score (P = 0.008), whereas other time and frequency domain measures of HRV showed no significant group effect. Also, no significant group × time interaction was found in any of the time and frequency domain HRV measures (Table 2).
5. Discussion
The present study was designed to determine the combined effect of aerobic training along with relaxing music as compared to only aerobic training on body composition, exercise HR, RPE, and autonomic function of the heart in overweight and obese individuals.
The present study indicates that aerobic training significantly reduced fat mass, which supports previous findings (10, 42, 43), but the addition of music over did not confer any additional benefits on fat mass. Body fat decreases after aerobic training as exercises add up to the ability of the body to use fat as a substrate. Total fat oxidation was increased during aerobic exercise due to increased lipoprotein lipase activity in the skeletal muscle and increased lipoprotein lipase mass index (44).
5.1. HR and RPE
A reduction in RPE and HR was observed in both the groups after four weeks. However, a decrease in exercise HR was higher in the aerobic plus music group. While most of the previous studies reported that there was a reduction in HR when exercise was incorporated with the music (21, 22, 36, 40). Nethery reported no difference from the motivational music on the HR during treadmill running at 50% VO2max (22). Figueroa et al. (20) also found a reduction in exercise HR after 16 weeks of endurance training in obese individuals. These results support our previous finding that aerobic exercise along with relaxing music reduces HR to a greater extent than aerobic exercise in athletes (36). The mechanism behind this could be the psychobiological impact that music has on exercise due to relaxation, reduced muscle tension, and increased blood flow, hence reduced heart rate when compared to the only aerobic group (36). Our study found overweight and obese individuals could be able to continue aerobic exercise for a longer time duration due to music intervention helping the distraction of mental stress during exercise bouts, which might help to achieve greater health benefits.
Whereas the present study found no difference between the two groups in terms of RPE, previous studies have reported a decrease in the perception of exertion (21, 36, 40, 45). This contrast in results could be due to the difference in population or the intensity of exercise, as previous findings were reported on the athletic population and the current study involved obese people. The stimulus of music and cognition of fatigue caused by exercise are two parallel sensory information (46). As the cognition of fatigue may predominate over the stimulation from music for the overweight and obese population, the effect of music on the perception of exertion could have been nullified.
5.2. HRV
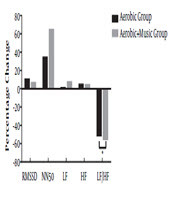
The NN50 and HF values of HRV after four weeks of exercise indicating an increase in parasympathetic activity during post-exercise recovery over the heart was similar in both the groups, showing no added effect of music (over just aerobic exercise) in HF values (Figure 2). However, there was an increment in the post-exercise HF power after training in both the groups (5.67% in the aerobic group and 5.15% in aerobic plus music group). Previously, Figueroa et al. (20) found an improvement in the HF power after endurance training in obese women with and without type 2 diabetes. Similarly, Yamamoto et al. (47) also found improvement in the post-exercise HF and SDNN values after four weeks of endurance training. Our data demonstrate a beneficial effect on post-exercise parasympathetic activity, wherein there was a larger change in NN50 in aerobic plus music group (65.54%) than aerobic group (35.2%). Even though the difference found was statistically non-significant, music did help enhance the parasympathetic activity over aerobic training through the pathways of relaxation, baroreflex activity, and increasing blood flow to the heart. The music helped in improving the anxiety (36) and stress which might help in improving the NN50 scores in overweight and obese individuals. Studies have shown that anxiety and stress have negative association with time domain HRV measures (48).
Relative percentage change after 4 weeks of training in time and frequency domain of HRV recovery. RMSSD: root mean square of successive differences; NN50: interval differences between of adjacent RR intervals > 50 ms derived from difference between consecutive RR intervals; LF, low frequency spectral power; HF, high frequency spectral power; LF/HF, low frequency/high frequency ratio.

Previous studies have shown decrease in the LF/HF values at rest after four weeks of aerobic training (49). Yamamoto et al. (47), for example, found a reduction in the LF/HF values 20 minutes post-exercise recovery after four weeks of endurance training. Our study revealed that after four weeks of training, LF/HF value decreased in both aerobic as well as aerobic plus music groups. Aerobic plus music group (56.17%) showed a larger lowering of LF/HF values than aerobic group (52%), indicating additional influence of music in decreasing sympathetic modulation and enhancing parasympathetic reactivation (Figure 2). The LF/HF score, which is a component of frequency domain indices, measures the sympathovagal balance to heart, reflecting an increase in sympathetic nerve activity.
In terms of the relevance of musical therapy and auditory stimulation, Chuang et al. (32) investigated the effects of an 8-month music intervention on anthracycline-treated breast cancer patients and showed improvement in the time and frequency domain indices of HRV. Okada et al. (50) found that music positively affected the autonomic activity and reduced incidences of heart failure in elderly patients with CVA and dementia. Similarly, the present study shows that music along with aerobic exercise, reversed sympathetic dominance, and parasympathetic withdrawal to a greater extent than aerobic exercise alone. These improvements in post-exercise cardiac autonomic modulation reduce the risk of cardiac complications and minimize cardiac mortality in obese individuals.
There are several limitations in our study; the results apply to the overweight and obese population, while overweight individuals were relatively less in number. Absence of control for various external (social evaluation, educational stress) and internal factors (dietary intake) that affect the autonomic functions of the body. Small sample size, considering the larger variability in the HRV measures. The study results, therefore, only provide preliminary evidence that relaxing music with aerobic exercise can promote autonomic functions in obese individuals. Further studies on different age groups and with different types of music (synchronous/asynchronous) or auditory stimulus could provide a better understanding. There is also scope for using other physiological parameters along with the autonomic profile.
In conclusion, four weeks of aerobic exercise complemented with music reduced exercise heart rate more than aerobic exercise alone. Regular aerobic exercise, when incorporated with relaxing music, appears to confer a more beneficial effect on the cardiac autonomic modulation apparently by post-exercise recovery LF/HF score. The overweight and obese individuals through this intervention gained benefits in cardiovascular modulation when recovering from moderate-intensity stress.
References
-
1.
WHO. Global estimates fact sheet: Obesity and overweight. 2018. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
-
2.
Nguyen NT, Magno CP, Lane KT, Hinojosa MW, Lane JS. Association of hypertension, diabetes, dyslipidemia, and metabolic syndrome with obesity: Findings from the National Health and Nutrition Examination survey, 1999 to 2004. J Am Coll Surg. 2008;207(6):928-34. [PubMed ID: 19183541]. https://doi.org/10.1016/j.jamcollsurg.2008.08.022.
-
3.
Bastien M, Poirier P, Lemieux I, Despres JP. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis. 2014;56(4):369-81. [PubMed ID: 24438728]. https://doi.org/10.1016/j.pcad.2013.10.016.
-
4.
Lavie CJ, Milani RV, Ventura HO. Obesity and cardiovascular disease: Risk factor, paradox, and impact of weight loss. J Am Coll Cardiol. 2009;53(21):1925-32. [PubMed ID: 19460605]. https://doi.org/10.1016/j.jacc.2008.12.068.
-
5.
Kottke TE, Wu LA, Hoffman RS. Economic and psychological implications of the obesity epidemic. Mayo Clin Proc. 2003;78(1):92-4. [PubMed ID: 12528882]. https://doi.org/10.4065/78.1.92.
-
6.
Wyatt SB, Winters KP, Dubbert PM. Overweight and obesity: Prevalence, consequences, and causes of a growing public health problem. Am J Med Sci. 2006;331(4):166-74. [PubMed ID: 16617231]. https://doi.org/10.1097/00000441-200604000-00002.
-
7.
[No author listed]. Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93(5):1043-65. [PubMed ID: 8598068].
-
8.
Rossi M, Marti G, Ricordi L, Fornasari G, Finardi G, Fratino P, et al. Cardiac autonomic dysfunction in obese subjects. Clin Sci (Lond). 1989;76(6):567-72. [PubMed ID: 2736876]. https://doi.org/10.1042/cs0760567.
-
9.
Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83(8):1242-7. [PubMed ID: 10215292]. https://doi.org/10.1016/s0002-9149(99)00066-1.
-
10.
Amano M, Kanda T, Ue H, Moritani T. Exercise training and autonomic nervous system activity in obese individuals. Med Sci Sports Exerc. 2001;33(8):1287-91. [PubMed ID: 11474328]. https://doi.org/10.1097/00005768-200108000-00007.
-
11.
Yadav RL, Yadav PK, Yadav LK, Agrawal K, Sah SK, Islam MN. Association between obesity and heart rate variability indices: an intuition toward cardiac autonomic alteration - a risk of CVD. Diabetes Metab Syndr Obes. 2017;10:57-64. [PubMed ID: 28255249]. [PubMed Central ID: PMC5322847]. https://doi.org/10.2147/DMSO.S123935.
-
12.
Arai Y, Saul JP, Albrecht P, Hartley LH, Lilly LS, Cohen RJ, et al. Modulation of cardiac autonomic activity during and immediately after exercise. Am J Physiol. 1989;256(1 Pt 2):H132-41. [PubMed ID: 2643348]. https://doi.org/10.1152/ajpheart.1989.256.1.H132.
-
13.
Pober DM, Braun B, Freedson PS. Effects of a single bout of exercise on resting heart rate variability. Med Sci Sports Exerc. 2004;36(7):1140-8. [PubMed ID: 15235317]. https://doi.org/10.1249/01.mss.0000132273.30827.9a.
-
14.
Piepoli M, Coats AJ, Adamopoulos S, Bernardi L, Feng YH, Conway J, et al. Persistent peripheral vasodilation and sympathetic activity in hypotension after maximal exercise. J Appl Physiol (1985). 1993;75(4):1807-14. [PubMed ID: 8282635]. https://doi.org/10.1152/jappl.1993.75.4.1807.
-
15.
Terziotti P, Schena F, Gulli G, Cevese A. Post-exercise recovery of autonomic cardiovascular control: A study by spectrum and cross-spectrum analysis in humans. Eur J Appl Physiol. 2001;84(3):187-94. [PubMed ID: 11320634]. https://doi.org/10.1007/s004210170003.
-
16.
Raczak G, Pinna GD, La Rovere MT, Maestri R, Danilowicz-Szymanowicz L, Ratkowski W, et al. Cardiovagal response to acute mild exercise in young healthy subjects. Circ J. 2005;69(8):976-80. [PubMed ID: 16041170]. https://doi.org/10.1253/circj.69.976.
-
17.
Schwartz PJ, La Rovere MT, Vanoli E. Autonomic nervous system and sudden cardiac death. Experimental basis and clinical observations for post-myocardial infarction risk stratification. Circulation. 1992;85(1 Suppl):I77-91. [PubMed ID: 1728509].
-
18.
Grassi G, Seravalle G, Colombo M, Bolla G, Cattaneo BM, Cavagnini F, et al. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation. 1998;97(20):2037-42. [PubMed ID: 9610534]. https://doi.org/10.1161/01.cir.97.20.2037.
-
19.
Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25(4 Pt 1):560-3. [PubMed ID: 7721398]. https://doi.org/10.1161/01.hyp.25.4.560.
-
20.
Figueroa A, Baynard T, Fernhall B, Carhart R, Kanaley JA. Endurance training improves post-exercise cardiac autonomic modulation in obese women with and without type 2 diabetes. Eur J Appl Physiol. 2007;100(4):437-44. [PubMed ID: 17406886]. https://doi.org/10.1007/s00421-007-0446-3.
-
21.
Szmedra L, Bacharach DW. Effect of music on perceived exertion, plasma lactate, norepinephrine and cardiovascular hemodynamics during treadmill running. Int J Sports Med. 1998;19(1):32-7. [PubMed ID: 9506797]. https://doi.org/10.1055/s-2007-971876.
-
22.
Nethery VM. Competition between internal and external sources of information during exercise: influence on RPE and the impact of the exercise load. J Sports Med Phys Fitness. 2002;42(2):172-8. [PubMed ID: 12032412].
-
23.
Knight WE, Rickard Ph D. Relaxing music prevents stress-induced increases in subjective anxiety, systolic blood pressure, and heart rate in healthy males and females. J Music Ther. 2001;38(4):254-72. [PubMed ID: 11796077]. https://doi.org/10.1093/jmt/38.4.254.
-
24.
Copeland BL, Franks BD. Effects of types and intensities of background music on treadmill endurance. J Sports Med Phys Fitness. 1991;31(1):100-3. [PubMed ID: 1861474].
-
25.
Miluk-Kolasa B, Matejek M, Stupnicki R. The effects of music listening on changes in selected physiological parameters in adult pre-surgical patients. J Music Ther. 1996;33(3):208-18. https://doi.org/10.1093/jmt/33.3.208.
-
26.
White JM. Effects of relaxing music on cardiac autonomic balance and anxiety after acute myocardial infarction. Am J Crit Care. 1999;8(4):220-30. [PubMed ID: 10392221].
-
27.
Schwartz SE, Fernhall B, Plowman SA. Effects of music on exercise performance. J Cardiopulm Rehabil. 1990;10(9):312-6. https://doi.org/10.1097/00008483-199009000-00002.
-
28.
Strauser JM. The effects of music versus silence on measures of state anxiety, perceived relaxation, and physiological responses of patients receiving chiropractic interventions. J Music Ther. 1997;34(2):88-105. https://doi.org/10.1093/jmt/34.2.88.
-
29.
Vanderark SD, Ely D. University biology and music majors' emotional ratings of musical stimuli and their physiological correlates of heart, rate, finger temperature, and blood pressure. Percept Mot Skills. 1994;79(3 Pt 1):1391-7. [PubMed ID: 7899024]. https://doi.org/10.2466/pms.1994.79.3.1391.
-
30.
Latha R, Tamilselvan K, Susiganeshkumar E, Sairaman H. Effect of classical music on heart rate variability between genders. Int J Biomed Res. 2015;6(3):192. https://doi.org/10.7439/ijbr.v6i3.1841.
-
31.
Roque AL, Valenti VE, Guida HL, Campos MF, Knap A, Vanderlei LC, et al. The effects of different styles of musical auditory stimulation on cardiac autonomic regulation in healthy women. Noise Health. 2013;15(65):281-7. [PubMed ID: 23771427]. https://doi.org/10.4103/1463-1741.113527.
-
32.
Chuang CY, Han WR, Li PC, Song MY, Young ST. Effect of long-term music therapy intervention on autonomic function in anthracycline-treated breast cancer patients. Integr Cancer Ther. 2011;10(4):312-6. [PubMed ID: 21382955]. https://doi.org/10.1177/1534735411400311.
-
33.
Yanagihashi R, Ohira M, Kimura T, Fujiwara T. Physiological and psychological assessment of sound. Int J Biometeorol. 1997;40(3):157-61. [PubMed ID: 9195862]. https://doi.org/10.1007/s004840050036.
-
34.
Iwanaga M, Kobayashi A, Kawasaki C. Heart rate variability with repetitive exposure to music. Biol Psychol. 2005;70(1):61-6. [PubMed ID: 16038775]. https://doi.org/10.1016/j.biopsycho.2004.11.015.
-
35.
Iwanaga M, Tsukamoto M. Effects of excitative and sedative music on subjective and physiological relaxation. Percept Mot Skills. 1997;85(1):287-96. [PubMed ID: 9293589]. https://doi.org/10.2466/pms.1997.85.1.287.
-
36.
Jamali SN, Solanky A, Ahmad I, Tayagi NK. Effect of music therapy, aerobic exercise and combined intervention on psychological and physiological parameters in collegiate athletes: A comparative study. Int J Curr Res Med Sci. 2016;3(11):65-75. https://doi.org/10.22192/ijcrms.2016.02.10.007.
-
37.
Dumith SC, Hallal PC, Reis RS, Kohl HW, 3rd. Worldwide prevalence of physical inactivity and its association with human development index in 76 countries. Prev Med. 2011;53(1-2):24-8. [PubMed ID: 21371494]. https://doi.org/10.1016/j.ypmed.2011.02.017.
-
38.
Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health. 2012;12:704. [PubMed ID: 23006411]. [PubMed Central ID: PMC3487794]. https://doi.org/10.1186/1471-2458-12-704.
-
39.
American College of Sports Medicine. ACSM's guidelines for exercise testing and prescription. New York: Lippincott Williams & Wilkins; 2013.
-
40.
Yamashita S, Iwai K, Akimoto T, Sugawara J, Kono I. Effects of music during exercise on RPE, heart rate and the autonomic nervous system. J Sports Med Phys Fitness. 2006;46(3):425-30. [PubMed ID: 16998447].
-
41.
Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59(2):178-93. [PubMed ID: 2874900]. https://doi.org/10.1161/01.res.59.2.178.
-
42.
Facchini M, Malfatto G, Sala L, Silvestri G, Fontana P, Lafortuna C, et al. Changes of autonomic cardiac profile after a 3-week integrated body weight reduction program in severely obese patients. J Endocrinol Invest. 2003;26(2):138-42. [PubMed ID: 12739741]. https://doi.org/10.1007/BF03345142.
-
43.
Maffiuletti NA, Agosti F, Marinone PG, Silvestri G, Lafortuna CL, Sartorio A. Changes in body composition, physical performance and cardiovascular risk factors after a 3-week integrated body weight reduction program and after 1-y follow-up in severely obese men and women. Eur J Clin Nutr. 2005;59(5):685-94. [PubMed ID: 15770221]. https://doi.org/10.1038/sj.ejcn.1602130.
-
44.
Marandi SM, Abadi NG, Esfarjani F, Mojtahedi H, Ghasemi G. Effects of intensity of aerobics on body composition and blood lipid profile in obese/overweight females. Int J Prev Med. 2013;4(Suppl 1):S118-25. [PubMed ID: 23717761]. [PubMed Central ID: PMC3665017].
-
45.
Mohammadzadeh H, Tartibiyan B, Ahmadi A. The effects of music on the perceived exertion rate and performance of trained and untrained individuals during progressive exercise. Facta Univ Ser Phys Educ Sport. 2008;6(1):67-74.
-
46.
Rejeski W. Perceived exertion: An active or passive process? J Sport Psychol. 1985;7(4):371-8. https://doi.org/10.1123/jsp.7.4.371.
-
47.
Yamamoto K, Miyachi M, Saitoh T, Yoshioka A, Onodera S. Effects of endurance training on resting and post-exercise cardiac autonomic control. Med Sci Sports Exerc. 2001;33(9):1496-502. [PubMed ID: 11528338]. https://doi.org/10.1097/00005768-200109000-00012.
-
48.
Chalmers JA, Quintana DS, Abbott MJ, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front Psychiatry. 2014;5:80. [PubMed ID: 25071612]. [PubMed Central ID: PMC4092363]. https://doi.org/10.3389/fpsyt.2014.00080.
-
49.
Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM, Bennett N, et al. Cardiac autonomic function and baroreflex changes following 4 weeks of resistance versus aerobic training in individuals with pre-hypertension. Acta Physiol (Oxf). 2009;195(3):339-48. [PubMed ID: 18774947]. https://doi.org/10.1111/j.1748-1716.2008.01897.x.
-
50.
Okada K, Kurita A, Takase B, Otsuka T, Kodani E, Kusama Y, et al. Effects of music therapy on autonomic nervous system activity, incidence of heart failure events, and plasma cytokine and catecholamine levels in elderly patients with cerebrovascular disease and dementia. Int Heart J. 2009;50(1):95-110. [PubMed ID: 19246850]. https://doi.org/10.1536/ihj.50.95.