Abstract
Objectives:
Alzheimer’s disease is one of the most common causes of dementia with metabolic disorders in the nervous system. Nutrition and physical activity are two main factors in the management of this disease. The aim of the present study was to investigate the effect of endurance training with crocin consumption on IGF-1 and glycogen expression in rat hippocampus tissue of a trimethyltin-treated model of Alzheimer’s disease.Methods:
In this experimental study, 30 male rats were selected and divided into 5 groups of 6 rats including (1) healthy control, (2) Alzheimer’s control, (3) endurance training, (4) crocin and (5) endurance training with crocin. At first, rats in groups 2 - 5 were induced Alzheimer’s disease by intraperitoneal injection of 8 mg/kg of trimethyltin. Then, during 8 weeks, rats in groups 3, 5, ran on treadmill for 3 sessions per week, each session 15 - 30 minutes at speeds of 15 - 20 m/min and groups 4 and 5 received 25 mg/kg of crocin daily. The results were analyzed by Shapiro-Wilk test and one-way ANOVA with Tukey post hoc (P ≤ 0.05).Results:
Alzheimer’s induction with trimethyltin had a significant effect on reduction of IGF-1 gene expression (P = 0.001) and glycogen (P = 0.001); endurance training had a significant effect on increase of IGF-1 (P = 0.001) and glycogen (P = 0.001); crocin consumption had no significant effect on IGF-1 (P = 0.48) and glycogen (P = 0.39); endurance training with crocin consumption had significant effect on increase of IGF-1 (P = 0.001) and glycogen (P = 0.02) as well as endurance training (P = 0.001) and endurance training with crocin consumption rather than crocin consumption had significant effect on increase of IGF-1.Conclusions:
Although endurance training results in a significant increase in IGF-1 and glycogen in the hippocampus tissue of Alzheimer’s rats, nonetheless, the use of crocin in combination with endurance training rather than crocin consumption alone can have a greater effect on increased IGF-1 content of the hippocampus in rats with Alzheimer’s.Keywords
1. Background
Alzheimer’s disease is one of the most common causes of dementia, which is rising dramatically throughout the world and has caused the world community to bear high costs for health care (1, 2). Recent evidence suggest the association between Alzheimer’s disease and metabolic abnormalities (3). Researchers have described oxidative damage as one of the main reasons for the progression of this disease (4). Oxidative damage seems to play a major role in degrading the enzymes of the energy supply routes of the central nervous system, especially the hippocampus. Glycolysis, the tricarboxylic acid cycle (TCA cycle) and ATP synthesis are some of the affected mechanism, which result in decreased synaptic function, sudden death and destruction of parts of the hippocampus (4). Although glucose is the main fuel of the brain, a significant amount of brain glycogen also exists in astrocytes (as cells that are the first line of defense of the brain against toxic compounds). Studies have also shown that glycogen is essential for some of the vital functions of the brain, memory and learning (5). Despite the role of insulin in regulating the metabolism of other tissues in the body, it seems to have a limited role in regulating the transfer of glucose in the brain. Hence, the researchers believe that insulin-like growth factor-1 (IGF-1) with insulin-like function can contribute to central nervous neurons in regulating glucose metabolism (6). Therefore, it seems that a disorder in these metabolic pathways is directly related to cognitive diseases, including Alzheimer’s disease (7). Considering the high financial costs and physical damage of Alzheimer’s disease, it seems necessary to find a way to prevent or treat this disease. Therefore, the desirable role of regular physical activity with moderate and high intensity in the improvement of Alzheimer’s disease has been studied in many studies (8). Researchers believe that exercise can increase the activity of the nervous system and increase the energy requirement of this member. Also researchers believed that the effect of physical activity on brain cells and the hippocampus (as a place for memory and learning) is related to improvement of neuroplasticity, neurogenesis and repair of damaged neurons, which, in turn, require energy (9). On the other hand, performing sports activities with the mechanism of increasing the catabolic enzymes of substrates such as glucose, fatty acids, ATP production, and increased glutamate and creatine levels, may improve the metabolism in hippocampus tissue (9). Controversial results regarding the effect of exercise on central nervous system metabolism have been reported, some suggesting that exercise activity is not effective on metabolic markers (9) and some suggesting the effect of exercise on glycogen (10, 11) and IGF-1 (11). On the other hand, given the limited information on the role of sports activities and the irreparable side effects of synthetic drugs, researchers in the field of sports sciences have recently become interested in using medicinal plants alongside sports activities. Saffron is a well-known herb that has antioxidant, anti-depressant, anti-inflammatory, anti-cancer and also has beneficial effects on the repair of damaged nerve cells in patients with cognitive impairment (12). Crocin is one of the main components of this medicinal plant, as researchers often attribute the properties of saffron to this substance (12). It seems that crocin can help improve cell metabolism through the mechanism of increased insulin sensitivity and increased glucose transport to the cell (13). In this regard, it has been reported that receiving 15 mg/kg and 30 mg/kg of crocin resulted in an improvement in serum glycemic indices and also in the improvement of the number of hippocampus neurons in diabetic rats (14). Also the presence of crocin had a significant effect on reducing glucose levels in the blood and increasing its entry into the cell were due to increased glucose transporters such as GLUT-4 in diabetic rats (15).
It seems that exercise activity and the use of medicinal plants are separately evaluated from a metabolic perspective in various diseases, and most studies have indicated the positive effects of these two factors in some diseases, however, due to the effect of Alzheimer’s disease on hippocampus tissue degradation and the lack of information on the role of exercise in combination with the use of crocin on the metabolism of the central nervous system, especially the hippocampus tissue; the present study aimed to investigate the effect of endurance training with crocin consumption on IGF-1 and glycogen expression in rat hippocampus tissue of a trimethyltin-treated model of Alzheimer’s disease.
2. Objectives
Therefore, the hypothesis of the present study is that endurance training with crocin consumption have interaction effects on the increase of IGF-1 and glycogen expression in rat hippocampus tissue of a trimethyltin-treated model of Alzheimer’s disease.
3. Methods
In this experimental study, 30 male Sprague Dawley rats with an average age of eight weeks and a weighing average of 250 ± 65.4 g were purchased from the Laboratory Animal Breeding and Animal Breeding Center of Islamic Azad University, Marvdasht Branch. After transferring the rats to the sports physiology lab of this unit, they were kept for one week in order to adapt to standard conditions. On day 8, 24 rats were injected with 1 mg/kg trimethyltin intraperitoneally and after 72 hours, learning and memory tests were performed to ensure memory deficits, by which full effect of trimethyltin on the hippocampus was assessed (16). Rats were randomly assigned to 4 groups of 6 rats including (1) Alzheimer’s control, (2) endurance training, (3) endurance training with crocin consumption, and (4) crocin consumption. It should be noted that in order to investigate the effects of trimethyltin injection on the levels of the research variables, 6 remaining were rats arranged in the healthy control group. Then, rats in groups 2 and 3 ran on treadmill for eight weeks, three sessions per week, each session 15 - 30 minutes with speed of 15 - 20 m/min and groups 3 and 4 received 25 mg/kg crocin daily for eight weeks (17). The basis for determining the sample size was, at first, the previous studies in this field, and then the estimates based on the G*Power software. In this software, the number of samples was calculated based on the meanings in each group and the determination of effect size and standard deviation; the minimum calculated number of animals for the current study was six rats in each group.
3.1. Endurance Training
To conduct endurance trainings, initially, before the onset of the research protocol, rats walked on treadmill without slope for 5 - 10 minutes at speeds of 5 - 8 m/min in order to learn how to run on the treadmill. Then, rats ran on treadmill for eight weeks, three sessions per week and 15 - 30 minutes at a speed of 15 - 20 m/min on each session. In fact, the rats in the first two weeks of research ran for 15 minutes in each session at a speed of 15 m/min, after that the intensity and duration of training gradually increased to 30 minutes and speed of 20 m/min in the eighth week. In addition, 5 minutes for warm up and 5 minutes for cool down were added to duration of the main trainings in each training session (18).
3.2. Crocin
Crocin (Sigma Aldrich; Cat-No.: 17024-4G) was dissolved in normal saline and 25 mg/kg injected peritoneally.
3.3. Measuring the Research Variables
Forty-eight hours after the last training session and crocin consumption, all rats were anesthetized by ketamine and xylazine and then heart tissue was extracted to measure the research variables.
For molecular analysis at the gene expression level, first, extraction of RNA from the heart tissue was carried out according to the manufacturer’s protocol (Sinagen, Iran). Then, drawing on the light absorbance property at wavelength of 260 nm, the concentration and degree of purity of the RNA sample was quantitatively obtained using the Equation 1:
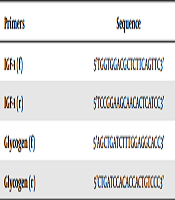
After extracting RNA with high purity and high concentration from all of the samples, cDNA synthesis steps were taken according to the manufacturer’s protocol, and then the synthesized cDNA was used for reverse transcription reaction. Initially, the designed primers for genes were examined, and then IGF-1 and glycogen genes expression was assessed using real time PCR. The sequence of primers used in the study is presented in Table 1.
Primer Sequence Used in the Study
| Primers | Sequence |
|---|---|
| IGF-1 (f) | 5’TGGTGGACGCTCTTCAGTTC3’ |
| IGF-1 (r) | 5’TCCGGAAGCAACACTCATCC3’ |
| Glycogen (f) | 5’AGCTGATCTTTGGAGGCACC3’ |
| Glycogen (r) | 5’CTGATCCACACCACTGTCCC3’ |
3.4. Analysis of Research Findings
The results of the research were analyzed by SPSS software using Shapiro-Wilk test and one-way ANOVA with Tukey post hoc tests (P ≤ 0.05).
4. Results
The results of Shapiro-Wilk test showed that distribution of IGF-1 (P = 0.25) and glycogen (P = 0.80) in research groups were normal. The levels of IGF-1 and glycogen in hippocampus tissue of rats are presented in Table 2. The results of one way ANOVA test showed that there were significant differences between IGF-1 (F = 52.30 and P = 0.001) and glycogen (F = 10.52 and P = 0.001) levels between research groups. The results of Tukey post hoc test showed that the IGF-1 in Alzheimer’s control group was significantly lower than the healthy control (P = 0.001); endurance training (P = 0.001) and endurance training with crocin (P = 0.001) groups; in endurance training group was significantly higher than crocin group (P = 0.001) as well as in endurance training with crocin group was significantly higher than crocin group (P = 0.001); also the glycogen in Alzheimer’s control group was significantly lower than the healthy control (P = 0.001); endurance training (P = 0.004) and endurance training with crocin (P = 0.02) groups (Table 3).
| Group | IGF1 | Glycogen |
|---|---|---|
| Healthy control | 2.61 ± 0.52 | 1.97 ± 0.81 |
| Alzheimer’s control | 0.31 ± 0.22 | 0.06 ± 0.04 |
| Endurance training | 2.21 ± 0.25 | 1.34 ± 0.58 |
| Crocin | 0.65 ± 0.35 | 0.63 ± 0.22 |
| Endurance training with crocin | 2.26 ± 0.32 | 1.09 ± 0.65 |
The Results of Tukey Post Hoc Test Compare the IGF-1 and Glycogen Levels in Research Groups
| Group | Mean Difference | P Value | 95% Confidence Interval | |
|---|---|---|---|---|
| Lower Bound | Upper Bound | |||
| IGF-1 | ||||
| Healthy control | ||||
| Alzheimer’s control | 2.29 | 0.001 | 1.69 | 2.89 |
| Endurance training | 0.39 | 0.32 | -0.20 | 0.99 |
| Endurance training with crocin | 0.35 | 0.44 | -0.25 | 0.95 |
| Crocin | 1.95 | 0.001 | 1.35 | 2.55 |
| Alzheimer’s control | ||||
| Endurance training | -1.89 | 0.001 | -2.49 | -1.29 |
| Endurance training with crocin | -1.94 | 0.001 | -2.54 | -1.34 |
| Crocin | -0.33 | 0.48 | -0.93 | 0.26 |
| Endurance training | ||||
| Endurance training with crocin | -0.04 | 0.99 | -0.64 | 0.55 |
| Crocin | 1.56 | 0.001 | 0.96 | 2.16 |
| Endurance training with Crocin | ||||
| Crocin | 1.60 | 0.001 | 1.01 | 2.20 |
| Glycogen | ||||
| Healthy control | ||||
| Alzheimer’s control | 1.91 | 0.001 | 0.98 | 2.83 |
| Endurance Training | 0.63 | 0.28 | -0.29 | 1.56 |
| Endurance training with crocin | 0.88 | 0.06 | -0.04 | 1.80 |
| Crocin | 1.34 | 0.002 | 0.42 | 2.27 |
| Alzheimer’s control | ||||
| Endurance training | -1.27 | 0.004 | -2.20 | -0.35 |
| Endurance training with crocin | -1.02 | 0.02 | -1.95 | -0.10 |
| Crocin | -0.56 | 0.39 | -1.49 | 0.35 |
| Endurance training | ||||
| Endurance training with crocin | 0.24 | 0.93 | -0.67 | 1.17 |
| Crocin | 0.71 | 0.19 | -0.21 | 1.63 |
| Endurance training with crocin | ||||
| Crocin | 0.46 | 0.59 | -0.46 | 1.38 |
5. Discussion
The results of present study showed that eight weeks of endurance training had a significant effect on the increase of IGF-1 and glycogen in the hippocampus tissue of rats with Alzheimer’s disease. IGF-1 is known as an essential ingredient in the development of the brain; however, the role of this hormone is not fully understood in the aging process. However, researchers suggest reducing levels of IGF-1 as a cause of aging and degenerative disorders. Researchers also believe that the simulation of Alzheimer’s disease in animal models has led to an increase in the accumulation of amyloid plaques, increased oxidative stress and increased inflammation, thereby reducing the activity of the receptor IGF-1 (IGF-1R) at the cell surface. This agent causes a defect in the metabolism of energy substrates, including glycogen in the brain, and particularly in the hippocampus (5, 19). On the other hand, the exercises increased the level of glycogen synthase kinase-3β (GSK-3β) and decreased Tau protein (Tau), which regulates and improves the brain glucose fuel as well as increase the amount of glucose fuel from the pathway that works through decreasing the ratio of inactive form of phosphoinositide 3-kinases (PI3Ks) to active form, activating the pathway of Akt, and increasing the glucose transporter in the hippocampus, prevents Alzheimer’s disease in obese people susceptible to this disease (20). According to the investigations, the researcher could not find a study specifically focused on these two variables in Alzheimer’s rats. However, consistent with the current study, the researchers stated that short-term and long-term aerobic exercises improved and increased the brain glycogen in obese animals that were prone to Alzheimer’s disease (20); endurance swimming trainings increased IGF-1 levels and decreased beta amyloid in the cerebellum tissue of diabetic rats susceptible to Parkinson’s disease (21). Also, eight weeks of exercises resulted in the recovery of glycogen stores and the increase of IGF-1 in the hippocampus of diabetic elderly rats (11). Fourteen days of exercise increased the expression of Akt and IGF-1 in the motor cortex of brain stroke rats (22).
On the other hand, the results showed that eight weeks of crocin consumption had no significant effect on IGF-1 and glycogen changes in the hippocampus tissue of rats with Alzheimer’s disease. Recent studies have shown that saffron and its products can have beneficial effects on the hippocampus, memory and learning in animal samples with degenerative disorders. Since Alzheimer’s disease is known to increase levels of beta-amyloid, it seems that strong antioxidant activity of crocin and crocetin (as saffron ingredients) can increase the flexibility of neurons and reduce the inflammation and death of neurons, and ultimately increase neuronal function (23). There is little information about the effect of saffron and crocin consumption in the central nervous system. However, previous studies have shown that saffron and its products can increase glucose uptake by increasing the expression of AMP-activated protein kinase (AMPK), increasing glucose transporter (GLUT-4) as well as inhibition of gluconeogenesis and phosphoenolpyruvate carboxykinase (PEPCK), which helps to increase the glycogen content. In other words, researchers believe that the effects of glucose uptake are dependent on activation of PI3K/AKT (24). Furthermore, crocin can increase insulin secretion from pancreatic cells and increase insulin sensitivity and increase glucose levels in the cells of the body. Researchers believe that saffron and its products can reduce insulin resistance by reducing inflammatory factors (25). Zhang et al. reported that 25, 50, and 100 mg/kg of crocin significantly increased sirtuin 1 (SIRT1) and peroxisome proliferator-activated receptor-gamma coactivator (PGC)-1α in hippocampus tissue of rats exposed to severe hypoxia and this increased expression was related to the crocin dosage and increased the level of SIRT1 expression (26); Moreover, 12.5, 25 and 50 mg/kg crocin consumption had a significant effect on antioxidant activity and oxidative stress reduction in the cerebellum and cortex of rats exposed to acrylamide poisoning (27). In addition, due to limited information on the mechanism of saffron and its products, especially crocin, most of the previous studies have examined these factors at serum levels and other tissues other than the hippocampus. Therefore, considering the different mechanisms of metabolism of the brain and hippocampus other studies seem to be necessary in this regard.
The results of the present study showed that endurance training with crocin consumption has significant effect on increasing the IGF-1 and glycogen in the hippocampus of rats with Alzheimer’s disease. It seems that endurance training and crocin consumption in a similar pathway, (such as increasing the expression of AMPK, GLUT-4, as well as inhibition of gluconeogenesis, PEPCK, and activation of the PI3K/AKT pathway) increase the absorption of glucose in the cell and also increase the glycogen synthase protein; which helps to increase the storage and rebuilding of glycogen (19, 20, 24) On the other hand, it has been reported that insulin resistance and similar illnesses have reduced the ability of brain for insulin absorption and the amount of insulin in the brain; and it is believed that insulin resistance is due to a change in the cascade insulin receptors and impairment of the message transmission path. Insulin in the brain has no role in the transfer of glucose, but it has an effect on the survival of the neuron and its function (28). Therefore, insulin and IGF-1 are administered by the mediator of the insulin receptor in the brain. Therefore, in present study endurance training with the activation pathway of Akt has been shown to increase the expression of IGF-1 in the hippocampus (22). Also, the dose of crocin seems to be an effective factor in changes of variables. Therefore, considering that the different dose of crocin with endurance training was one of the limitations of this study, it is recommended that future studies review the effects of different doses of crocin on IGF-1 and glycogen in hippocampus tissue. Regarding the effect of PI3K, Akt, AMPK and PI3K proteins on glycogen and IGF-1 levels in hippocampus tissue of rats, failure to measure these factors is also one of the limitations of present study; therefore, measurement of these factors is recommended in future studies. Considering the fact that in previous studies, the effects of exercises and crocin consumption on IGF-1 and glycogen were investigated separately, the strengths of this study can be reviewed and compared to the effect of endurance training and crocin consumption on IGF-1 and glycogen; Additionally, the weaknesses of the present study may be the lack of measurement of IGF-1 and glycogen by using different methods such as ELISA and Western blot.
5.1. Conclusions
Although endurance training leads to a significant increase in IGF-1 and glycogen in the hippocampus tissue of rats with Alzheimer’s disease, however, the consumption of crocin along with endurance exercises rather than crocin consumption alone can have a greater effect on the increase of IGF-1 in the hippocampus tissue of the rats with Alzheimer’s disease.
Acknowledgements
References
-
1.
Perneczky R, Kempermann G, Korczyn AD, Matthews FE, Ikram MA, Scarmeas N, et al. Translational research on reserve against neurodegenerative disease: Consensus report of the International Conference on Cognitive Reserve in the Dementias and the Alzheimer's Association Reserve, Resilience and Protective Factors Professional Interest Area working groups. BMC Med. 2019;17(1):47. [PubMed ID: 30808345]. [PubMed Central ID: PMC6391801]. https://doi.org/10.1186/s12916-019-1283-z.
-
2.
Theurey P, Connolly NMC, Fortunati I, Basso E, Lauwen S, Ferrante C, et al. Systems biology identifies preserved integrity but impaired metabolism of mitochondria due to a glycolytic defect in Alzheimer's disease neurons. Aging Cell. 2019;18(3). e12924. [PubMed ID: 30793475]. [PubMed Central ID: PMC6516149]. https://doi.org/10.1111/acel.12924.
-
3.
de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer's disease. Biochem Pharmacol. 2014;88(4):548-59. [PubMed ID: 24380887]. [PubMed Central ID: PMC4550323]. https://doi.org/10.1016/j.bcp.2013.12.012.
-
4.
Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20(3):148-60. [PubMed ID: 30737462]. https://doi.org/10.1038/s41583-019-0132-6.
-
5.
Bak LK, Walls AB, Schousboe A, Waagepetersen HS. Astrocytic glycogen metabolism in the healthy and diseased brain. J Biol Chem. 2018;293(19):7108-16. [PubMed ID: 29572349]. [PubMed Central ID: PMC5950001]. https://doi.org/10.1074/jbc.R117.803239.
-
6.
Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A. 2000;97(18):10236-41. [PubMed ID: 10954733]. [PubMed Central ID: PMC27834]. https://doi.org/10.1073/pnas.170008497.
-
7.
Muhic M, Vardjan N, Chowdhury HH, Zorec R, Kreft M. Insulin and insulin-like growth factor 1 (IGF-1) modulate cytoplasmic glucose and glycogen levels but not glucose transport across the membrane in astrocytes. J Biol Chem. 2015;290(17):11167-76. [PubMed ID: 25792745]. [PubMed Central ID: PMC4409273]. https://doi.org/10.1074/jbc.M114.629063.
-
8.
Chen WW, Zhang X, Huang WJ. Role of physical exercise in Alzheimer's disease. Biomed Rep. 2016;4(4):403-7. [PubMed ID: 27073621]. [PubMed Central ID: PMC4812200]. https://doi.org/10.3892/br.2016.607.
-
9.
Matura S, Fleckenstein J, Deichmann R, Engeroff T, Fuzeki E, Hattingen E, et al. Effects of aerobic exercise on brain metabolism and grey matter volume in older adults: Results of the randomised controlled SMART trial. Transl Psychiatry. 2017;7(7). e1172. [PubMed ID: 28934191]. [PubMed Central ID: PMC5538117]. https://doi.org/10.1038/tp.2017.135.
-
10.
Ghanbari-Niaki A, Saeidi A, Tartibian B, Qujeq D, Naghizadeh Qomi M. The response of brain kisspeptin and glycogen at different times to acute aerobic exercise with and without glucose solution consumption in male rats. J Clin Res Paramed Sci. 2018;7(2). e85193. https://doi.org/10.5812/jcrps.85193.
-
11.
Gomes RJ, de Oliveira CA, Ribeiro C, Mota CS, Moura LP, Tognoli LM, et al. Effects of exercise training on hippocampus concentrations of insulin and IGF-1 in diabetic rats. Hippocampus. 2009;19(10):981-7. [PubMed ID: 19437499]. https://doi.org/10.1002/hipo.20636.
-
12.
Azari H, Ebrahimi S, Saeb S, Ghanbari A, Peyravian F, Mokarram P. The effect of saffron aquatic extract and crocin on the differentiation of neural stem cells into oligodendrocyte precursor cells. Shiraz E-Med J. 2018;19(3). e60190. https://doi.org/10.5812/semj.60190.
-
13.
Samadi H, Javadi S, Asri S. [Evaluation of the effects of crocin on the serum levels of glucose, insulin, urea, creatinine and β2m in healthy and streptozotocin-induced diabetic rats]. J Urmia Uni Med Sci. 2015;26(9):802-12. Persian.
-
14.
Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16(1):91-100. [PubMed ID: 23638297]. [PubMed Central ID: PMC3637909].
-
15.
Shirali S, Zahra Bathaie S, Nakhjavani M. Effect of crocin on the insulin resistance and lipid profile of streptozotocin-induced diabetic rats. Phytother Res. 2013;27(7):1042-7. [PubMed ID: 22948795]. https://doi.org/10.1002/ptr.4836.
-
16.
Bazyar Y, Rafiei S, Hosseini A, Edalatmanesh MA. [Effect of endurance exercise training and gallic acid on tumor necrosis factor-α in an animal model of Alzheimer’s disease]. Neurosci J Shefaye Khatam. 2015;3(3):21-6. Persian. https://doi.org/10.18869/acadpub.shefa.3.3.21.
-
17.
Salahshoor MR, Khashiadeh M, Roshankhah S, Kakabaraei S, Jalili C. Protective effect of crocin on liver toxicity induced by morphine. Res Pharm Sci. 2016;11(2):120-9. [PubMed ID: 27168751]. [PubMed Central ID: PMC4852656].
-
18.
Baziyar Y, Edalatmanesh MA, Hosseini SA, Zar A. The effects of endurance training and gallic acid on BDNF and TNF-a in male rats with Alzheimer. Int J Appl Exerc Physiol. 2016;5(4):45-54.
-
19.
Wrigley S, Arafa D, Tropea D. Insulin-like growth factor 1: At the crossroads of brain development and aging. Front Cell Neurosci. 2017;11:14. [PubMed ID: 28203146]. [PubMed Central ID: PMC5285390]. https://doi.org/10.3389/fncel.2017.00014.
-
20.
Kim DY, Jung SY, Kim TW, Lee KS, Kim K. Treadmill exercise decreases incidence of Alzheimer's disease by suppressing glycogen synthase kinase-3beta expression in streptozotocin-induced diabetic rats. J Exerc Rehabil. 2015;11(2):87-94. [PubMed ID: 25960981]. [PubMed Central ID: PMC4415755]. https://doi.org/10.12965/jer.150198.
-
21.
Borges ME, Ribeiro AM, Pauli JR, Arantes LM, Luciano E, de Moura LP, et al. Cerebellar insulin/IGF-1 signaling in diabetic rats: Effects of exercise training. Neurosci Lett. 2017;639:157-61. [PubMed ID: 28034783]. https://doi.org/10.1016/j.neulet.2016.12.059.
-
22.
Chang HC, Yang YR, Wang PS, Kuo CH, Wang RY. Insulin-like growth factor I signaling for brain recovery and exercise ability in brain ischemic rats. Med Sci Sports Exerc. 2011;43(12):2274-80. [PubMed ID: 21606872]. https://doi.org/10.1249/MSS.0b013e318223b5d9.
-
23.
Al-Snafi AE. The pharmacology of Crocus sativus-A review. IOSR J Pharm. 2016;6(6):8-38.
-
24.
Dehghan F, Hajiaghaalipour F, Yusof A, Muniandy S, Hosseini SA, Heydari S, et al. Saffron with resistance exercise improves diabetic parameters through the GLUT4/AMPK pathway in-vitro and in-vivo. Sci Rep. 2016;6:25139. [PubMed ID: 27122001]. [PubMed Central ID: PMC4848502]. https://doi.org/10.1038/srep25139.
-
25.
Asishirazi I, Hosseini S, Keikhosravi F. [Hypoglycemic interactional effects of saffron (Crocus sativus) aqueous extract and swimming training in streptozotocin induced diabetic rats]. J Sabzevar Univ Med Sci. 2017;24(4):273-9. Persian.
-
26.
Zhang XY, Zhang XJ, Xv J, Jia W, Pu XY, Wang HY, et al. Crocin attenuates acute hypobaric hypoxia-induced cognitive deficits of rats. Eur J Pharmacol. 2018;818:300-5. [PubMed ID: 29106903]. https://doi.org/10.1016/j.ejphar.2017.10.042.
-
27.
Mehri S, Abnous K, Khooei A, Mousavi SH, Shariaty VM, Hosseinzadeh H. Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci. 2015;18(9):902-8. [PubMed ID: 26523222]. [PubMed Central ID: PMC4620190].
-
28.
Parvaneh Tafreshi A, Jalal R, Darvishalipour S, Sepehri H, Adeli K. [Level of the brain IGF-I protein expression in the insulin resistant animal model]. Int J Endocrinol Metab. 2010;12(1):71-6. Persian.