Abstract
Background:
Acute kidney injury (AKI) due to antibiotic nephrotoxicity is a complication that can be avoided or managed properly if diagnosed early.Objectives:
We aimed to determine the incidence and risk factors of AKI and to assess the possible effects of nephrotoxic antibiotic therapy on its development in a large group of patients with infective endocarditis (IE).Methods:
Patients with definite or possible IE diagnosed based on the Duke criteria were included in this retrospective cohort study at a tertiary referral center from 2007 to 2017. Data were derived from the single-center Iranian Registry of Infective Endocarditis (IRIE). Baseline risk factors for AKI were assessed via repeated serum creatinine measurements. Patients (n = 22) with end-stage renal failure undergoing dialysis were excluded. AKI was defined and staged in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) classification.Results:
Totally, 498 patients at a mean age of 45 ± 16 years were studied. The baseline creatinine level was 1.26 ± 0.72 mg/dL. AKI occurred in 126 (26.3%) patients 1 week after the initiation of antibiotic therapy. There was a significant relationship between AKI and the use of gentamicin (P = 0.01) and gentamicin and vancomycin concomitantly (P = 0.01). At the end of the treatment, after dose adjustment and additional treatments, the incidence of AKI decreased to 22.7%, whereas this improvement was less remarkable in the patients with prior renal failure. Some independent variables, including age (P = 0.04), diabetes (P < 0.0001), prior renal failure (creatinine > 2 mg/dL), anemia (P = 0.003), left-sided IE (P = 0.04), and positive blood cultures with Staphylococcus aureus (P = 0.04) had a statistically significant association with AKI.Conclusions:
Close monitoring of the renal function is essential in IE patients receiving treatment with nephrotoxic antibiotics, especially patients with advanced age, diabetes, chronic renal failure, anemia, and left-sided IE.Keywords
Infective Endocarditis Acute Kidney Injury Antibiotic Therapy Nephrotoxic Antibiotics Gentamycin Vancomycin
1. Background
Despite significant improvements in the surgical and medical management of infective endocarditis (IE), it continues to create high mortality and morbidity rates, especially when complicated by acute kidney injury (AKI).
Acute renal failure in IE is a growing complication and occurs in approximately 6% - 30% of cases; moreover, albeit often reversible, it is associated with poor prognosis (1, 2). The causes of acute renal failure in IE are multifactorial, ranging from bacterial infection-related, immune complex-mediated glomerulonephritis and renal infarction from septic emboli to renal cortical necrosis. In addition, drug-induced acute interstitial nephritis is notably related to aminoglycosides, vancomycin (synergistic toxicity with aminoglycosides), and even high-dose penicillin. With aminoglycosides administered for acute tubular necrosis, AKI can develop as a result of the treatment of the infection and the nephrotoxicity of contrast agents used for imaging purposes (1, 3, 4). Approximately one-third of the patients in a previous study developed AKI (of any cause), with the complication observed most often among older patients and those infected with Staphylococcus aureus (1).
Drug-induced interstitial nephritis -usually with penicillin, cephalosporin, or quinolone- shares many of the clinical findings of acute glomerulonephritis: hematuria, mild proteinuria, and renal insufficiency. Pyuria and white cell casts can be seen in both disorders; nonetheless, they typically constitute the major finding in acute interstitial nephritis. These disorders can usually be distinguished from each other by the timing of the renal manifestations. Glomerular involvement is typically near or at its peak of severity just before the initiation of appropriate antimicrobial therapy (3, 5, 6). By contrast, acute interstitial nephritis is a later event, generally requiring 10 or more days of drug treatment (3, 7). Aminoglycoside-induced acute tubular necrosis also occurs late (after at least 5-7 days of therapy) and is associated with different urinary findings from either glomerulonephritis or interstitial nephritis (8-10).
Monitoring of the renal function through repeated serum creatinine measurements is essential for the early detection of renal dysfunction. Gentamicin and vancomycin are excreted almost entirely by glomerular filtration and might exhibit nephrotoxic adverse effects. It is expected that the prevalence of this clinical problem will increase (11, 12). For the mitigation of this complication, antibiotic doses should be adjusted for creatinine clearance with careful monitoring of serum levels (aminoglycosides and vancomycin). Imaging with nephrotoxic contrast agents and high-dose diuretics should be avoided when possible in patients with hemodynamic impairment or previous renal insufficiency (9, 11).
2. Objectives
In the current study, we sought to determine the incidence of AKI to investigate the possible effects of nephrotoxic antibiotic treatment on its development, and to assess the role of optimizing management in its reduction in a large group of Iranian patients with IE who derived from the single-center Iranian Registry of Infective Endocarditis (IRIE).
3. Methods
The current infective endocarditis registry retrospectively collected all cases admitted to the Iranian Registry of Infective Endocarditis (IRIE) in Rajaie Cardiovascular, Medical, and Research Center (a tertiary hospital in Tehran, Iran) from April 2007 to June 2017. The recruited subjects were 498 consecutive patients with definite or possible IE based on the Duke criteria. Data were collected regarding demographic and clinical characteristics, medical history, IE signs and symptoms, echocardiographic and surgical information, microbiological findings, antibiotic regimens, complications, and follow-up outcomes.
The renal function was evaluated via repeated serum creatinine measurements and was recorded before and weekly during treatment. AKI was defined and staged in accordance with the Kidney Disease: Improving Global Outcomes (KDIGO) score. The KDIGO criteria for urine output and the glomerular filtration rate were not utilized for our study definitions due to the infeasibility of accurate retrospective data collection. The baseline creatinine level was defined as a plasma creatinine level > 2 mg/dL.
The KDIGO definition and staging system is the most recent and preferred definition. AKI is staged as follows:
• Stage 1- Increase in serum creatinine by ≥ 0.3 mg/dL or 50% - 99%;
• Stage 2- Increase in serum creatinine to 2.0 - 2.9 times the baseline or increase in serum creatinine > 100% - 199%; and
• Stage 3- Increase in serum creatinine to 3.0 times the baseline, or an increase in serum creatinine ≥ 4.0 mg/dL, or an increase in serum creatinine > 200% (13-15).
All the patients received antibiotic and surgical treatments following the recent international guidelines on IE. The choice of antibiotics was based on the results of blood culture samples obtained for high-level disk susceptibility tests. The patients were assessed for the development of AKI from their baseline creatinine at any point during their inpatient bacterial endocarditis treatment course.
The study protocol was approved by the Ethics Committee of our center, and written informed consent was obtained from all the patients.
3.1. Statistical Analysis
The statistical analyses were performed with SPSS version 15 for Windows (SPSS Inc, Chicago, Illinois). The mean ± the standard deviation and frequencies were used for the descriptive analysis. For the evaluation of data distribution, the one-sample Kolmogorov-Smirnov test was utilized. Group comparisons were done using the student t-test or the Wilcoxon test for the numeric variables and the χ2 or Fisher exact test for the categorical variables.
4. Results
4.1. Patients’ Demographic and Clinical Characteristics
The present infective endocarditis registry recruited 498 patients with the mean age ± standard deviation 45 ± 16 years. Women comprised 67.8% (n = 337) of the study population. The mean length of hospital stay was 40.9 ± 14.5 days (range = 9 - 78 days). The baseline blood urea nitrogen level was 21.77 ± 17.67 mg/dL, and the baseline creatinine level was 1.26 ± 0.72 mg/dL. Surgical intervention was performed on 295 (59.3%) patients due to IE during their initial hospitalization. Eighty-one (14.8%) patients were intravenous drug abusers.
4.2. AKI
One week after antibiotic therapy initiation, AKI occurred in 126 (26.3%) patients. In this period, 269 (54.1%) cases exhibited no rise in their creatinine level, whereas 102 (20.5%) subjects had an increase < 50% in creatinine compared with the baseline (Figure 1).
Changes in creatinine (Cr) levels and the incidence of acute kidney injury (AKI) during antibiotic (AB) therapy

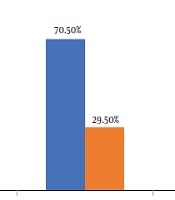
AKI was observed in 147 (29.5%) patients 2 weeks after the initiation of antibiotic therapy. Within 2 weeks, while 258 (51.9%) patients had no elevation in creatinine, 92 (18.5%) cases had an increase < 50% in their creatinine level compared with the baseline (Figure 1).
Three weeks after antibiotic therapy, 113 (22.7%) subjects had elevated creatinine levels, whereas 248 (49.9%) patients had no increase in creatinine and 136 (27.4%) cases had an increase < 50% in creatinine by comparison with the baseline (Figure 1). The staging of AKI based on the KDIGO is depicted in Table 1.
Staging Criteria for AKI Based on the KDIGO
| Staging Criteria for Creatinine | No. (%) |
|---|---|
| One week after antibiotic therapy (N = 126) | |
| Stage I | 92 (73) |
| Stage II | 22 (17.5) |
| Stage III | 12 (9.5) |
| Two weeks after antibiotic therapy (N = 147) | |
| Stage I | 101 (68.7) |
| Stage II | 27 (18.4) |
| Stage III | 19 (12.9) |
| Three weeks after antibiotic therapy (N = 113) | |
| Stage I | 58 (51.3) |
| Stage II | 35 (31) |
| Stage III | 20 (17.7) |
The patients with AKI were older (P = 0.04) and had a higher prevalence of diabetes (P < 0.0001), anemia (P = 0.003), and left-sided IE (P = 0.04) (Table 2).
| Clinical Characteristic | AKI | No AKI | P Value |
|---|---|---|---|
| Age, y | 47.53 ± 17.04 | 44.25 ± 15.87 | 0.04 |
| Female sex | 149 (65.6) | 188 (69.6) | 0.34 |
| Hypotension: systolic blood pressure < 90 mmHg | 54 (23.7) | 41 (15.8) | 0.63 |
| Anemia: hemoglobin < 10 g/dL | 114 (50.2) | 58 (21.4) | 0.003 |
| Diabetes | 110 (48.4) | 61 (22.5) | < 0.0001 |
| Ejection fraction | 47 ± 10.49 | 48.3 ± 9.5 | 0.21 |
| Prior renal failure (creatinine > 2 mg/dL) | 40 (17.6) | 12 (5.9) | 0.01 |
| Left-sided IE | 80 (63.5) | 141 (35.7) | 0.04 |
| Right-sided IE | 31 (24.6) | 86 (23.2) | 0.74 |
Aortic valve IE (34 patients) had a significant relationship with the severe stages of AKI (stage II and stage III) (P = 0.05).
Finally, 87 (17.5%) patients were discharged with a creatinine level > 2 mg/dL. Of these patients, 33 cases had renal failure in their history (creatinine > 2 mg/dL).
4.3. Blood Culture and AKI
Positive blood cultures were reported in 214 (43.05%) patients. Staphylococcus aureus was the most commonly isolated primary pathogen (25.7%) in the cases with positive cultures. Enterococcus and Streptococcus viridans were identified in 17.3% and 15.9% of the patients, respectively (Table 3).
. Frequency of Primary Pathogens in the Patients with Positive Cultures (N = 214)
| Primary Pathogen Isolated | No. (%) |
|---|---|
| Staphylococcus aureus | 57 (25.9) |
| Enterococcus | 39 (17.7) |
| Streptococcus viridians | 34 (15.5) |
| Coagulase-negative Staphylococci | 27 (12.3) |
| Pseudomonas | 14 (6.4) |
| Fungal infection (candida, aspergillus) | 9 (4.1) |
| Staph saprophyticus | 8 (3.6) |
| Klebsiella | 6 (2.7) |
| Streptococcus sp. | 4 (1.8) |
| Streptococcus alpha haemolyticus | 4 (1.8) |
| Brucella | 3 (1.4) |
| E. coli | 3 (1.4) |
| str_B | 2 (0.9) |
| aci_A | 2 (0.9) |
| Streptococcus pneumonia | 2 (0.9) |
| Serratia marcescens | 2 (0.9) |
| HACEK group | 2 (0.9) |
| Non-hemolytic streptococcus | 1 (0.5) |
There was a significant relationship between AKI and blood culture (P = 0.04). Positive blood cultures with S. aureus were significantly more frequent among the subjects with AKI (Table 4).
Association Between AKI and Blood Culture After Antibiotic Therapy Initiation
| Type of Microorganism | AKI | No AKI | P Value |
|---|---|---|---|
| Negative blood culture | 66 (52.3) | 211 (58.2) | 0.04 |
| Streptococcus viridans | 14 (11.1) | 36 (10.5) | |
| Staphylococcus aureus | 14 (11.1) | 18 (5.2) | |
| Coagulase-negative Staphylococci | 6 (4.7) | 17 (4.9) | |
| Enterococci | 9 (7.1) | 20 (5.8) | |
| Pseudomonas | 3 (2.3) | 9 (2.6) | |
| Other microorganism | 14 (11.1) | 30 (8.7) |
4.4. Antibiotic Therapy and AKI
As prior therapy, gentamicin was administered to 227 (48.6%) and vancomycin to 361 (72.6%) patients. A total of 157 (31.5%) cases received gentamicin and vancomycin concomitantly. Rifampin was administered empirically to 128 (25.7%) subjects.
There was a significant relationship between AKI at 1 week after antibiotic therapy initiation and gentamicin usage. The cases that received gentamicin had severe renal failure (stage II or III of AKI) (P = 0.01). It was also seen in the administration of gentamicin and vancomycin concomitantly (P = 0.01). There was no significant relationship between AKI and the administration of rifampin or vancomycin alone at 1 week after antibiotic therapy (P > 0.05) (Table 5).
Relationship Between the Staging of AKI and the Most Common Antibiotic Used in the First, Second, and Third Weeks After Antibiotic Therapy Initiation
| Stage I | Stage II | Stage III | P Value | |
|---|---|---|---|---|
| One week after antibiotic therapy | ||||
| Rifampin | 25 (27.5) | 7 (31.8) | 4 (36.4) | 0.48 |
| Gentamycin | 40 (44.0) | 14 (66.7) | 8 (72.7) | 0.01 |
| Vancomycin | 60 (65.2) | 16 (72.7) | 10 (90.9) | 0.11 |
| Gentamycin + vancomycin | 27 (37.0) | 10 (50) | 8 (80) | 0.01 |
| Two weeks after antibiotic therapy | ||||
| Rifampin | 25 (25.3) | 12 (44.4) | 6 (33.3) | 0.10 |
| Gentamycin | 45 (45.9) | 14 (51.9) | 14 (77.8) | 0.03 |
| Vancomycin | 63 (63.6) | 19 (70.4) | 17 (94.4) | 0.02 |
| Gentamycin + vancomycin | 30 (38.5) | 11 (50) | 14 (82.4) | 0.003 |
| Three weeks after antibiotic therapy | ||||
| Rifampin | 13 (22.4) | 12 (35.3) | 5 (25) | 0.44 |
| Gentamycin | 28 (49.1) | 18 (54.5) | 12 (60) | 0.38 |
| Vancomycin | 38 (65.5) | 21 (61.8) | 14 (70) | 0.90 |
| Gentamycin + vancomycin | 20 (43.5) | 13 (50) | 9 (52.9) | 0.45 |
Two weeks after antibiotic therapy, there were more severe stages of AKI (stage III) significantly in the patients who received gentamicin or vancomycin alone and gentamicin and vancomycin concomitantly (P = 0.03, P = 0.02, and P = 0.003, respectively) (Table 5).
Three weeks after antibiotic therapy initiation, no significant association was found between any antibiotics and AKI (P > 0.05) (Table 5).
Upon multivariate analysis, after adjustments were made for 8 independent variables prior to renal failure (OR: 1.39, 95% CI: 1.25 to 1.590), the use of gentamicin (OR: 1.014, 95% CI: 1.001 to 1.028), and left-sided IE (OR: 1.32, 95% CI: 1.208 to 1.507) were associated significantly with the development of AKI (Table 6).
Association Between the Adjusted Variables and AKI by Logistic Regression
| B | Odds Ratio | 95% CI | P Value | |
|---|---|---|---|---|
| Age | 0.042 | 1.001 | 0.277 - 1.201 | 0.64 |
| Diabetes mellitus | 0.377 | 1.014 | 0.98 - 1.028 | 0.32 |
| Anemia | 0.561 | 1.403 | 0.645 - 1.850 | 0.33 |
| Prior renal failure (creatinine > 2 mg/dL) | 0.942 | 1.390 | 1.25 - 1.590 | < 0.001 |
| Gentamycin | 0.014 | 1.014 | 1.001 - 1.028 | 0.03 |
| Vancomycin | 0.055 | 1.057 | 0.679 - 1.646 | 0.80 |
| Staphylococcus aureus | 0.371 | 1.448 | 0.747 - 2.810 | 0.27 |
| Left-sided IE | 1.126 | 1.32 | 1.208 - 1.507 | 0.001 |
Eighty-seven (17.5%) cases expired during hospitalization. There was a significant relationship between death and AKI in that death was more prevalent among the patients with AKI than among those without AKI (48 [21.1%] vs. 39 [14.4%]; P = 0.05).
5. Discussion
The results of our study are derived from the Iranian Registry of Infective Endocarditis, which is the largest case-series study of patients with IE in Middle Eastern countries.
The causes of AKI can usually be distinguished from each other on the basis of the timing of the renal manifestations. Glomerular involvement is typically near or at its peak of severity just before the initiation of appropriate antimicrobial therapy (3, 5, 6). By contrast, AKI induced by antibiotic therapy is a later event (3, 7).
In the current study, we found that the incidence of AKI was 26.3% one week after the initiation of antibiotic therapy. Treatment with aminoglycosides for over 48 hours, not least when vancomycin is added to it, increases the risk of AKI significantly. In our investigation, this increase was more observed during the first week in the patients who had positive S. aureus blood cultures. Nonetheless, we significantly reduced AKI in these patients (particularly stage I) by adjusting the antibiotic dose and carefully monitoring the serum level of nephrotoxic antibiotics and hydration according to our nephrology consult at the end of the fourth week. Indeed, monitoring of the renal function by repeated serum creatinine measurements is essential for the early detection of renal dysfunction.
Most of the previous studies have used the absolute values of serum creatinine for AKI diagnosis. In our study, we drew upon the KDIGO classification, which is the most recent and preferred staging system. Our finding regarding the incidence of AKI is consistent with previous studies, including those conducted by Conlon et al. (33%) (16), Fernandez-Hidalgo et al. (33% - 46%) (17), Korem et al. (31.4%) (18), and Goenaga Sanchez et al. (38.7%) (19), as well as the estimated figure (30%) by the European guidelines (1).
The most commonly used antibiotics in our center for patients with IE as a part of the combination therapy regimen are vancomycin and gentamycin. The occurrence of renal failure in our study was associated with such independent risk factors as advanced age, a history of diabetes mellitus, chronic renal failure (creatinine > 2 mg/dL), anemia (hemoglobin < 10 g/dL), and positive S. aureus blood cultures. This is consistent with previous studies (18, 20).
Additionally, the involvement of the aortic valve was independently associated with severe AKI (stage > 2).
In the current study, having adjusted the variables in the multivariate analysis, we found that prior renal failure and gentamicin use were independently associated with the occurrence of AKI in our patients with IE. Nonetheless, there was no significant improvement in serum creatinine levels during hospital admission in our patients with chronic renal failure.
The limitation of our retrospective study was the paucity of some important data, the most notable of which were the KDIGO criteria for urine output and the glomerular filtration rate; Having employed these criteria, we could have identified more patients with AKI. Additionally, the infeasibility of accurate retrospective data collection precluded access to information regarding the duration of concomitant nephrotoxic exposure (e.g., loop diuretics and NSAID therapy). It is worthy to note, however, that since April 2015, the Iranian Registry of Infective Endocarditis has included all necessary data on IE patients.
Some of the risk factors for AKI identified in our study are modifiable; these risk factors include antibiotic choice, dosing regimens, and the duration of therapy, as well as concomitant nephrotoxic exposure, especially for prolonged lengths of the treatment. Patient outcomes may be optimized through the consideration of these risk factors in patients treated for IE.
5.1. Conclusions
Acute renal failure associated with bacterial endocarditis remains a frequent clinical problem that is often associated with a fatal outcome. In the present study, the incidence rate of AKI was 26.3% and had a significant association with gentamicin use, especially when used concomitantly with vancomycin. Some independent risk factors such as advanced age, a history of diabetes mellitus, prior chronic renal failure (creatinine > 2 mg/dL), anemia (hemoglobin < 10 g/dL), positive Staphylococcus aureus blood cultures, and left-sided IE were associated with AKI. Further, aortic valve IE had a significant relationship with higher stages of AKI. Therefore, the renal function and trough levels of nephrotoxic antibiotics should be closely monitored in patients treated for IE, specifically those with other renal injury risk factors.
References
-
1.
Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-128. [PubMed ID: 26320109]. https://doi.org/10.1093/eurheartj/ehv319.
-
2.
Legrand M, Pirracchio R, Rosa A, Petersen ML, Van der Laan M, Fabiani JN, et al. Incidence, risk factors and prediction of post-operative acute kidney injury following cardiac surgery for active infective endocarditis: An observational study. Crit Care. 2013;17(5):R220. [PubMed ID: 24093498]. [PubMed Central ID: PMC4056899]. https://doi.org/10.1186/cc13041.
-
3.
Radhakrishnan J, Fervenza FC. Kidney disease in the setting of infective endocarditis or an infected ventriculoatrial shunt. UpToDate; 2013. Available from: https://www.uptodate.com/contents/kidney-disease-in-the-setting-of-infective-endocarditis-or-an-infected-ventriculoatrial-shunt.
-
4.
Majumdar A, Chowdhary S, Ferreira MA, Hammond LA, Howie AJ, Lipkin GW, et al. Renal pathological findings in infective endocarditis. Nephrol Dial Transplant. 2000;15(11):1782-7. [PubMed ID: 11071965]. https://doi.org/10.1093/ndt/15.11.1782.
-
5.
Hammond DA, Smith MN, Painter JT, Meena NK, Lusardi K. Comparative incidence of acute kidney injury in critically Ill patients receiving vancomycin with concomitant piperacillin-tazobactam or cefepime: A retrospective cohort study. Pharmacotherapy. 2016;36(5):463-71. [PubMed ID: 26952639]. https://doi.org/10.1002/phar.1738.
-
6.
Randjelovic P, Veljkovic S, Stojiljkovic N, Sokolovic D, Ilic I. Gentamicin nephrotoxicity in animals: Current knowledge and future perspectives. EXCLI J. 2017;16:388-99. [PubMed ID: 28507482]. [PubMed Central ID: PMC5427480]. https://doi.org/10.17179/excli2017-165.
-
7.
Rybak MJ, Albrecht LM, Boike SC, Chandrasekar PH. Nephrotoxicity of vancomycin, alone and with an aminoglycoside. J Antimicrob Chemother. 1990;25(4):679-87. [PubMed ID: 2351627]. https://doi.org/10.1093/jac/25.4.679.
-
8.
Cosgrove SE, Vigliani GA, Fowler VJ, Abrutyn E, Corey GR, Levine DP, et al. Initial low-dose gentamicin for Staphylococcus aureus bacteremia and endocarditis is nephrotoxic. Clin Infect Dis. 2009;48(6):713-21. [PubMed ID: 19207079]. https://doi.org/10.1086/597031.
-
9.
Picard W, Bazin F, Clouzeau B, Bui HN, Soulat M, Guilhon E, et al. Propensity-based study of aminoglycoside nephrotoxicity in patients with severe sepsis or septic shock. Antimicrob Agents Chemother. 2014;58(12):7468-74. [PubMed ID: 25288085]. [PubMed Central ID: PMC4249539]. https://doi.org/10.1128/AAC.03750-14.
-
10.
Balakumar P, Rohilla A, Thangathirupathi A. Gentamicin-induced nephrotoxicity: Do we have a promising therapeutic approach to blunt it? Pharmacol Res. 2010;62(3):179-86. [PubMed ID: 20434560]. https://doi.org/10.1016/j.phrs.2010.04.004.
-
11.
Jegier B, Jaszewski R, Lelonek M. Acute kidney failure after vancomycin therapy in a patient with infective endocarditis treated surgically. Clin Exp Med Lett. 2011;52(1-2):71-4.
-
12.
Lopez-Novoa JM, Quiros Y, Vicente L, Morales AI, Lopez-Hernandez FJ. New insights into the mechanism of aminoglycoside nephrotoxicity: An integrative point of view. Kidney Int. 2011;79(1):33-45. [PubMed ID: 20861826]. https://doi.org/10.1038/ki.2010.337.
-
13.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-84. [PubMed ID: 22890468]. https://doi.org/10.1159/000339789.
-
14.
Eckardt KU, Berns JS, Rocco MV, Kasiske BL. Definition and classification of CKD: The debate should be about patient prognosis--a position statement from KDOQI and KDIGO. Am J Kidney Dis. 2009;53(6):915-20. [PubMed ID: 19406541]. https://doi.org/10.1053/j.ajkd.2009.04.001.
-
15.
James M, Bouchard J, Ho J, Klarenbach S, LaFrance JP, Rigatto C, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO clinical practice guideline for acute kidney injury. Am J Kidney Dis. 2013;61(5):673-85. [PubMed ID: 23518195]. https://doi.org/10.1053/j.ajkd.2013.02.350.
-
16.
Conlon PJ, Jefferies F, Krigman HR, Corey GR, Sexton DJ, Abramson MA. Predictors of prognosis and risk of acute renal failure in bacterial endocarditis. Clin Nephrol. 1998;49(2):96-101. [PubMed ID: 9524779].
-
17.
Fernandez-Hidalgo N, Almirante B, Gavalda J, Gurgui M, Pena C, de Alarcon A, et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating enterococcus faecalis infective endocarditis. Clin Infect Dis. 2013;56(9):1261-8. [PubMed ID: 23392394]. https://doi.org/10.1093/cid/cit052.
-
18.
Korem M, Israel S, Gilon D, Cahan A, Moses AE, Block C, et al. Epidemiology of infective endocarditis in a tertiary-center in Jerusalem: A 3-year prospective survey. Eur J Intern Med. 2014;25(6):550-5. [PubMed ID: 24931808]. https://doi.org/10.1016/j.ejim.2014.05.011.
-
19.
Goenaga Sanchez MA, Kortajarena Urkola X, Bouza Santiago E, Munoz Garcia P, Verde Moreno E, Farinas Alvarez MC, et al. Aetiology of renal failure in patients with infective endocarditis. The role of antibiotics. Med Clin (Barc). 2017;149(8):331-8. [PubMed ID: 28431897]. https://doi.org/10.1016/j.medcli.2017.03.009.
-
20.
Ritchie BM, Hirning BA, Stevens CA, Cohen SA, DeGrado JR. Risk factors for acute kidney injury associated with the treatment of bacterial endocarditis at a tertiary academic medical center. J Chemother. 2017;29(5):292-8. [PubMed ID: 28245728]. https://doi.org/10.1080/1120009X.2017.1296916.