Abstract
Context:
COVID-19 severe manifestations must be detected as soon as possible. One of the essential poor characteristics is the involvement of coagulopathy. Simple coagulation parameters, including prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), and platelet, are widely accessible in many health centers.Objectives:
This meta-analysis aimed to determine the association between simple coagulation profiles and COVID-19 in-hospital mortality.Method:
We systematically searched five databases for studies measuring simple coagulation parameters in COVID-19 on admission. The random-effects and inverse-variance weighting were used in the study, which used a standardized-mean difference of coagulation profile values. The odds ratios were computed using the Mantel-Haenszel formula for dichotomous variables.Results:
This meta-analysis comprised a total of 30 studies (9,175 patients). In our meta-analysis, we found that non-survivors had a lower platelet count [SMD = -0.56 (95% CI: -0.79 to -0.33), P < 0.01; OR = 3.00 (95% CI: 1.66 to 5.41), P < 0.01], prolonged PT [SMD = 1.22 (95%CI: 0.71 to 1.72), P < 0.01; OR = 1.86 (95%CI: 1.43 to 2.43), P < 0.01], prolonged aPTT [SMD = 0.24 (95%CI: -0.04 to 0.52), P = 0.99], and increased INR [SMD = 2.21 (95%CI: 0.10 to 4.31), P = 0.04] than survivors.Conclusions:
In COVID-19 patients, abnormal simple coagulation parameters on admission, such as platelet, PT, and INR, were associated with mortality outcomes.Keywords
COVID-19 Coagulopathy Coagulation Profile Platelet Mortality
1. Context
Rapid growing numbers of COVID-19 patients and limited infrastructure resources provide significant challenges for healthcare institutions. It would be beneficial if any clinical or laboratory parameters would help us rapidly triage patients to appropriate units. The COVID-19 severe manifestations must be detected as soon as possible to predict each case's prognosis. Although the underlying pathophysiology of severe COVID-19 is poorly defined, some studies (1) reported that severe COVID-19 is related to significant coagulopathy.
A previous meta-analysis (2) demonstrated that advanced coagulation parameters such as D-dimer were associated with severity and mortality of COVID-19. However, most hospitals in peripheral areas, especially in developing countries, might not be able to test D-dimer. Simple coagulation parameters, including Prothrombin Time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), and platelets, are widely accessible in many health centers (3). Based on early reports, moderate to severe COVID-19 patients were likely to have prolonged PT, elevated INR, prolonged aPTT, and decreased platelets with subsequent poorer outcomes (4-6).
2. Objectives
We aimed to identify if basic coagulation profiles have a prognostic value in COVID-19 in-hospital mortality.
3. Method
We selected observational studies or trials on adult COVID-19 patients presenting some details on coagulation profiles, including platelet (PLT), PT, aPTT, and INR, for in-hospital mortality outcomes. Any study that had incomplete required data or lacked coagulation profile information on admission was removed. This meta-analysis was written as per the Preferred Reporting Items for systematic reviews and meta-analyses (PRISMA) guidelines (7).
A systematic literature search was finalized on November 20, 2021, following the approval of the institutional review board. We searched five different databases (PubMed, Science Direct, Scopus, ProQuest, and medRxiv) using the keywords "COVID 19" OR "Sars-Cov-2" OR "Novel coronavirus" AND "Laboratory parameter" OR "Coagulation" AND "Mortality" OR "Death" OR "Survivor." We also examined reference lists of the included studies to recognize any relevant studies to be added. Before full-text retrieval, three investigators evaluated titles and abstracts. Three investigators reviewed titles and abstracts before retrieving full-text papers. Two investigators then collected the data in each comparison category from full-text studies, including the authors, publication year, location, study design, peer-reviewed publication status, study outcome, and coagulation profile data.
The coagulation profile focusing on survival and non-survival outcomes was the primary outcome in our meta-analysis. The NIH quality assessment tool for observational Cohort and cross-sectional studies was used to determine the methodological quality of the studies. The visual analysis of funnel plots and the Egger regression test were used to assess publication bias (8).
Data analysis was carried out utilizing review manager (RevMan v5.4 2020) and Stata v.16. A standardized mean difference (SMD) for coagulation profile values was used in the meta-analysis. According to Wan et al. (9), sample size, median, and interquartile range (IQR) were used to calculate the mean and standard deviation (SD). We used inverse-variance weighting and random-effects models. The pooled odds ratios (ORs) were calculated using the Mantel-Haenszel formula for dichotomous variables.
We carried out a subgroup analysis by study design. Sensitivity analysis was performed using the leave-one-out method or dependent on peer-review status to evaluate the reason for heterogeneity. We assessed the heterogeneity using the I2 statistic. Restricted maximum likelihood random-effects meta-regression was performed for age, sex, cardiovascular disease (CVD), hypertension (HTN), and diabetes mellitus (DM) comorbidities in coagulation profiles, with a significant result and more than 10 studies included (10). In this meta-analysis, all p values less than 0.05 were statistically significant (except for heterogeneity using P < 0.10).
4. Results
Initial searches showed 88 PubMed records, 14 Science Direct records, 34 ProQuest records, 14 Scopus records, 262 medRxiv records, and 53 other records (Figure 1). After removing 39 duplicates and excluding 326 records, we retrieved 100 records for full-text screening. A total of 14 studies were excluded due to incorrect patient population, 13 due to unavailability of data on coagulation parameters, and 43 due to no outcome of interest. Thereby, we included the remaining 30 studies (9,175 patients) for analysis (11-40).
Study flow chart (as per PRISMA guideline)
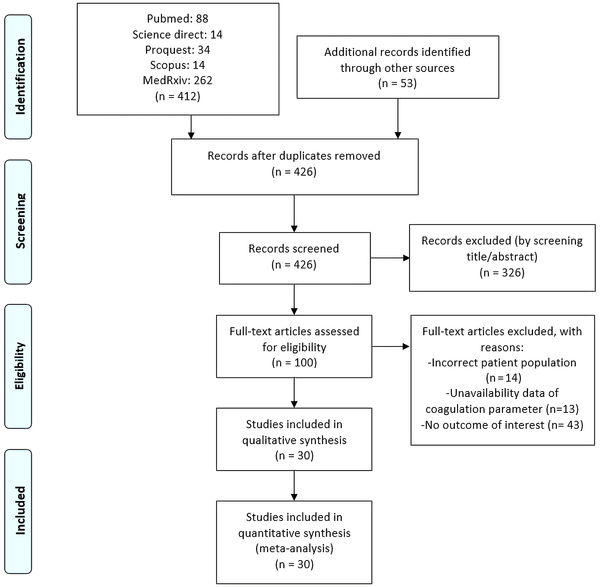
Tables 1 and 2 show the baseline characteristics of the included studies. There were 28 retrospective studies and two prospective observational studies. Peer review had already been completed on 21 studies. We assessed all methodologically acceptable studies (Table 1). The analyses and conclusions drawn were reliable. Nonetheless, due to their cross-sectional designs, most studies did not assess exposure before evaluating the outcome and would most likely lack adequate periods for the outcome.
| No | Author | Study Design | Hospital | Town, Country | Period | Samples (n) | Samples with a Lab Value | Male (%) | Age (y) | HTNo. (%) | CVD (%) | DM (%) | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Zhang et al. 2020 (14) | Retro | Wuhan Pulmonary Hospital | Wuhan, China | February 7 - March 27, 2020 | 53 (13 vs. 40) | aPTT 42(10 vs. 32), PT 53(40 vs. 13), Platelet 53(40 vs. 13) | N/A | N/A | N/A | N/A | N/A | Fair |
| 2 | Yan et al. 2020 (15) | Retro | Tongji Hospital | Wuhan, China | January 10 - February 24, 2020 | 193 (108 vs. 85) | 48 (39 vs. 9) | 76.9 vs. 33.3 | 70.5 ± 10 vs. 64.7 ± 7.3 | 52.8 vs. 18.8 | 25 vs. 4.7 | 36.1 vs. 10.6 | Good |
| 3 | Tang et al. 2020 (16) | Retro | Tongji Hospital | Wuhan, China | Jan 1 - Feb 3 2020 | 449 (134 vs. 315) | 449 (134 vs. 315) | 67.1 vs. 56.5 | 68.7 ± 11.4 vs. 63.7 ± 12.2 | N/A | N/A | N/A | Good |
| 4 | Wu et al. 2020 (17) | Retro | Jinyintan Hospital | Wuhan, China | Dec 25, 2019 - Jan 26, 2020 | 84 (44 vs. 40) | 84 (44 vs. 40) | 65.9 vs. 77.5 | 67.6 ± 12 vs. 49.03 ± 12.69 | 36.4 vs. 17.5 | 9.1 vs. 2.5 | 25 vs. 12.5 | Good |
| 5 | Tang et al. 2020 (13) | Retro | Tongji Hospital | Wuhan, China | Jan 1 - Feb 3 2020 | 183 (21 vs. 162) | 183 (21 vs. 162) | 76.19 vs. 50.61 | 64.0 ± 20.7 vs. 52.4 ± 15.6 | N/A | N/A | N/A | Good |
| 6 | Fan et al. 2020 (18) | Retro | Jinyintan Hospital | Wuhan, China | Dec 30, 2019 - Feb 16, 2020 | 73 (47 vs. 26) | 73 (47 vs. 26) | 68.09 vs. 65.38 | 65.46 ± 9.74 vs. 46.23 ± 12.01 | 44.68 vs. 11.54 | 14.89 vs. 0 | 21.28 vs. 7.69 | Good |
| 7 | Li et al. 2020 (19) | Retro | Wuhan Fourth Hospital | Wuhan, China | Jan 25 - Feb 26, 2020 | 74 (14 vs. 60) | 74(14 vs. 60) | 78.6 vs. 55 | 72.33 ± 6.59 vs. 61.67 ± 12.91 | 71.4 vs. 41.7 | 28.6 vs. 3.3 | 21.4 vs. 18.3 | Good |
| 8 | Satici et al. 2020 (20) | Retro | Gaziosmanpasa Research and Training Hospital | Istanbul, Turkey | April 2 - May 1, 2020 | 681 (55 vs. 626) | 681 (55 vs. 626) | 60 vs. 50.2 | 65.8 ± 12 vs. 56.1 ± 15.8 | 50.9 vs. 32.9 | 14.5 vs. 8.6 | 41.8 vs. 26.8 | Good |
| 9 | Du et al. 2020 (21) | Pros | Wuhan Pulmonary Hospital | Wuhan, China | Dec 25, 2019 - Feb 7, 2020 | 179 (21 vs. 158) | 179 (21 vs. 158) | 47.6 vs. 55.1 | 70.2 ± 7.7 vs. 56 ± 13.5 | 61.9 vs. 28.5 | 57.1 vs. 10.8 | 28.6 vs. 17.1 | Good |
| 10 | Pan et al. 2020 (22) | Retro | Union Hospital, Tongji Medical College, Huazhong University of Science and Technology | Shanghai, China | Jan 27-Mar 19, 2020 | 124 (89 vs. 35) | 124 (89 vs. 35) | 75.3 vs. 51.4 | 69 (61-73) vs. 65 (49-77) | 52.8 vs. 42.9 | 14.6 vs. 17.1 | 21.3 vs. 17.1 | Good |
| 11 | Chen et al. 2020 (12) | Retro | Tongji, Hospital | Shanghai, China | Jan 13-Feb 12, 2020 | 274 (113 vs. 161) | 274 (113 vs. 161) | 73 vs. 55 | 68.0 (62.0-77.0) vs. 51.0 (37.0-66.0) | 48 vs. 24 | 14 vs. 4 | 21 vs. 14 | Good |
| 12 | Gil et al. 2020 (11) | Retro | Montefiore Medical Center/ University Hospital for Albert Einstein College of Medicine, Moses Campus | New York, USA | Mar 20-31, 2020 | 217 (70 vs. 147) | 217 (70 vs. 147) | 67.1 vs. 53.7 | 68.71 ± 12.44 vs. 57.71 ± 15.56 | 74.3 vs. 61.2 | N/A | 45.7 vs. 33.3 | Fair |
| 13 | Alshukry et al. 2020 (23) | Retro | Jaber Al-Ahmad Hospital | Kuwait City, Kuwait | Feb 24-May 24, 2020 | 417 (60 vs. 357) | 88 (60 vs. 22) | 90 vs. 68.2 | 54.20 ± 11.09 vs. 52.32 ± 13.51 | 46.7 vs. 22.7 | 21.7 vs. 4.5 | 40.0 vs. 22.7 | Fair |
| 14 | Ayed et al. 2020 (24) | Retro | Jaber Al-Ahmad Al Sabah Hospital | Kuwait City, Kuwait | Mar 1-Apr 30, 2020 | 103 (45 vs. 47) | 92 (45 vs. 47) | 91 vs. 79 | 56 (48-63) vs. 51 (40-61) | 51.1 vs. 23.4 | 17.8 vs. 6.5 | 51.1 vs. 30.4 | Good |
| 15 | Shi et al. 2020 (25) | Retro | Renmin Hospital of Wuhan University | Wuhan, China | before February 15, 2020 | 101 (48 vs. 53) | 101 (48 vs. 53) | 58.3 vs. 60.4 | 72.0 (59.0-78.0) vs. 71.0 (59.0-81.0) | 56.3 vs. 60.4 | 18.8 vs. 26.4 | 18.8 vs. 22.6 | Fair |
| 16 | Luo et al. 2020 (26) | Retro | Renmin Hospital of Wuhan University | Wuhan, China | Jan 30-Feb 25, 2020 | 403 (100 vs. 303) | PLT: 403 (100 vs. 303) | 57 vs. 44.9 | 71 (65-80) vs. 49 (37-62) | 60 vs. 17.5 | 16 vs. 6.6 (CAD) | 25 vs. 10.6 | Good |
| 17 | Zhang et al. 2020 (27) | Retro | Wuhan No.1 Hospital | Wuhan, China | Dec 25, 2019- Feb 15, 2020 | 48 (17 vs. 31) | PLT: 48 (17 vs. 31) | 70.6 vs. 67.7 | 78.65 ± 8.31 vs. 66.16 ± 13.66 | 70.6 vs. 64.5 | 23.5 vs. 29.0 (CAD) | 29.4 vs. 16.1 | Fair |
| 18 | Paranjpe et al. 2020 (28) | Retro | Mount Sinai Hospital | New York, USA | Feb 27-April 2, 2020 | 1078 (310 vs. 768) | PLT:1008 (282 vs. 726); PT: 446 (142 vs. 304); aPTT: 442 (140 vs. 302) | 61.6 vs. 56.8 | 75 (64-85) vs. 59 (45-72) | 45.2 vs. 30.3 | 26.8 vs. 10.9 | 33.9 vs. 19.7 | Fair |
| 19 | Hu et al. 2020 (29) | Retro | Tongji Hospital | Wuhan, China | Jan 28-Mar 11, 2020 | 183 (68 vs. 115) | 183 (68 vs. 115) | 73.53 vs. 49.57 | 68.44 ± 9.94 vs. 60.54 ± 13.19 | 44.12 vs. 37.39 | N/A | 20.59 vs. 18.26 | Good |
| 20 | Fu et al. 2020 (30) | Retro | Third Batch of Chongqing Medical Aid Team | Wuhan, China | February 4- February 16, 2020 | 85 (14 vs. 71) | 85 (14 vs. 71) | 78.57 vs. 53.52 | 67(50.75-74.25) vs. 62(55-70) | 50 vs. 33.8 | 28.57 vs. 11.23 | 28.57 vs. 12.68 | Good |
| 21 | Luo et al. 2020 (31) | Retro | Eastern Campus of Renmin Hospital of Wuhan University | Wuhan, China | Jan 30-Feb 20, 2020 | 298 (84 vs. 214) | 298 (84 vs. 214) | 60.7 vs. 46.3 | 71 (64-80) vs. 51 (37-63) | 58.3 vs. 17.3 | 15.5 vs. 6.1 | 21.4 vs. 12.6 | Good |
| 22 | Wang et al. 2020 (32) | Retro | Renmin Hospital | Wuhan, China | Jan 1-Feb 6, 2020 | 339 (65 vs. 274) | 339 (65 vs. 274) | 60 vs. 46.4 | 76 (70–83) vs. 68 (64–74) | 50 vs. 38.8 | 32.8 vs. 11.7 | 17.2 vs. 15.8 | Good |
| 23 | Yang et al. 2020 (33) | Retro | Wuhan Jin Yin-tan hospital | Wuhan, China | Dec 24, 2019-Jan 26, 2020 | 52 (32 vs. 20) | 52 (32 vs. 20) | 66 vs. 70 | 64.6 ± 11.2 vs. 51.9 ± 12.9 | N/A | 9 vs. 10 | 22 vs. 10 | Good |
| 24 | Zhou et al. 2020 (34) | Retro | Jinyintan Hospital and Wuhan Pulmonary Hospital | Wuhan, China | December 29, 2019-Jan 31, 2020 | 191 (54 vs. 137) | PLT: 191(54 vs. 137); PT: 182(54 vs. 128) | 70 vs. 59 | 69 (63–76) vs. 52 (45–58) | 48 vs. 23 | 24 vs. 1 | 31 vs. 14 | Good |
| 25 | Wang et al. 2020 (35) | Retro | Sino-French New City Branch of Tongji Hospital | Wuhan, China | Jan 28-Mar 4, 2020 | 199 (24 vs. 175) | 199 (24 vs. 175) | 66.7 vs. 49.1 | 69.5 (64.5-82.75) vs. 64.0 (51.0-71.0) | 50.0 vs. 37.9 | 8.3 vs. 12 | 37.5 vs. 18.9 | Good |
| 26 | Sai et al. 2021 (36) | Retro | Leishenshan Hospital | Wuhan, China | Feb 24-April 5, 2020 | 47 (15 vs. 32) | 47 (15 vs. 32) | 46.7 vs. 71.9 | 70.64 ± 12.33 vs. 69.67 ± 12.91 | 46.7 vs. 56.3 | 20 vs. 15.6 | 40 vs. 37.5 | Good |
| 27 | Peiró et al. 2021 (37) | Retro | Joan XXIII University Hospital | Tarragona, Spain | Mar 16-May 15, 2020 | 196 (37 vs. 159) | 196 (37 vs. 159) | 62.2 vs. 59.1 | 76.5 (68.5–82.5) vs. 61.5 (51.5–75.5) | 64.9 vs. 39.6 | 18.9 vs. 7.6 | 35.1 vs. 20.8 | Good |
| 28 | Velasco-Rodríguez et al. 2021 (38) | Retro | 4 hospitals in Madrid | Madrid, Spain | Feb 27-Apr 17, 2020 | 2070 (393 vs. 1677) | 2070 (393 vs. 1677) | 20.92 vs. 79.08 | 81 (72–87) vs. 63 (51–75) | 27.75 vs. 72.25 | 31.49 vs. 68.51 | 29.1 vs. 80.9 | Good |
| 29 | Violi et al. 2021 (39) | Pros | University hospitals located in Rome (2 centers), Latina, Perugia, and Chieti | Italy | Mar 1-31, 2020 | 373 (75 vs. 298) | 373 (75 vs. 298) | 72 vs. 59 | 75.3 ± 13.9 vs. 65.5 ± 17.0 | 61 vs. 51 | 22 vs. 13 | 25 vs. 15 | Good |
| 30 | Gayam et al. 2021 (40) | Retro | inner-city teaching hospital Brooklyn | New York, USA | Mar 1-Apr 9, 2020 | 408 (132 vs. 276) | 408 (132 vs. 276) | 32.9 vs. 67.1 | 71 (62-80) vs. 63 (53-73) | 64.9 vs. 39.6 | 37.04 vs. 62.92 | 40.91 vs. 59.09 | Good |
| No. | Author | aPTT (s) | PT (s) | PT Cut-Off | PLT (109/L) | PLT Cut-Off | INR |
|---|---|---|---|---|---|---|---|
| 1 | Zhang et al. 2020 (14) | 39.99 ± 7.12 vs. 40.25 ± 4.65 | 14.95 ± 1.70 vs. 13.70 ± 1.04 | NR | 109.42 ± 112.33 vs. 176.75 ± 54.40 | NR | N/A |
| 2 | Yan et al. 2020 (15) | 40.16 ± 8.3 vs. 37.63 ± 6.77 | 15.47 ± 3.15 vs. 13.73 ± 0.92 | NR | 167 ± 88.51 vs. 202.33 ± 111,08 | NR | N/A |
| 3 | Tang et al. 2020 (16) | N/A | 16.5 ± 8.4 vs. 14.6 ± 2.1 | NR | 178 ± 92 vs. 231 ± 99 | NR | N/A |
| 4 | Wu et al. 2020 (17) | 24.9 ± 4.67 vs. 29.78 ± 9.03 | 11.72 ± 1.03 vs. 11.72 ± 1.15 | NR | 167.83 ± 92.35 vs. 201.33 ± 96.5 | NR | N/A |
| 5 | Tang et al. 2020 (13) | 45.33 ± 8.59 vs. 40.7 ± 5.31 | 15.4 ± 1.51 vs. 13.63 ± 0.97 | NR | N/A | N/A | N/A |
| 6 | Fan et al. 2020 (18) | N/A | 11.88 ± 1.55 vs. 11.13 ± 1.41 | NR | 168.33 ± 65 vs. 207 ± 93.33 | NR | N/A |
| 7 | Li et al. 2020 (19) | 37.47 ± 7.17 vs. 35.13 ± 6.30 | 13.93 ± 2.80 vs. 13.33 ± 1.37 | NR | N/A | N/A | 1.13 ± 0.16 vs. 0.69 ± 0.76 |
| 8 | Satici et al. 2020 (20) | N/A | N/A | N/A | 196 ± 47.96 vs. 198.33 ± 60.93 | NR | N/A |
| 9 | Du et al. 2020 (21) | 36.7 ± 8.51 vs. 35.1 ± 6.14 | 14.17 ± 3.18 vs. 13.77 ± 2.09 | NR | N/A | N/A | N/A |
| 10 | Pan et al. 2020 (22) | 37.45 ± 1.86 vs. 38.63 ± 1.69 | 14.15 ± 0.43 vs. 13.67 ± 0.29 | > 13.9 | 187.33 ± 58.78 vs. 191.33 ± 70.34 | ≤187 | N/A |
| 11 | Chen et al. 2020 (12) | 40.92 ± 1.99 vs. 40.72 ± 1.25 | 15.6 ± 0.56 vs. 13.85 ± 0.21 | NR | 160.78 ± 18.95 vs. 203 ± 16.92 | NR | 1.23 ± 0.05 vs. 1.08 ± 0.02 |
| 12 | Gil et al. 2020 (11) | 32.63 ± 1.10 vs. 34.13 ± 1.29 | 13.85 ± 0.22 vs. 14.68 ± 0.45 | NR | N/A | N/A | N/A |
| 13 | Alshukry et al. 2020 (23) | 45.81 ± 3.05 vs. 32.63 ± 1.3 | 15.87 ± 1.04 vs. 13.64 ± 0.35 | NR | 260.35 ± 22.89 vs. 323.92 ± 24.27 | NR | N/A |
| 14 | Ayed et al. 2020 (24) | 41.5 ± 8.5 vs. 38.75 ± 6.68 | N/A | N/A | 216.5 ± 20.66 vs. 261.75 ± 24.9 | NR | 1.16 ± 0.10 vs. 1.03 ± 0.03 |
| 15 | Shi et al. 2020 (25) | 30.48 ± 1.02 vs. 30.03 ± 1.10 | 13.32 ± 0.38 vs. 12.63 ± 0.42 | NR | 168 ± 26.78 vs. 159.75 ± 16.19 | NR | N/A |
| 16 | Luo et al. 2020 (26) | N/A | N/A | N/A | 169.67 ± 73.72 vs. 207.33 ± 82.68 | < 125 | N/A |
| 17 | Zhang et al. 2020 (26) | N/A | N/A | N/A | 140 ± 100.24 vs. 182.33 ± 57.51 | < 125 | N/A |
| 18 | Paranjpe et al. 2020 (28) | 33.57 ± 5.54 vs. 31.63 ± 4.54 | 14.7 ± 2.02 vs. 13.63 ± 1.04 | NR | 189.33 ± 70.79 vs. 197.67 ± 69.82 | NR | N/A |
| 19 | Hu et al. 2020 (29) | N/A | 15.6 ± 2.42 vs. 13.83 ± 0.98 | NR | 171.33 ± 78.39 vs. 211 ± 80.33 | NR | N/A |
| 20 | Fu et al. 2020 (30) | N/A | N/A | N/A | 165.33 ± 50.67 vs. 226 ± 72.64 | NR | N/A |
| 21 | Luo et al. 2020 (31) | N/A | N/A | N/A | 159.33 ± 76.94 vs. 202.67 ± 75.38 | NR | N/A |
| 22 | Wang et al. 2020 (32) | 29.43 ± 3.26 vs. 28.37 ± 4.17 | 12.97 ± 1.64 vs. 12.17 ± 0.52 | NR | 164.67 ± 86.36 vs. 212.67 ± 81.15 | NR | N/A |
| 23 | Yang et al. 2020 (33) | N/A | 12.9 ± 2.9 vs. 10.9 ± 2.7 | NR | 191 ± 63 vs. 164 ± 74 | NR | N/A |
| 24 | Zhou et al. 2020 (34) | N/A | 12.33 ± 1.86 vs. 11.47 ± 1.65 | ≥16 | 167.17 ± 92.92 vs. 219.67 ± 77.17 | < 100 | N/A |
| 25 | Wang et al. 2020 (35) | 41.3 ± 7.32 vs. 39.3 ± 6.05 | 39.37 ± 6.05 vs. 14.9 ± 1.26 | NR | 221 ± 114.0 vs. 230.5 ± 86.5 | NR | N/A |
| 26 | Sai et al. 2021 (36) | 35.87 ± 14.51 vs. 33.63 ± 9.75 | 13.63 ± 3.52 vs. 12.53 ± 1.77 | NR | 173.47 ± 107.84 vs. 225.47 ± 98.79 | NR | N/A |
| 27 | Peiró et al. 2021 (37) | N/A | N/A | N/A | 226.33 ± 118.77 vs. 215.67 ± 90.52 | NR | N/A |
| 28 | Velasco-Rodríguez et al. 2021 (38) | 30.07 ± 4.17 vs. 30.47 ± 3.34 | 13.3 ± 1.41 vs. 12.87 ± 1.19 | > 14 | 199.17 ± 82.96 vs. 198.08 ± 128.54 | < 140 | N/A |
| 29 | Violi et al. 2021 (39) | N/A | N/A | N/A | 204 ± 119 vs. 211 ± 75 | NR | N/A |
| 30 | Gayam et al. 2021 (40) | 31.42 ± 4.76 vs. 31.41 ± 3.95 | N/A | N/A | 215.33 ± 83.19 vs. 226 ± 89.43 | NR | N/A |
Funnel plots for INR and aPTT showed an asymmetrical appearance indicating publication bias (Appendix 1). Since less than 10 studies were involved, we did not conduct Egger's regression test for INR. The publication bias for aPTT was also shown by the Egger's test (P = 0.007), but not for PT (P = 0.395) and PLT (P = 0.896).
4.1. Platelet
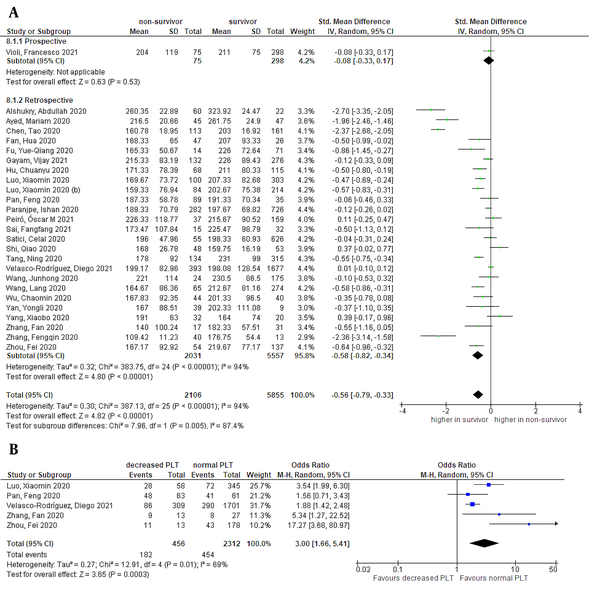
Random-effects meta-analysis revealed significantly lower platelet counts on admission in the non-survivor group than in the survivor group, as shown in Figure 2 [26 studies, SMD = -0.56 (95% CI: -0.79 to -0.33), P < 0.01; I2 = 94%, P < 0.01]. A similar result was shown in retrospective subgroup analysis. Categorical data of platelet count were found in five studies. Decreased platelet counts were associated with increased mortality [OR = 3.00 (95% CI: 1.66 to 5.41), P < 0.01; I2 = 69%, P = 0.01] (Figure 2). The sensitivity of 58% (95% CI: 38 to 76%) and specificity of 70% (95% CI: 54 to 83%) were obtained from a pooled analysis of multiple cut-off points (Appendix 2). Decreased platelet had a positive likelihood ratio (LR) of 1.9 and a negative LR of 0.6. According to a meta-regression analysis, unlike age (P = 0.023) and HTN (P = 0.014), sex (P = 0.412), CVD (P = 0.580) and DM (P = 0.935) had no impacts on the relationship between decreased platelet count and mortality.
Forest plot of platelet level for mortality outcome. A, Non-survivors had a lower platelet level than survivors; and B, Decreased platelet was associated with increased mortality.
4.2. Prothrombin Time
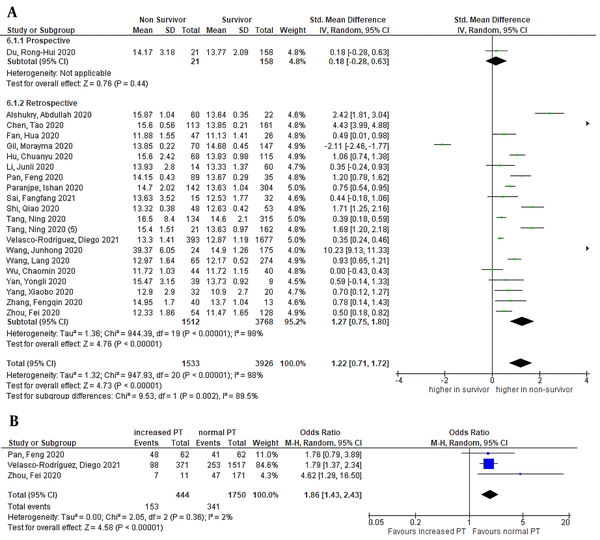
The pooled effect size demonstrated that PT was significantly higher in non-survivors than in survivors, as shown in Figure 3 [21 studies, SMD = 1.22 (95% CI: 0.71 to 1.72), P < 0.01; I2 = 98%, P < 0.01]. A similar result was shown in retrospective subgroup analysis. Sensitivity analysis by removing Gil et al.’ study (11) showed no improvement in heterogeneity. Pooled analysis of three studies with categorical data of PT demonstrated increased PT in the non-survivor group [OR = 1.86 (95% CI: 1.43 to 2.43), P < 0.01; I2 = 2%, P = 0.36] (Figure 3). According to a meta-regression analysis, age (P = 0.964), sex (P = 0.422), CVD (P = 0.889), DM (P = 0.955), and HTN (P = 0.910) comorbidities had no impact on the relationship between decreased platelet count and mortality.
Forest plot of PT level for mortality outcome. A, Non-survivors had a higher PT level than survivors; and B, Increased PT and mortality (PT, prothrombin time).
4.3. Activated Partial Thromboplastin Time
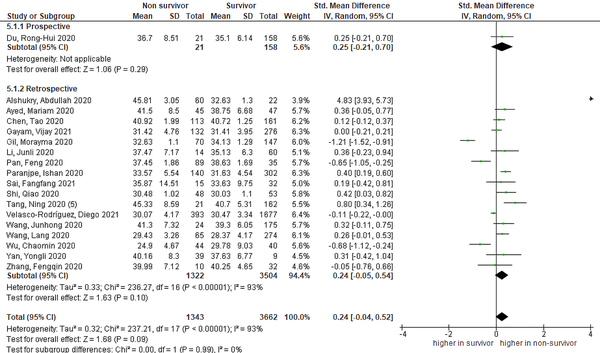
The pooled effect size demonstrated that aPTT was non-significantly higher in non-survivors than in survivors, as shown in Figure 4 [18 studies, SMD = 0.24 (95% CI: -0.04 to 0.52), P = 0.09; I2 = 93%, P < 0.01]. The prospective group did not differ from the retrospective subgroup, as shown in subgroup analysis based on study design. Nevertheless, the removal of Gil et al.’s study (11) demonstrated a significant result of higher aPTT in non-survivors [SMD = 0.43 (95% CI: 0.06 to 0.58), P = 0.02; I2 = 91%, P < 0.01].
Forest plot of aPTT level for mortality outcome. Non-survivors had a non-significantly higher aPTT level than survivors (aPTT, activated partial thromboplastin time).
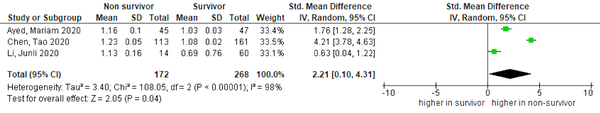
4.4. International Normalized Ratio
Higher mean INR was found in non-survivors than in survivors, as shown in Figure 5 [three studies, SMD = 2.21 (95% CI: 0.10 to 4.31), P = 0.04; I2 = 98%, P < 0.01]. Sensitivity analysis by removing Chen et al.’ study (12) showed improvement in heterogeneity [SMD = 1.21 (95% CI: 0.10 to 2.32), P = 0.03; I2 = 88%, P < 0.01].
Forest plot of INR for mortality outcome. Non-survivors had a higher INR level than survivors (INR, international normalized ratio).
5. Discussion
This meta-analysis found that COVID-19 patients with prolonged PT and aPTT, elevated INR, and a lower platelet level on admission had a higher mortality rate. Our results are similar to previous studies (6, 41). The prolongation of PT in the non-survivor group was consistent with another meta-analysis (4). However, the degree of PT prolongation is less prominent in COVID-19 than in bacterial sepsis-induced coagulopathy or disseminated intravascular coagulation (DIC) (42). Mild prolongation of aPTT demonstrated in COVID-19 subjects is possibly explained by the involvement of severe consumption or inhibition to specific coagulation factors (43).
Along with the emerging evidence of SARS-CoV-2, the presence of coagulopathy is one of the major factors responsible for high mortality rates other than cytokine storms (44). Severe infection activates the coagulation cascade and increases DIC risk, consequently increasing the fatality rates (13). Besides, COVID-19 increases the risk of thromboembolism in several organs, as it causes abnormal activation of coagulation and secondary hyperfibrinolysis (45). A first autopsy series to COVID-19-related deaths in New Orleans (46) reported the presence of significant diffuse alveolar damage and pulmonary microvascular thrombosis, possibly contributing to death.
Decreased platelet counts in COVID-19 are possibly caused by hematopoiesis suppression in the bone marrow by the virus. As known, COVID-19 increases autoantibodies and immune complexes, leading to specific immune system disruption of platelets. Lung tissue and pulmonary endothelial cells damage in COVID-19 can activate platelets in the lungs, leading to microthrombi aggregation and formation and increased platelet consumption (47).
In addition, PT and aPTT are beneficial for the early detection of DIC in COVID-19-associated coagulopathy (48). Laboratory characteristics in DIC vary depending on the stage. In early DIC, hemostatic system activation is compensated. As DIC develops into the decompensated stage, which might be found in the late stage of COVID-19, decreased thrombocyte, elevated PT and aPTT, increased fibrinogen, increased fibrin degradation product, and reduced protease inhibition are found (49). Besides, PT, aPTT, and INR are excellent parameters describing clot formation. These parameters do not provide information about fibrin crosslinking or clot dissolution and will thus be insensitive to abnormalities of fibrinolysis. On the other hand, D-dimer indicates recent or ongoing intravascular coagulation and fibrinolysis (50).
Our findings suggest that the abnormality of routine coagulation parameters on admission can be used as risk stratification tools in adult COVID-19 patients. Risk stratification in triage would help health workers allocate resources and sort the patients in the appropriate critical care or modified units, therefore maximizing the use of acute care beds (51). We encourage further studies to develop a prognostic model involving coagulation profiles in COVID-19 outcomes.
To the authors’ knowledge, our review of 30 studies is the largest meta-analysis on the elaboration of coagulation profiles and in-hospital mortality of COVID-19. However, several limitations are found in our study. Publication bias was noted in several coagulation parameters. There was also substantial heterogeneity across studies. Some of the included studies in this meta-analysis were published at the preprint server. The majority of the included studies were retrospective and had limited sample sizes. Furthermore, China was the source of the majority of the studies. Differences in ethnicity and geography can skew the analysis results.
5.1. Conclusions
In COVID-19 patients, abnormal simple coagulation parameters on admission, such as increased PPT and INR and decreased platelets, were related to a higher risk of in-hospital mortality. We recommend clinicians closely monitor routine coagulation parameters as markers for potential progression to critical illness.
References
-
1.
Marchandot B, Sattler L, Jesel L, Matsushita K, Schini-Kerth V, Grunebaum L, et al. COVID-19 Related Coagulopathy: A Distinct Entity? J Clin Med. 2020;9(6). [PubMed ID: 32486469]. [PubMed Central ID: PMC7356260]. https://doi.org/10.3390/jcm9061651.
-
2.
Nugroho J, Wardhana A, Maghfirah I, Mulia EPB, Rachmi DA, A'Yun M Q, et al. Relationship of D-dimer with severity and mortality in SARS-CoV-2 patients : A meta-analysis. Int J Lab Hematol. 2021;43(1):110-5. [PubMed ID: 32931146]. https://doi.org/10.1111/ijlh.13336.
-
3.
Abdullah W. Shortened Activated Partial Thromboplastin Time (APTT): A Simple but Important Marker of Hypercoagulable State During Acute Coronary Event. Coronary Artery Disease - New Insights and Novel Approaches. London, UK: IntechOpen; 2012. https://doi.org/10.5772/27887.
-
4.
Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021-8. [PubMed ID: 32286245]. https://doi.org/10.1515/cclm-2020-0369.
-
5.
Deng Y, Liu W, Liu K, Fang YY, Shang J, Zhou L, et al. Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 in Wuhan, China: a retrospective study. Chin Med J (Engl). 2020;133(11):1261-7. [PubMed ID: 32209890]. [PubMed Central ID: PMC7289311]. https://doi.org/10.1097/CM9.0000000000000824.
-
6.
Velavan TP, Meyer CG. Mild versus severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304-7. [PubMed ID: 32344011]. [PubMed Central ID: PMC7194601]. https://doi.org/10.1016/j.ijid.2020.04.061.
-
7.
Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777-84. [PubMed ID: 26030634]. https://doi.org/10.7326/M14-2385.
-
8.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-34. [PubMed ID: 9310563]. [PubMed Central ID: PMC2127453]. https://doi.org/10.1136/bmj.315.7109.629.
-
9.
Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. [PubMed ID: 25524443]. [PubMed Central ID: PMC4383202]. https://doi.org/10.1186/1471-2288-14-135.
-
10.
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane handbook for systematic reviews of interventions. New Jersey, USA: John Wiley & Sons; 2019.
-
11.
Gil MR, Gonzalez-Lugo JD, Rahman S, Barouqa M, Szymanski J, Ikemura K, et al. Correlation of coagulation parameters with clinical outcomes in Coronavirus-19 affected minorities in United States: Observational cohort. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.05.01.20087932.
-
12.
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. [PubMed ID: 32217556]. [PubMed Central ID: PMC7190011]. https://doi.org/10.1136/bmj.m1091.
-
13.
Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-7. [PubMed ID: 32073213]. [PubMed Central ID: PMC7166509]. https://doi.org/10.1111/jth.14768.
-
14.
Zhang F, Xiong Y, Wei Y, Hu Y, Wang F, Li G, et al. Obesity predisposes to the risk of higher mortality in young COVID-19 patients. J Med Virol. 2020;92(11):2536-42. [PubMed ID: 32437016]. [PubMed Central ID: PMC7280697]. https://doi.org/10.1002/jmv.26039.
-
15.
Yan Y, Yang Y, Wang F, Ren H, Zhang S, Shi X, et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1). [PubMed ID: 32345579]. [PubMed Central ID: PMC7222577]. https://doi.org/10.1136/bmjdrc-2020-001343.
-
16.
Tang N, Bai H, Chen X, Gong J, Li D, Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-9. [PubMed ID: 32220112]. https://doi.org/10.1111/jth.14817.
-
17.
Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934-43. [PubMed ID: 32167524]. [PubMed Central ID: PMC7070509]. https://doi.org/10.1001/jamainternmed.2020.0994.
-
18.
Fan H, Zhang L, Huang B, Zhu M, Zhou Y, Zhang H, et al. Cardiac injuries in patients with coronavirus disease 2019: Not to be ignored. Int J Infect Dis. 2020;96:294-7. [PubMed ID: 32437935]. [PubMed Central ID: PMC7211636]. https://doi.org/10.1016/j.ijid.2020.05.024.
-
19.
Li J, Xu G, Yu H, Peng X, Luo Y, Cao C. Clinical Characteristics and Outcomes of 74 Patients With Severe or Critical COVID-19. Am J Med Sci. 2020;360(3):229-35. [PubMed ID: 32653160]. [PubMed Central ID: PMC7832924]. https://doi.org/10.1016/j.amjms.2020.05.040.
-
20.
Satici C, Demirkol MA, Sargin Altunok E, Gursoy B, Alkan M, Kamat S, et al. Performance of pneumonia severity index and CURB-65 in predicting 30-day mortality in patients with COVID-19. Int J Infect Dis. 2020;98:84-9. [PubMed ID: 32553714]. [PubMed Central ID: PMC7293841]. https://doi.org/10.1016/j.ijid.2020.06.038.
-
21.
Du RH, Liang LR, Yang CQ, Wang W, Cao TZ, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur Respir J. 2020;55(5). [PubMed ID: 32269088]. [PubMed Central ID: PMC7144257]. https://doi.org/10.1183/13993003.00524-2020.
-
22.
Pan F, Yang L, Li Y, Liang B, Li L, Ye T, et al. Factors associated with death outcome in patients with severe coronavirus disease-19 (COVID-19): A case-control study. Int J Med Sci. 2020;17(9):1281-92. [PubMed ID: 32547323]. [PubMed Central ID: PMC7294915]. https://doi.org/10.7150/ijms.46614.
-
23.
Alshukry A, Ali H, Ali Y, Taweel TA, Abu-Farha M, AbuBaker J, et al. Clinical characteristics of Coronavirus Disease 2019 (COVID-19) patients in Kuwait. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.06.14.20131045.
-
24.
Ayed M, Borahmah AA, Yazdani A, Sultan A, Mossad A, Rawdhan H. Assessment of clinical characteristics and mortality-associated factors in COVID-19 Critical cases in Kuwait. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.06.17.20134007.
-
25.
Shi Q, Zhao K, Yu J, Jiang F, Feng J, Zhao K, et al. Clinical characteristics of 101 COVID-19 non-survivors in Wuhan, China: a retrospective study. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.03.04.20031039.
-
26.
Luo X, Xia H, Yang W, Wang B, Guo T, Xiong J, et al. Characteristics of patients with COVID-19 during epidemic ongoing outbreak in Wuhan, China. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.03.19.20033175.
-
27.
Zhang F, Yang D, Li J, Gao P, Chen T, Cheng Z, et al. Myocardial injury is associated with in-hospital mortality of confirmed or suspected COVID-19 in Wuhan, China: A single center retrospective cohort study. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.03.21.20040121.
-
28.
Paranjpe I, Russak AJ, De Freitas JK, Lala A, Miotto R, Vaid A, et al. Clinical Characteristics of Hospitalized Covid-19 Patients in New York City. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.04.19.20062117.
-
29.
Hu C, Liu Z, Jiang Y, Zhang X, Shi O, Xu K, et al. Early prediction of mortality risk among severe COVID-19 patients using machine learning. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.04.13.20064329.
-
30.
Fu Y, Sun Y, Lu S, Yang Y, Wang Y, Xu F. Impact of blood analysis and immune function on the prognosis of patients with COVID-19. medRxiv. 2020;Preprint. https://doi.org/10.1101/2020.04.16.20067587.
-
31.
Luo X, Zhou W, Yan X, Guo T, Wang B, Xia H, et al. Prognostic Value of C-Reactive Protein in Patients With Coronavirus 2019. Clin Infect Dis. 2020;71(16):2174-9. [PubMed ID: 32445579]. [PubMed Central ID: PMC7314209]. https://doi.org/10.1093/cid/ciaa641.
-
32.
Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639-45. [PubMed ID: 32240670]. [PubMed Central ID: PMC7118526]. https://doi.org/10.1016/j.jinf.2020.03.019.
-
33.
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-81. [PubMed ID: 32105632]. [PubMed Central ID: PMC7102538]. https://doi.org/10.1016/S2213-2600(20)30079-5.
-
34.
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054-62. [PubMed ID: 32171076]. [PubMed Central ID: PMC7270627]. https://doi.org/10.1016/S0140-6736(20)30566-3.
-
35.
Wang J, Zhang H, Qiao R, Ge Q, Zhang S, Zhao Z, et al. Thrombo-inflammatory features predicting mortality in patients with COVID-19: The FAD-85 score. J Int Med Res. 2020;48(9):300060520955037. [PubMed ID: 32960106]. [PubMed Central ID: PMC7511832]. https://doi.org/10.1177/0300060520955037.
-
36.
Sai F, Liu X, Li L, Ye Y, Zhu C, Hang Y, et al. Clinical characteristics and risk factors for mortality in patients with coronavirus disease 2019 in intensive care unit: A single- center, retrospective, observational study in China. Ann Palliat Med. 2021;10(3):2859-68. [PubMed ID: 33548994]. https://doi.org/10.21037/apm-20-1575.
-
37.
Peiro OM, Carrasquer A, Sanchez-Gimenez R, Lal-Trehan N, Del-Moral-Ronda V, Bonet G, et al. Biomarkers and short-term prognosis in COVID-19. Biomarkers. 2021;26(2):119-26. [PubMed ID: 33426934]. [PubMed Central ID: PMC7832452]. https://doi.org/10.1080/1354750X.2021.1874052.
-
38.
Velasco-Rodriguez D, Alonso-Dominguez JM, Vidal Laso R, Lainez-Gonzalez D, Garcia-Raso A, Martin-Herrero S, et al. Development and validation of a predictive model of in-hospital mortality in COVID-19 patients. PLoS One. 2021;16(3). e0247676. [PubMed ID: 33661939]. [PubMed Central ID: PMC7932507]. https://doi.org/10.1371/journal.pone.0247676.
-
39.
Violi F, Ceccarelli G, Cangemi R, Cipollone F, D'Ardes D, Oliva A, et al. Arterial and venous thrombosis in coronavirus 2019 disease (Covid-19): Relationship with mortality. Intern Emerg Med. 2021;16(5):1231-7. [PubMed ID: 34218413]. [PubMed Central ID: PMC8255055]. https://doi.org/10.1007/s11739-020-02621-8.
-
40.
Gayam V, Chobufo MD, Merghani MA, Lamichhane S, Garlapati PR, Adler MK. Clinical characteristics and predictors of mortality in African-Americans with COVID-19 from an inner-city community teaching hospital in New York. J Med Virol. 2021;93(2):812-9. [PubMed ID: 32672844]. [PubMed Central ID: PMC7405133]. https://doi.org/10.1002/jmv.26306.
-
41.
Sakka M, Connors JM, Hekimian G, Martin-Toutain I, Crichi B, Colmegna I, et al. Association between D-Dimer levels and mortality in patients with coronavirus disease 2019 (COVID-19): A systematic review and pooled analysis. J Med Vasc. 2020;45(5):268-74. [PubMed ID: 32862984]. [PubMed Central ID: PMC7250752]. https://doi.org/10.1016/j.jdmv.2020.05.003.
-
42.
Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103-9. [PubMed ID: 32558075]. [PubMed Central ID: PMC7323352]. https://doi.org/10.1111/jth.14975.
-
43.
Shimura T, Kurano M, Kanno Y, Ikeda M, Okamoto K, Jubishi D, et al. Clot Waveform of APTT Has Abnormal Patterns in Subjects with COVID-19. Research Square. 2020;Preprint. https://doi.org/10.21203/rs.3.rs-43405/v1.
-
44.
Magro G. Cytokine Storm: Is it the only major death factor in COVID-19 patients? Coagulation role. Med Hypotheses. 2020;142:109829. [PubMed ID: 32428809]. [PubMed Central ID: PMC7217113]. https://doi.org/10.1016/j.mehy.2020.109829.
-
45.
Wang J, Saguner AM, An J, Ning Y, Yan Y, Li G. Dysfunctional Coagulation in COVID-19: From Cell to Bedside. Adv Ther. 2020;37(7):3033-9. [PubMed ID: 32504450]. [PubMed Central ID: PMC7274265]. https://doi.org/10.1007/s12325-020-01399-7.
-
46.
Fox SE, Akmatbekov A, Harbert JL, Li G, Quincy Brown J, Vander Heide RS. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681-6. [PubMed ID: 32473124]. [PubMed Central ID: PMC7255143]. https://doi.org/10.1016/S2213-2600(20)30243-5.
-
47.
Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205-8. [PubMed ID: 32296910]. [PubMed Central ID: PMC7156897]. https://doi.org/10.1007/s00277-020-04019-0.
-
48.
Long H, Nie L, Xiang X, Li H, Zhang X, Fu X, et al. D-Dimer and Prothrombin Time Are the Significant Indicators of Severe COVID-19 and Poor Prognosis. Biomed Res Int. 2020;2020:6159720. [PubMed ID: 32596339]. [PubMed Central ID: PMC7301188]. https://doi.org/10.1155/2020/6159720.
-
49.
Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50(1):54-67. [PubMed ID: 32415579]. [PubMed Central ID: PMC7225095]. https://doi.org/10.1007/s11239-020-02134-3.
-
50.
Weitz JI, Fredenburgh JC. Factors XI and XII as Targets for New Anticoagulants. Front Med (Lausanne). 2017;4:19. [PubMed ID: 28286749]. [PubMed Central ID: PMC5323386]. https://doi.org/10.3389/fmed.2017.00019.
-
51.
Chilimuri S, Sun H, Alemam A, Mantri N, Shehi E, Tejada J, et al. Predictors of Mortality in Adults Admitted with COVID-19: Retrospective Cohort Study from New York City. West J Emerg Med. 2020;21(4):779-84. [PubMed ID: 32726241]. [PubMed Central ID: PMC7390589]. https://doi.org/10.5811/westjem.2020.6.47919.