Abstract
Background:
Kawasaki disease (KD) is a frequent cause of acquired heart disease in infants and young children, which may be triggered by infectious agents, with the highest incidence in Asian countries. Several genotypes are identified as susceptibility genes for KD, but none is known in the Iranian population.Objectives:
Due to the racial differences in the frequency and significance of the identified genes, we aimed to investigate the 8p22-23-rs2254546 genotypes in a sample of Iranian children and their association with aneurysm and resistance to treatment.Methods:
In this prospective cross-sectional study, 100 children with KD as the sample group were compared with 100 matched unrelated healthy Iranian children with no history of KD or immune-related disease. The control subjects were ethnically recruited from the same hospital at the time of a routine physical examination. The 8p22-23-rs2254546 genotypes were analyzed using the polymerase chain reaction (PCR) and direct sequencing techniques, and the frequency of the three genotypes (GA, AA, and GG) was reported. Finally, the frequency of aneurysm and IVIG resistance was reported, and their associations with 8p22-23-rs2254546 genotypes were tested by SPSS software.Results:
Of 100 patients with a mean age of 1.9 ± 1.7 (0.1 - 10.2) years, 57% were boys and 43% were girls. Of them, 62% had GG, 30% GA, and 8% AA genotypes. Besides, 21% of the studied patients were resistant to IVIG, and 19% had aneurysm, but resistance to IVIG and aneurysm was not associated with GG and GA genotypes of 8p22-23-rs2254546 (P = 0.29 and 0.88, respectively).Conclusions:
The majority of the children with KD were shown to have this genetic susceptibility, which shows the importance of the 8p22-23-rs2254546 genotype in Iranian children. However, this genotype was not associated with the risk of resistance to IVIG and aneurysm.Keywords
Mucocutaneous Lymph Node Syndrome Coronary Aneurysm Genotype
1. Background
Kawasaki disease (KD) is a frequent cause of acquired heart disease in infants and young children (1). It is reported in many countries of the world, including Asian, European, and American countries, with the highest incidence in Asian countries, including Japan and Korea, with an incidence rate of 265:100,000 children aged < 5 years (2). This is while in the USA, it is estimated at 19 per 100,000 children aged < 5-years-old (3). The incidence of KD in other countries depends on the dominant race and ethnicity (4). Furthermore, it is reported to be six times more in boys with seasonal peaks in incidence (5, 6). Kawasaki disease has been introduced since 1967 (7), although its etiology is still not well understood, which hinders appropriate diagnosis and treatment (8). However, some reports have shown the association of KD with bacterial and viral triggers (9). In a case report, the author described a child with a KD diagnosis that was complicated with streptococcal infection and pleural effusion comorbidity (10). It has been reported that adenoviruses, enteroviruses, rhinoviruses, and coronaviruses are the etiologic triggers of KD (11).
Prolonged fever, mucocutaneous presentations such as redness, rash, and erythema of the skin and mucosa, bilateral conjunctival injection, periungual desquamation, and nonsuppurative enlargement of the cervical lymph nodes are the main symptoms of KD (9). However, the most important complication of KD, which may cause serious cardiovascular sequelae, is coronary artery vasculitis (10). If left untreated, vasculitis may occur in one of every five children after a few days. Therefore, the early administration of intravenous immunoglobulin (IVIG) and aspirin should be done to minimize the risk of vasculitis (12). However, 13% to 21% of KD patients may be resistant to IVIG, and vasculitis can still be observed in 3% - 5% of treated children (13).
As the main step towards effective treatment is to target the disease etiology, the possible etiology of KD has been the focus of research for many years (14). Evidence suggests that KD is developed by the immunologic reaction in genetically susceptible hosts after exposure to environmental triggers (15). After the identification of genes associated with a significantly higher risk of KD and coronary artery aneurysm, like ITPKC (16), the Genome-wide Association studies (GWASs) introduced the association of single nucleotide polymorphisms (SNPs) with KD or coronary artery lesions (9, 17, 18). Among these six novel SNPs, rs2254546 located at 8p22-23 between FAM167A and B lymphoid tyrosine kinase (BLK) was first discovered by Onouchi et al. (19) in Japan and the USA and subsequently confirmed to be associated with the risk of KD in a Chinese population (19). However, these studies have not investigated the association of this genotype with the risk of aneurysm and resistance to IVIG (9, 19). Furthermore, to our knowledge, the significance of this genotype has not been addressed in the Iranian population (20).
2. Objectives
Therefore, this study aimed to investigate the genetic profile of an Iranian sample of children with KD and examine the frequency of 8p22-23-rs2254546 and its association with aneurysm and resistance to IVIG.
3. Methods
3.1. Study Design
The current prospective cross-sectional study was performed at Mofid Children’s Hospital, Tehran, Iran, affiliated with Shahid Beheshti University of Medical Sciences. This hospital is the main referral medical center for pediatric diseases. The Ethics Committee of the Mofid Children’s Hospital approved the study protocol, and the study was performed in line with the principles of the latest version of the Helsinki Declaration on human studies.
The study compared 100 children with KD as the sample group and 100 matched unrelated healthy Iranian children with no history of KD or immune-related disease. The control subjects were ethnically recruited from the same hospital at the time of a routine physical examination. All children who were diagnosed with typical KD based on the diagnostic criteria of the American Heart Association undergoing treatment at this center were considered as the study population. The study objectives and steps were explained to the parents of children who were referred from October 2015 to October 2016, and they were asked to read and sign informed consent if they were willing their child participates in the study. The patients were recruited in the study by a convenience sampling method until saturation of the sample size. Any patient who refused to complete the study was excluded from the study. The demographics of children, including sex and age, were recorded. The presence of an aneurysm was determined by two-dimensional echocardiography, performed by an expert specialist.
3.2. Genotyping and Quality Control
Two milliliters of the peripheral blood were taken from the cubitus area of patients, collected in EDTA tubes, and transferred in a cold box within 60 min to Gholhak laboratory. A Roche High Pure PCR template preparation kit was used for the extraction of genomic DNA from the blood sample, following the manufacturer’s protocol for the whole blood. A NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) was used to confirm the concentration and optical density (OD) of DNA. The OD levels > 1.7 - 2 were considered optimal for the PCR.
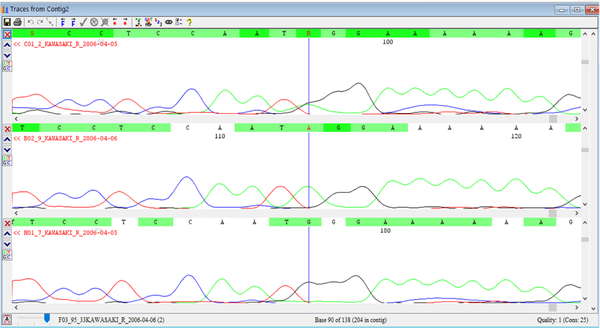
The 8p22-23-rs2254546 SNP genotype was determined by direct sequencing (Applied Biosystems, Foster City, CA, USA) as a gold standard method for the detection of nucleotide changes, which uses very specific primers, designed for the flanking region of this genotype (Figure 1).
3.3. Polymerase Chain Reaction and Sanger Sequencing
For genotype in grs 2254546, the PCR and Sanger sequencing were performed based on the method reported by Fong et al. (21).
3.4. Statistical Analysis
For descriptive reports, the mean ± standard deviation (SD) was reported for quantitative variables and frequency (percentage) for categorical variables. Among the three genotypes, between and within-group associations were calculated using the ANOVA test with testing P-value. For the statistical analysis, the IBM SPSS statistical software for Windows version 21.0 (IBM Corp. 2012. Armonk, NY: IBM Corp.) was used.
4. Results
Among 100 patients who completed the study, the frequency of the genotypes was as follows: GG: 62%, AG: 30%, and AA: 8%. Among 100 controls, GG was 31%, AG was 41%, and AA was 28% (Table 1). Considering resistance to IVIG, 21% of the studied patients were resistant. Of all patients, 19% had an aneurysm. Their mean age at diagnosis was 1.9 ± 1.7 years (3 - 105 months), including 57% boys and 43% girls. In the next step, the association of genotypes with IVIG resistance and aneurysm was tested by ANOVA, the results of which showed no significant association (Tables 2-5).
Association Between 8p22-23-rs2254546 and Kawasaki Disease Risk in the Current Study
| KD | Odds Ratio | Std. Err. | Z | P > z | 95% CI | |
|---|---|---|---|---|---|---|
| AG | 2.560976 | 1.196932 | 2.01 | 0.044 | 1.024652 | 6.400802 |
| GG | 7 | 3.200932 | 4.26 | 0.000 | 2.856695 | 17.15268 |
| -cons | 0.2857143 | 0.114541 | -3.12 | 0.002 | 0.130224 | 0.6268617 |
| KD | ||||||
| AG | 0.3658537 | 0.119176 | -3.09 | 0.002 | 0.193211 | 0.6927606 |
| AA | 0.1428572 | 0.065325 | -4.26 | 0.000 | 0.0583 | 0.3500548 |
| -cons | 2 | 0.439941 | 3.15 | 0.002 | 1.299543 | 3.078005 |
Intravenous Immunoglobulin Resistance in Kawasaki Disease Patients
| No. (%) | Valid Percent | ||
|---|---|---|---|
| Valid | Sensitive | 79 (79.0) | 79.0 |
| Resistant | 21 (21.0) | 21.0 | |
| Total | 100 (100.0) | 100.0 |
Aneurysm in Kawasaki Disease Patients
| No. (%) | Valid Percent | ||
|---|---|---|---|
| Valid | Normal | 81 (81.0) | 81.0 |
| Dilated | 19 (19.0) | 19.0 | |
| Total | 100 ()100.0 | 100.0 |
Association Between Genotypes and Intravenous Immunoglobulin Resistance (Analysis of Variance)a
| IVIGres | Genotype | |||
|---|---|---|---|---|
| 1 | 2 | 3 | Total | |
| 0 | 48 | 26 | 5 | 79 |
| 1 | 14 | 4 | 3 | 21 |
| Total | 62 | 30 | 8 | 100 |
| IVIGres | 0 | 1 | Total |
|---|---|---|---|
| 0 | 68 | 11 | 79 |
| 1 | 13 | 8 | 21 |
| Total | 81 | 19 | 100 |
Genotyping of 8p22-23-rs2254546 SNP
5. Discussion
Kawasaki disease also called mucocutaneous lymph node syndrome, is an acute, self-limited vasculitis that predominately affects infants and young children (9). The present study examined the frequency of three genotypes of 8p22-23-rs2254546 in 100 Iranian children with KD at a referral pediatric hospital. The results showed that only 8% had an AA genotype, while 62% had a GG genotype and 30% a GA genotype. These results are similar to those reported by Wang et al. (20). In their study, they investigated the genotype of 892 Chinese children (428 with KD and 478 healthy controls). In the case group, 63% were GG, 33% GA, and 4% AA, and in the control group, 60% were GG, 33% GA, and 7% AA (20). Among 100 controls, GG was in 31%, AG in 41%, and AA in 28% (Table 1). These results are similar to the results of the present study, although the frequency of patients with the AA genotype was higher in our study and closer to their control group, which can be justified by the racial differences in the frequency of different genotypes. Furthermore, they determined that the GG genotype was significantly associated with an increased risk of KD (20), which confirms the results of previous studies indicating the association of rs2254546 with KD (9, 21). We examined the association of GG and GA genotypes with two important conditions in KD, namely, the risk of aneurysm and resistance to IVIG.
Coronary artery aneurysm is an important complication of KD, as it results in adulthood cardiac sequelae (22). As the results of the present study indicated, 19% had an aneurysm. In an Iranian study on 159 children with KD, Moradinejad and Kiani (23) reported that 66.7% of the studied patients had an aneurysm, which is much higher than the frequency of patients with an aneurysm in our study. The low rate of patients with an aneurysm can be attributed to the low frequency of IVIG-resistant patients, as according to our results, 21% of the studied patients were resistant to IVIG, which is considered the main cause of aneurysm in treated patients (23). In the study by Tremoulet et al. (24) in San Diego County, 38.3% of patients with KD were IVIG-resistant, which is much higher than the frequency of IVIG-resistant patients in our study, and in the study by Dionne et al. (25), 24% of children were found to be IVIG-resistant, which is a little higher than the IVIG-resistance rate in our study (21%). This difference in the rates of resistance to IVIG can originate from different population characteristics, including age, serum levels of inflammatory markers, and liver enzymes, which have been suggested to affect the risk of resistance to IVIG (25). Although concurrent infections are associated with KD, the clinical presentations, prognosis, and even response to IVIG treatment have been reported to be the same as in non-confirmed infectious patients (26).
As vasculitis can develop few days after the onset of KD, it is important to start treatment as soon as possible in diagnosed cases (11); however, 15% - 25% of patients are suggested to be resistant to IVIG (27). The rate of resistance to IVIG in our study was also within the proposed range, although other Iranian studies have reported lower rates. In a study on 64 patients in Shiraz, Iran, Kashef et al. (28) reported that 9.4% of children remained febrile after the initial dose of IVIG, while in another study by Saffar and Reshidighader (29), 2/25 patients (8%) remained febrile after the initial dose and one (4%) after two doses. The frequency of resistance to IVIG in our study was higher than in the two mentioned Iranian studies (26, 28); nonetheless, the sample sizes of these studies were small, and further Iranian studies are required for their results to be comparable to ours. Due to the significance of IVIG resistance on the complications of KD, different studies have suggested different scoring systems to estimate the possibility of unresponsiveness to IVIG, such as Kobayashi, Egami, and Sano scores. Nevertheless, these scoring systems have a low sensitivity in non-Japanese populations (29), and the accuracy of these scoring systems has not been investigated in Iranians, and they are not routinely used.
The results of the present study indicated that aneurysm and resistance to IVIG were not associated with GG or GA genotypes of 8p22-23-rs2254546. The association of this gene with aneurysm and resistance to IVIG has not been previously studied for the results to be comparable to ours; however, according to the results of studies, the identified genes are not only genetically associated with the risk of KD but also are associated functionally. ITPKC, encoding a negative regulator of T cell activation (1,4,5-triphosphate 3-kinase C), has been associated with an increased risk of an aneurysm (15). In addition, LNX1, CAMK2D, ZFHX3, CSMD1, and TCP1 have also been shown to be related to inflammation, apoptosis, and cardiovascular pathology (16). A review of the articles has also determined that the gene loci, FCGR2A, BLK, CD40, and HLA, predict not only the risk of KD but also that of coronary artery lesions and resistance to IVIG (30). Accordingly, we hypothesized that 8p22-23-rs2254546 genotype would also be associated with an aneurysm; however, the results did not confirm our initial hypothesis.
The advantages of the present study included studying the 8p22-23-rs2254546 gene in an Iranian population for the first time, as well as studying its association with aneurysm and resistance to IVIG. But, this study may also have some limitations, including the fact that we had no control group to compare the results with and examine whether the gene is associated with KD risk. Furthermore, the inclusion of patients into the study was not random, and all patients were selected from consecutive patients of one center; therefore, the results may not be generalized to the entire Iranian children with KD.
5.1. Conclusions
Kawasaki disease is an important disease-causing vasculitis and cardiac sequelae. One important issue in KD is genetic susceptibilities, identified in different populations, including Japanese and Chinese populations. Meanwhile, the susceptibility gene loci have not been investigated in Iranian children with KD. In this study, we identified that the majority of the patients with KD had GG or GA genotypes of 8p22-23-rs2254546, and the frequency of aneurysm and resistance to IVIG was 19% and 21%, respectively. In addition, we examined the association of this genotype with aneurysm and resistance to IVIG. However, the identified genotype in our study was associated neither with an aneurysm nor with resistance to IVIG. Further case-control studies are required to add to the results of this study.
Acknowledgements
References
-
1.
Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016;67(14):1738-49. [PubMed ID: 27056781]. https://doi.org/10.1016/j.jacc.2015.12.073.
-
2.
Makino N, Nakamura Y, Yashiro M, Ae R, Tsuboi S, Aoyama Y, et al. Descriptive epidemiology of Kawasaki disease in Japan, 2011-2012: from the results of the 22nd nationwide survey. J Epidemiol. 2015;25(3):239-45. [PubMed ID: 25716368]. [PubMed Central ID: PMC4341001]. https://doi.org/10.2188/jea.JE20140089.
-
3.
Uehara R, Belay ED. Epidemiology of Kawasaki disease in Asia, Europe, and the United States. J Epidemiol. 2012;22(2):79-85. [PubMed ID: 22307434]. [PubMed Central ID: PMC3798585]. https://doi.org/10.2188/jea.je20110131.
-
4.
Holman RC, Christensen KY, Belay ED, Steiner CA, Effler PV, Miyamura J, et al. Racial/ethnic differences in the incidence of Kawasaki syndrome among children in Hawaii. Hawaii Med J. 2010;69(8):194-7. [PubMed ID: 20845285]. [PubMed Central ID: PMC3118023].
-
5.
Nakamura Y, Yashiro M, Uehara R, Sadakane A, Tsuboi S, Aoyama Y, et al. Epidemiologic features of Kawasaki disease in Japan: results of the 2009-2010 nationwide survey. J Epidemiol. 2012;22(3):216-21. [PubMed ID: 22447211]. [PubMed Central ID: PMC3798622]. https://doi.org/10.2188/jea.je20110126.
-
6.
Park YW, Han JW, Hong YM, Ma JS, Cha SH, Kwon TC, et al. Epidemiological features of Kawasaki disease in Korea, 2006-2008. Pediatr Int. 2011;53(1):36-9. [PubMed ID: 20534021]. https://doi.org/10.1111/j.1442-200X.2010.03178.x.
-
7.
Kawasaki T. [Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children]. Arerugi. 1967;16(3):178-222. Japanese. [PubMed ID: 6062087].
-
8.
Rowley AH, Shulman ST. Pathogenesis and management of Kawasaki disease. Expert Rev Anti Infect Ther. 2010;8(2):197-203. [PubMed ID: 20109049]. [PubMed Central ID: PMC2845298]. https://doi.org/10.1586/eri.09.109.
-
9.
Shiari R. Kawasaki Disease; A Review Article. Arch Pediatr Infect Dis. 2013;2(3):154-9. https://doi.org/10.5812/pedinfect.11628.
-
10.
Cox JR, Sallis RE. Recognition of kawasaki disease. Perm J. 2009;13(1):57-61. [PubMed ID: 21373247]. [PubMed Central ID: PMC3034467]. https://doi.org/10.7812/tpp/08-042.
-
11.
Tsuda E, Matsuo M, Naito H, Noguchi T, Nonogi H, Echigo S. Clinical features in adults with coronary arterial lesions caused by presumed Kawasaki disease. Cardiol Young. 2007;17(1):84-9. [PubMed ID: 17244380]. https://doi.org/10.1017/S1047951107000169.
-
12.
Bal AK, Prasad D, Umali Pamintuan MA, Mammen-Prasad E, Petrova A. Timing of intravenous immunoglobulin treatment and risk of coronary artery abnormalities in children with Kawasaki disease. Pediatr Neonatol. 2014;55(5):387-92. [PubMed ID: 24636168]. https://doi.org/10.1016/j.pedneo.2013.11.007.
-
13.
Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831-835 e3. [PubMed ID: 21168857]. [PubMed Central ID: PMC3075321]. https://doi.org/10.1016/j.jpeds.2010.10.031.
-
14.
Takahashi K, Oharaseki T, Yokouchi Y. Pathogenesis of Kawasaki disease. Clin Exp Immunol. 2011;164 Suppl 1:20-2. [PubMed ID: 21447126]. [PubMed Central ID: PMC3095860]. https://doi.org/10.1111/j.1365-2249.2011.04361.x.
-
15.
Rowley AH. Kawasaki disease: novel insights into etiology and genetic susceptibility. Annu Rev Med. 2011;62:69-77. [PubMed ID: 20690826]. [PubMed Central ID: PMC3021097]. https://doi.org/10.1146/annurev-med-042409-151944.
-
16.
Onouchi Y, Gunji T, Burns JC, Shimizu C, Newburger JW, Yashiro M, et al. ITPKC functional polymorphism associated with Kawasaki disease susceptibility and formation of coronary artery aneurysms. Nat Genet. 2008;40(1):35-42. [PubMed ID: 18084290]. [PubMed Central ID: PMC2876982]. https://doi.org/10.1038/ng.2007.59.
-
17.
Kim JJ, Hong YM, Sohn S, Jang GY, Ha KS, Yun SW, et al. A genome-wide association analysis reveals 1p31 and 2p13.3 as susceptibility loci for Kawasaki disease. Hum Genet. 2011;129(5):487-95. [PubMed ID: 21221998]. https://doi.org/10.1007/s00439-010-0937-x.
-
18.
Kim KY, Bae YS, Ji W, Shin D, Kim HS, Kim DS. ITPKC and SLC11A1 Gene Polymorphisms and Gene-Gene Interactions in Korean Patients with Kawasaki Disease. Yonsei Med J. 2018;59(1):119-27. [PubMed ID: 29214786]. [PubMed Central ID: PMC5725348]. https://doi.org/10.3349/ymj.2018.59.1.119.
-
19.
Onouchi Y, Ozaki K, Burns JC, Shimizu C, Terai M, Hamada H, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012;44(5):517-21. [PubMed ID: 22446962]. https://doi.org/10.1038/ng.2220.
-
20.
Wang W, Lou J, Lu XZ, Qi YQ, Shen N, Zhong R, et al. 8p22-23-rs2254546 as a susceptibility locus for Kawasaki disease: a case-control study and a meta-analysis. Sci Rep. 2014;4:4247. [PubMed ID: 24577620]. [PubMed Central ID: PMC3937782]. https://doi.org/10.1038/srep04247.
-
21.
Fong WY, Ho CC, Poon WT. Comparison of Direct Sequencing, Real-Time PCR-High Resolution Melt (PCR-HRM) and PCR-Restriction Fragment Length Polymorphism (PCR-RFLP) Analysis for Genotyping of Common Thiopurine Intolerant Variant Alleles NUDT15 c.415C>T and TPMT c.719A>G (TPMT*3C). Diagnostics (Basel). 2017;7(2). [PubMed ID: 28498350]. [PubMed Central ID: PMC5489947]. https://doi.org/10.3390/diagnostics7020027.
-
22.
Yan Y, Ma Y, Liu Y, Hu H, Shen Y, Zhang S, et al. Combined analysis of genome-wide-linked susceptibility loci to Kawasaki disease in Han Chinese. Hum Genet. 2013;132(6):669-80. [PubMed ID: 23456091]. https://doi.org/10.1007/s00439-013-1279-2.
-
23.
Moradinezhad MH, Kiani A. Kawasaki disease in 159 Iranian children. Iran J Pediatr. 2007;17(3):241-6.
-
24.
Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117-21. [PubMed ID: 18571548]. [PubMed Central ID: PMC2526555]. https://doi.org/10.1016/j.jpeds.2007.12.021.
-
25.
Dionne A, Le CK, Poupart S, Autmizguine J, Meloche-Dumas L, Turgeon J, et al. Profile of resistance to IVIG treatment in patients with Kawasaki disease and concomitant infection. PLoS One. 2018;13(10). e0206001. [PubMed ID: 30332473]. [PubMed Central ID: PMC6192641]. https://doi.org/10.1371/journal.pone.0206001.
-
26.
Navaeifar MR, Rezai MS. Intravenous immunoglobulin resistant Kawasaki disease. J Pediatr Rev. 2013;1(1):44-52.
-
27.
Baek JY, Song MS. Meta-analysis of factors predicting resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. Korean J Pediatr. 2016;59(2):80-90. [PubMed ID: 26958067]. [PubMed Central ID: PMC4781736]. https://doi.org/10.3345/kjp.2016.59.2.80.
-
28.
Kashef S, Safari M, Amin R. Initial intravenous gamma-globulin treatment failure in Iranian children with Kawasaki disease. Kaohsiung J Med Sci. 2005;21(9):401-4. [PubMed ID: 16248123]. https://doi.org/10.1016/S1607-551X(09)70141-X.
-
29.
Saffar MJ, Reshidighader F. Kawasaki disease in East Mazandaran, Islamic Republic of Iran, 1997-2002. EMHJ-East Mediterr Health J. 2005;11(1-2):28-35.
-
30.
Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99(1):74-83. [PubMed ID: 24162006]. [PubMed Central ID: PMC3888612]. https://doi.org/10.1136/archdischild-2012-302841.

