Abstract
Background:
Pneumonia presents high oxidative stress, which directly affects the lung injury and oxygenation status. Evidence has shown the correlation of oxidative stress markers in tracheal aspirates (TA) in infant patients, however, not before or after chest physical therapy (CPT).Objectives:
The objective of this study was to evaluate the correlation between lung injury score (LIS) or PvO2/FiO2 ratio and oxidative stress parameters in TA before and after CPT in infant patients during recovery from pneumonia.Methods:
TA samples from 40 intubated patients aged 5.4 ± 0.15 months were collected before and after CPT for evaluating oxidative stress parameters; as a glutathione (GSH), vitamin E, hyarulonic acid (HA), and malondialdehyde (MDA). Furthermore, the LIS and PvO2/FiO2 ratio were recorded. The correlation between oxidative stress markers and LIS or PvO2/FiO2 ratio was evaluated before and after CPT.Results:
The results before CPT showed no significant correlation between LIS and all parameters, whereas, the PvO2/FiO2 ratio correlated with the thiol group (r = -0.566, P = 0.000) and HA (r = -0.507, P = 0.000). After CPT, LIS correlated with GSH and HA (r = -0.396 and -0.409, P = 0.01) and the PvO2/FiO2 ratio correlated significantly with the GSH, HA, and MDA (r = 0.609, 0.768, -0.482, P = 0.000).Conclusions:
Some oxidative stress markers, such as the GSH, HA, and MDA in TA possibly reflected lung injury and oxygenation and may respond with more correlation after CPT intervention than before it.Keywords
1. Background
Evidence from previous reviews has suggested that clinical chest physical therapy (CPT) is used widely in the treatment of post-pneumonia to eliminate inflammatory exudate or secretion and prevent lung atelectasis, as well as reduce airway resistance, enhance gas exchange, and reduce breathing effort (1-3). Basic CPT techniques such as manual percussion, vibration on the chest wall, and postural drainage (PD) are performed commonly (4-6). Previous reviews evaluated the efficiency of CPT by duration of hospital stay, time to clinical resolution, lung sounds, chest X-ray, and cough frequency. However, whether these evaluations benefit cases of pneumonia is still controversial (6). Although some studies reported that the length of hospital stay, oxygen requirements, and the severity of clinical score in infants with acute bronchiolitis did not changed from CPT (7), an updated study in 2015 showed that CPT increased clinical resolution and reduced respiratory rate, including significantly increased arterial oxygen saturation in 15 infant patients with pneumonia (8). Therefore, the benefits of CPT in physiological parameters are controversial.
The pathology of pneumonia in infants or neonatal patients, who were treated with prolonged intubation via a mechanical ventilator, presented recurrent pneumonia (ventilator-associated pneumonia; VAP) (9). Infection induces oxidative stress in the lung, therefore, free and non-free radicals, such as hypochlorous acid, hydrogen peroxide, superoxide, and hydroxyl radicals are released dominantly in the alveolar area (10, 11) and can cause damage from the oxidative process to lipid, protein, and DNA, including extra-cellular matrixes such as glycosaminoglycan (GAG) and hyaluronic acid (HA) (12-14). Therefore, end products such as malondialdehyde (MDA) or 4-hydroxynonenal (4-NHE) can be detected in tracheal aspirates (TA) or bronchoalvelar lavage (BAL) (12, 15). Whereas, some antioxidant enzymes, such as superoxide dismutase (SOD), catalase, and glutathione peroxidase (GPX) or non-enzymes, e.g. glutathione (GSH) or alpha-tocopherol (Vitamin E), can regulate by scavenging or modifying propagated intermediate molecules (15), especially in epithelial lining fluid (ELF) (16). Vitamin E is a lipid-soluble antioxidant that can also scavenge peroxyl radicals or inhibit lipid peroxide as an MDA product (17, 18). Therefore, higher oxidative stress in the alveolar space within the lung affects gas exchange and oxygen diffusion. Clinical investigation basically evaluated lung function by checking the PaO2/FiO2 ratio, which has been used clinically to predict severity of lung injury (19). An interesting problem for infant patients after pneumonia is recurrent infection from retained secretion and inability to remove secretion by coughing effectively (20). Interesting results from a previous study of 22 mechanically ventilated patients showed a positive correlation between ventilation-perfusion mismatch and deoxygenation in patients with much lung secretion (21). This possibly supported the benefits of clear secretion and may help improve oxygenation by CPT (22). In particular, the changes and correlation of PvO2/FiO2 ratio oxygenation index or lung injury score on any oxidative stress parameters either before or after CPT have never been presented before. Therefore, the aim of this study was to make those present.
2. Objectives
The objective was to evaluate the correlation between all oxidative stress parameters in tracheal aspirate samples and the LIS or PvO2/FiO2 ratio either before or after chest physical therapy.
3. Methods
3.1. Study design and Subjects
The protocol in this study was approved by the research ethics committee at the faculty of Medicine, Chiang Mai University (ethical number 57/2000), Thailand. The pediatric patients were consulted routinely in physical therapy through their parents by physicians at the Intensive Care Unit at the department of pediatric, Maharhaj Nakorn Chiang Mai hospital, Chiang Mai province, Thailand. Forty cases were included in the program after being diagnosed with pneumonia and completing antibiotic drug administration with no fever and no complications in the liver or renal function. They were in need of CPT by a special pediatric physician after chest X-ray had identified secretion infiltration and/or atelectasis. Patients with severe anemia and a very low platelet count or unstable clinical condition from any laboratory results were excluded. All subjects gave permission through their parents, who signed the consent form. Of the 40 infant patients, a nurse diluted a secretion in 19 of them by 10 minutes of aerosol treatment with normal saline solution (NSS) (0.9%) in a metered dose inhaler (MDI) via an endotracheal tube before CPT. Clinical CPT was performed continuously 3 times daily for 6 days. A pooled TA sample was collected on, before, and after 6 days of CPT for evaluating oxidative stress parameters such as glutathione (GSH), malondialdehyde (MDA), vitamin E, as well as hyaluronic acid (HA), and the lung injury score (LIS) and PvO2/FiO2 ratio were calculated from the capillary blood gas (CBG) results.
3.2. Chest Physical Therapy Program and Sample Collection
Standardized chest physical therapy (CPT) with postural drainage (PD), percussion, and vibration by hands before suction was performed under the clinical guideline of the American association for respiratory care (AARC) (5, 23). All programs were treated by a specialized chest physical therapist that had more than 15 years’ of experience. Modified PD with head bent down 30 degrees and leaning forward on a pillow was performed for both sides of the body before 5 minutes of percussion (80 - 100 time per minute) (24) as well as vibration on the chest wall. TA samples were collected before or after CPT from the bronchial or tracheal airway with a negative pressure suctor at -100 cmH2O and kept in a sterile mucus extractor #6 (Endomed Company Limited, USA) at -20°C before analysis.
3.3. Evaluation of Oxidative Stress Parameters
3.3.1. Glutathione (GSH) Assay
Total sulfhydryl (TS) or thiol group of GSH in aspirated secretion or tracheal aspirate (TA) samples was determined by co-reaction with dithionitrobenzoic acid (DTNB) reagent following the Beutler protocol (25) with modifications from the protocol of Leelarungrayub (26). The 400 µL of TA was mixed with distilled water (1.6 mL) before protein precipitation with precipitating solution. The pellet was removed after precipitation and short centrifugation at 10,000 rpm. Furthermore, the pellet was removed and 1.0 mL of clear supernatant (0.5 mL) was mixed with phosphate solution (pH 8.0) (0.5 mL) before adding to the DTNB solution (0.5 mL). After incubation at room temperature for 5 minutes, the total thiol group or GSH was quantified by spectrophotometry at 412 nm, and compared with standard glutathione (GSH) (Sigma; St. Louis, MO).
3.3.2. Malondialdehyde (MDA) Assay
MDA in TA from lipid perioxidation was determined by the protocol of high performance liquid chromatography (HPLC) (27). The TA sample (200 µL) was added in ortho-phosphoric acid (2.5% v/v) (750 µL) and thiobarbituric acid (TBA) (0.2 mol/L) solution (200 µL). After heating for 30 minutes at 90°C, clear yellowed supernatant of MDA-TBA adduct (20 µL) was identified at 532 nm by a reverse-phase column (C18) with pure methanol (LabScan HPLC grade) as a mobile phase at 1.0 mL/min flow rate. The MDA concentration in TA was calculated by being compared with standard tetramethoxypropone (TMP) (Sigma, St. Louis).
3.3.3. Hyaluronic acid (HA) Assay
HA in TA was determined with ELISA-based techniques (28). The TA or standard competitor (Haelon) was added at 175 µL to an equal volume of biothylated HA binding protein (B-HABP) (1: 200) before incubating for 60 minutes at room temperature. Microtiter plates (Maxisorp NUNC®, Denmrk) were pre-coated with an umbilical cord HA (Sigma; St Louis, MO) (100 µg/mL) and blocked with a Bovine serum albumin (BSA) solution (1%) (150 µL/well) in a phosphate-Tween buffer. After the plates were washed with PBS-Tween buffer (150 µL), a peroxidase-mouse monoclonal anti-biotin solution (Zymed Laboratories. INC, CA) (100 µL/well) (1: 2000) was added to each well before incubating in a cool room at 25°C for 1 hour. Then the plates were washed with PBS-Tween buffer and the peroxidase solution (100 µL) as well as o-phenylebdiamine (OPD) substrate (1.2 mg/mL) (50 µL) (pH 5.5) and H2O2 (5 µL) were added. The H2SO4 (4 mol/L) (50 µL) was added finally to stop the reaction within 10 minutes after incubation at 37°C. The HA concentration was calculated from absorbance by reading a microtiter plate reader (Multiskan®MCC/340, Northland) at 492/690 nm and comparing the absorbance of standard HA.
3.3.4. α-tocopherol (Vitamin E) Assay
Vitamin E in TA was determined by the HPLC method (29). Tocopherol acetate (Sigma. St. Louis, MO) (10 mg/L) (100 µL) was added in the TA sample (200 µL). Total vitamin E was extracted with pure hexane by shaking and separating in order to evaporate by short high speed centrifugation (30°C). Total vitamin content was re-dissolved in absolute ethanol (200 µL) and filtrated through a 0.45 µm of pre-cut membrane Nylon PTFE before injection into HPLC system. The peak of total alpha-tocopherol was identified by C-18 reverse-phase column and Conta Meric LDL Analyzer at 294 nm using a (7%; v/v) dichloromethane (Labscan, Island) mobile phase at a flow rate of 1.0 mL/min. Alpha-tocopherol concentrations in TA were calculated by comparing with the peak and area curve of standard alpha-tocopherol acetate (Sigma, St. Louis).
3.4. Lung Injury Score (LIS) and PvO2/FiO2 ratio Evaluation
The lung injury score (LIS) was performed following a previous Murry protocol (19) and calculated from 4 clinical components; (1) chest radiograph score (no alveolar consolidation to alveolar consolidation in all 4 quadrants = 0 - 4), (2) hypoxemia score (PaO2/FiO2 > 300 to < 100 = 0 - 4), (3) PEEP score (< 5 to > 15 cmH2O = 0 - 4), and/or (4) static compliance of the respiratory system (> 80 to < 19 mL/cmH2O = 0 - 4). The final LIS was calculated by dividing the aggregate sum by the number of all components. The PvO2/FiO2 ratio was calculated from the FiO2 (inspired oxygen concentration on ventilator setting) as recorded by a physician, with the PvO2 such as oxygen tension in capillary or venous blood being analyzed by a Gas Analyzer.
3.5. Statistical Analysis
The Kolmogorov-Sirmov test was used to analyze normal data distribution of all outcomes before presenting the data with mean and standard deviation (SD). The Pearson correlation test was applied for checking the correlation and p values of all 40 samples and all parameters before CPT on day 1 or after 6 days of CPT. The statistical package for social science (SPSS) for Windows (version 10.0) was used for the statistical tests with a P value of less than 0.05.
4. Results
Forty infant patients required clinical physical therapy by pediatric physicians to remove secretion and re-expand collapsed lungs after recovery from pneumonia. Nineteen of them needed aerosol treatment due to thickened secretion before CPT. The mean age of all 40 infant patients was 5.4 ± 0.15 months. The results in Table 1 show the medical diagnosis and problems acquired of all patients with pneumonia and respiratory distress syndrome (RDS) (n = 5) as well as bronchopulmonary dysplasia (BPD) (n = 21). The criteria for receiving CPT for general infiltrated secretion and lung atelectasis in the right upper (n = 11), right lower (n = 2), and left upper lung (n = 3) are presented in Table 1. After the Kolmogorov-Smirnov test was used to pre-screen all variables for normal distribution, non-significant results were presented (P > 0.05). Therefore, the mean and standard deviation (SD) are documented in Table 2.
Characteristics of 40 Infant Patients Who Received CPT (N = 21) and Aerosol Treatment Before CPT (n = 19)
| Variables | CPT | Aerosol-CPT | Total |
|---|---|---|---|
| Sex (M/F) | 14/6 ( n = 21) | 12/8 ( n = 19) | 26/14 (n = 40) |
| Age, mo | 5.3 ± 0.62 (2 - 12) | 5.5 ± 0.65 (1 - 11) | 5.4 ± 0.15 (1 - 12) |
| Diseases and Co-morbid diseases | |||
| Pneumonia | 21 | 19 | 40 |
| Respiratory distress syndrome (RDS) | 2 | 3 | 5 |
| Bronchopulmonary dysplasia (BPD) | 11 | 10 | 21 |
| Secretion and Atelectasis | |||
| General infiltration | 21 | 19 | 40 |
| Right upper/lower lung atelectasis | 5/0 | 6/2 | 11/2 |
| Left upper /lower lung atelectasis | 1/0 | 2/0 | 3/0 |
LIS, PvO2/FiO2 ratio and Oxidative Stress Parameters in TA Before and After 6 Days of CPT in 40 Infant Patientsa
| Parameters | Before | After |
|---|---|---|
| LIS | 1.09 ± 0.19, (0.9 - 1.5) | 1.00 ± 0.09, (0.8 - 1.4) |
| PvO2/FiO2 ratio | 129.77 ± 10.65, (110 - 152) | 162.85 ± 16.46, (145 - 195) |
| - FiO2 | 0.40 ± 0.07, (0.25 - 0.55) | 0.41 ± 0.06, (0.30 - 0.55) |
| - PvO2 | 52.86 ± 11.00, (27.5 - 72.0) | 66.70 ± 11.56, (43.0 - 85.0) |
| Thiol group, mg/dL | 2.61 ± 0.53, (1.8 - 3.4) | 5.26 ± 0.98, (3.7 - 7.5) |
| Vitamin E, mg/dL | 20.45 ± 2.86, (15 - 28) | 27.72 ± 2.87, (24 - 39) |
| HA, x102 ng/mL | 711.72 ± 110.14, (459 - 995) | 364.17 ± 200.34, (125 - 825) |
| TBARs - MDA, µmol/L | 31.40 ± 1.64, (28 - 36) | 29.37 ± 2.41, (25 - 34) |
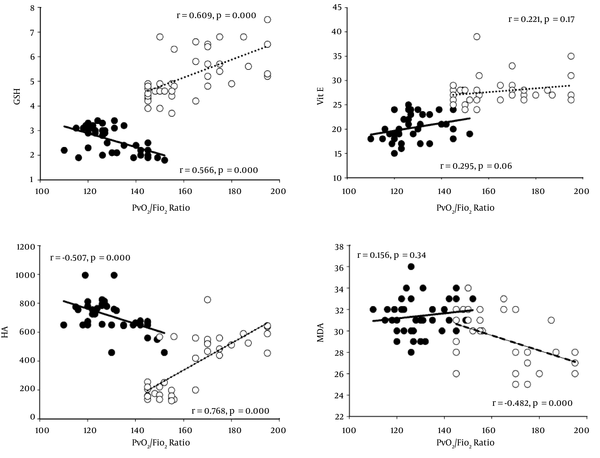
Results of the correlation between LIS or PvO2/FiO2 ratio and all oxidative stress markers are shown in Table 3 as well as Figures 1 and 2. CPT showed a negative correlation between LIS and HA (P < 0.01) before CPT, except for the thiol group, vitamin E, and MDA, whereas, the PvO2/FiO2 ratio had a significant correlation to the thiol group and HA. Vitamin E and MDA did not correlate with the PvO2/FiO2 ratio. Results after 6 days of CPT showed a significantly negative correlation between LIS and the thiol group (P < 0.05) and HA (P < 0.01). However, vitamin E and MDA did not correlate with LIS (P > 0.05), whereas, the PvO2/FiO2 ratio showed a positive correlation with the thiol group (P < 0.01) and HA (P < 0.01), negative correlation with MDA (P <0.01), and no correlation with vitamin E (P > 0.05).
Correlation Between all Oxidative Stress Markers and Lung Injury Score (LIS) or PvO2/FiO2 ratio in all 40 Pediatric Patients Before and After 6 Days of CPT
| Variables | Thiol Group | Vitamin E | HA | TBARs-MDA |
|---|---|---|---|---|
| Before CPT | ||||
| LIS | ||||
| Pearson correlation | -0.29 | 0.004 | -0.007 | - 0.124 |
| Sig. (2-tailed) | 0.86 | 0.98 | 0.68 | 0.45 |
| PvO2/FiO2 ratio | ||||
| Pearson correlation | -0.566a | 0.295 | -0.507a | 0.156 |
| Sig. (2-tailed) | 0.000 | 0.06 | 0.000 | 0.34 |
| After 6 days of CPT | ||||
| LIS | ||||
| Pearson correlation | -0.396b | 0.113 | -0.409a | 0.169 |
| Sig. (2-tailed) | 0.01 | 0.49 | 0.01 | 0.29 |
| PvO2/FiO2 ratio | ||||
| Pearson correlation | 0.609a | 0.221 | 0.768a | -0.482a |
| Sig. (2-tailed) | 0.000 | 0.17 | 0.000 | 0.000 |
Correlation Between Lung Injury Score (LIS) and All Oxidative Stress Parameters; GSH, Vitamin E, HA, and MDA Before (Black Circles) and After 6 Days of CPT (White Circles) (N = 40). r = Pearson Correlation, and P = Significant Level

Correlation Between PvO2/FiO2 Ratio and All Oxidative Stress Parameters; GSH, Vitamin E, HA, and MDA Before (Black Circles) and After 6 Days of CPT (White Circles) (N = 40). r = Pearson Correlation, and P = Significant Level
5. Discussion
This study showed comparison between, before, and after CPT, however, it did not present the main interest of all correlations between oxidative stress markers and LIS or the PvO2/FiO2 ratio before or after CPT. However, some difference in correlations should be presented before and after CPT. Although the pediatric physicians requested a different protocol for 10 minutes of aerosol treatment with MDI before CPT, the aim of this study did not target different protocols. The goals of CPT in this study were to remove secretion and re-expand collapsed lungs in a way that was similar to previous CPT guidelines for resolving post pneumonia complications (1). The CPT program in this study used the standardized technique with adjustment to the patients’ position for draining secretion by postural drainage, manual percussion, and vibration that moved secretions from peripheral airways to larger bronchi before suction (5, 24). Overall, approximately 30 minutes of CPT were performed in each case with 5 - 10 minutes in each position, thus, as much secretion as possible was removed from the upper and lower lung in 6 days of CPT.
Although there is still controversial evidence of CPT in pneumonia cases, such as no reduced length of hospital stay, oxygen requirement, or security of clinical score (7), an updated study reported that arterial oxygen saturation increased after CPT in 15 patients with pneumonia (8). Calculation of LIS and the PaO2/FiO2 ratio were followed in previous studies (19, 30). Unfortunately, arterial oxygen (PaO2) in neonatal patients could not be evaluated, thus mixed oxygen from suitable vein (PvO2) was assayed clinically in this study with an instant the PaO2/FiO2 ratio. Moreover, PvO2/FiO2 ratio was classified into a score of 3, if the ratio fell in the range of 100 - 174 (19). Table 2 shows the mean of the PvO2/FiO2 ratio within a score range either before (129.77 ± 10.65) or after CPT (162.85 ± 16.46), which did not change the LIS score. Therefore, less secretion and better ventilation in the lung of these patients possibly related to the increased ventilation and oxygenation in pneumonia combined with either BPD or acute respiratory distress syndrome (ARDS) (31). However, the PvO2/FiO2 ratio in all of the patients was less than 200 indicating ARDS, as classified by the European consensus conference (AECC) in 1994 (32).
Interesting evidence showed that pneumonia relating to oxidative stress status still provokes the case of retained secretion and recurrent pneumonia dominantly in preterm pediatric patients with chronic lung disease (33) as in the case of BPD (34). Confirmed data in a previous study of 32 intubated preterm and newborn infants with respiratory distress syndrome (RDS) and admitted to a neonatal intensive care unit (NICU) showed a low total GSH level in TA when compared with 11 control children (35). The MDA level in alveolar fluid was significantly high from ventilator-associated pneumonia (VAP) (36). However, a previous report suggested that HA is the extracellular matrix within the lung, which is released possibly through oxidative stress (18), as in osteoarthritis (OA) and rheumatoid arthritis (RA). It also expressed tissue remolding, including that of the lung (37). Therefore, a high level of HA in TA can possibly be referred to either the degradative or adaptive processes. Whereas, a hydrophobic antioxidant vitamin E is located in the bilayer membrane and can be excreted in alveolar surfactant during oxidative stress propagation. Thus, GSH and vitamin E are dominant antioxidant compounds in lung epithelial fluid that can inhibit lipid peroxidation by trapping the lipid peroxyl radical (LOO) and alpha-tocopheroxyl radical (α-TO) (38). Therefore, the above evidence shows that GSH, vitamin E, MDA and HA are possibly interesting parameters, presenting the oxidative stress in TA samples.
This study found interesting correlative results between the LIS, PvO2/FiO2 ratio, and all parameters (Table 3). Different correlative data were shown either before CPT or after 6 days of it. There was no correlation between LIS and any oxidative stress parameters (P > 0.05), whereas, the PvO2/FiO2 ratio owed significantly negative correlation with the GSH (r= -0.566, P = 0.000) and HA (r = -0.507, P = 0.000). On the other hand, vitamin E and MDA levels showed no statistical correlation with LIS or PvO2/FiO2 ratio either before or after CPT.
LIS had a significantly negative correlation with the GSH (r = - 0.396, P = 0.01) and HA (r = -0.409, P = 0.01) after 6 days of CPT, however, the PvO2/FiO2 ratio showed a positive correlation with both (r = 0.609, P = 0.000 and r = 0.768, P = 0.000, respectively). Moreover, the PvO2/FiO2 ratio also presented a negative correlation with the MDA level in TA (r = - 0.482, P = 0.000).
This means that LIS possibly does not correlate with oxidative stress markers before CPT, whereas the PvO2/FiO2 ratio probably presents a correlation with the thiol group and HA in TA samples. The data also presented a correlation between the increased thiol group or HA levels in TA samples and a lower LIS score when CPT had cleared secretion. The higher thiol group and HA concentration and lower TBARs-MDA level in TA correlated interestingly with a higher PvO2/FiO2 ratio. In addition, the non-significant correlation of vitamin E in this study is still unclear and controversial. Increased GSH within alveolar fluid indicates improvement of antioxidant status for controlling pro-inflammatory processes in the lungs, important immune modulation, extracellular matrix remodeling, apoptosis, and mitochondrial respiration. During this study, all patients received only a bronchodilator, Berodual or Ventolin, and no antibiotics or dexamethasone treatment previously, thus the oxidative stress or inflammatory activity is possibly not involved.
5.1. Conclusion and Limitations
The results showed interesting correlative alternations in some markers relating to oxidative stress in TA samples, especially in the thiol group, HA, and MDA before CPT. High concentrations of the thiol group and HA levels as well as lower MDA level in TA reflect better oxygenation and less lung injury. Furthermore, these correlations, also presented after secretion, have been cleared by CPT, which possibly reduces oxidative stress in the lung. Therefore, these parameters are potentially useful for a follow up in the future. As the sample size was small in this study, a larger one with similar pathology is still needed for confirmation. The correlation between all oxidative stress parameter and LIS or PvO2/FiO2/ ratio in various pathological conditions from different bacterial lung infection is also very interesting for future studies.
Acknowledgements
References
-
1.
Balachandran A, Shivbalan S, Thangavelu S. Chest physiotherapy in pediatric practice. Indian Pediatr. 2005;42(6):559-68. [PubMed ID: 15995272].
-
2.
Wallis C, Prasad A. Who needs chest physiotherapy? Moving from anecdote to evidence. Arch Dis Child. 1999;80(4):393-7. [PubMed ID: 10086954]. https://doi.org/10.1136/adc.80.4.393.
-
3.
Spearman CB, Sheldon RL, Egan DF. Humidity and aerosol therapy. Egan's fundamentals of respiratory therapy. 4th ed. Princeton: Mosby; 1982. p. 335-75.
-
4.
Kirilloff LH, Owens GR, Rogers RM, Mazzocco MC. Does chest physical therapy work? Chest. 1985;88(3):436-44. [PubMed ID: 3896680]. https://doi.org/10.1378/chest.88.3.436.
-
5.
Strickland SL, Rubin BK, Drescher GS, Haas CF, O'Malley CA, Volsko TA, et al. AARC clinical practice guideline: effectiveness of nonpharmacologic airway clearance therapies in hospitalized patients. Respir Care. 2013;58(12):2187-93. [PubMed ID: 24222709]. https://doi.org/10.4187/respcare.02925.
-
6.
Chaves GS, Fregonezi GA, Dias FA, Ribeiro CT, Guerra RO, Freitas DA, et al. Chest physiotherapy for pneumonia in children. Cochrane Database Syst Rev. 2013;(9). CD010277. [PubMed ID: 24057988]. https://doi.org/10.1002/14651858.CD010277.pub2.
-
7.
Postiaux G, Zwaenepoel B, Louis J. Chest physical therapy in acute viral bronchiolitis: an updated review. Respir Care. 2013;58(9):1541-5. [PubMed ID: 23287014]. https://doi.org/10.4187/respcare.01890.
-
8.
Abdelbasset W, Elnegamy T. Effect of chest physical therapy on pediatrics hospitalized with Pneumonia. Int J Health Rehabil Sci. 2015;4(4):219. https://doi.org/10.5455/ijhrs.000000095.
-
9.
Koenig SM, Truwit JD. Ventilator-associated pneumonia: diagnosis, treatment, and prevention. Clin Microbiol Rev. 2006;19(4):637-57. [PubMed ID: 17041138]. https://doi.org/10.1128/CMR.00051-05.
-
10.
Nelson S, Mason CM, Kolls J, Summer WR. Pathophysiology of pneumonia. Clin Chest Med. 1995;16(1):1-12. [PubMed ID: 7768083].
-
11.
Schraufatatter UI, Cochrane GC. Oxidants; types, sources, and mechanisms of injury. In: Crystal GR, Barnes JP, editors. The lung. 2nd ed. New York: Lippincott-Roven; 1997. p. 2251-8.
-
12.
Halliwell B, Gutteridge JMC. Lipid peroxidation. Free radicals in biology and medicine. 2nd ed. Oxford Clarendon press; 1989. p. 260-3.
-
13.
Hallgren R, Samuelsson T, Laurent TC, Modig J. Accumulation of hyaluronan (hyaluronic acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139(3):682-7. [PubMed ID: 2923370]. https://doi.org/10.1164/ajrccm/139.3.682.
-
14.
Haslett C. Introduction--the paradox of inflammation. Semin Cell Biol. 1995;6(6):315-6. [PubMed ID: 8748138]. https://doi.org/10.1016/S1043-4682(05)80001-7.
-
15.
Frank L. Antioxidants, nutrition, and bronchopulmonary dysplasia. Clin Perinatol. 1992;19(3):541-62. [PubMed ID: 1526071].
-
16.
Cetinkale O, Belce A, Konukoglu D, Senyuva C, Gumustas MK, Tas T. Evaluation of lipid peroxidation and total antioxidant status in plasma of rats following thermal injury. Burns. 1997;23(2):114-6. [PubMed ID: 9177876]. https://doi.org/10.1016/S0305-4179(96)00084-8.
-
17.
Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. 4th ed. Oxford: Clarendon Press; 2006.
-
18.
Zimmerman JJ. Oxyradical pathophysiology. Adv Pediatr. 1995;42:243-302. [PubMed ID: 8540430].
-
19.
Murray JF, Matthay MA, Luce JM, Flick MR. An expanded definition of the adult respiratory distress syndrome. Am Rev Respir Dis. 1988;138(3):720-3. [PubMed ID: 3202424]. https://doi.org/10.1164/ajrccm/138.3.720.
-
20.
Spector TD, Axford JS. Introduction to general pathology. 4th ed. PA: Churchill Livingstone; 1999. p. 10-1.
-
21.
Connors AJ, Hammon WE, Martin RJ, Rogers RM. Chest physical therapy. The immediate effect on oxygenation in acutely ill patients. Chest. 1980;78(4):559-64. [PubMed ID: 7418480].
-
22.
Branson RD. Secretion management in the mechanically ventilated patient. Respir Care. 2007;52(10):1328-42. discussion 1342-7. [PubMed ID: 17894902].
-
23.
AARC (American Association for Respiratory Care) clinical practice guideline. Postural drainage therapy. Respir Care. 1991;36(12):1418-26. [PubMed ID: 10145593].
-
24.
Blazey S, Jenkins S, Smith R. Rate and force of application of manual chest percussion by physiotherapists. Aust J Physiother. 1998;44(4):257-64. [PubMed ID: 11676741]. https://doi.org/10.1016/S0004-9514(14)60385-8.
-
25.
Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882-8. [PubMed ID: 13967893].
-
26.
Leelarungrayub D, Saidee K, Pothongsunun P, Pratanaphon S, YanKai A, Bloomer RJ. Six weeks of aerobic dance exercise improves blood oxidative stress status and increases interleukin-2 in previously sedentary women. J Bodyw Mov Ther. 2011;15(3):355-62. [PubMed ID: 21665113]. https://doi.org/10.1016/j.jbmt.2010.03.006.
-
27.
Chirico S. High-performance liquid chromatography-based thiobarbituric acid tests. Methods Enzymol. 1994;233:314-8. [PubMed ID: 8015465]. https://doi.org/10.1016/S0076-6879(94)33035-2.
-
28.
Pothacharoen P, Teekachunhatean S, Louthrenoo W, Yingsung W, Ong-Chai S, Hardingham T, et al. Raised chondroitin sulfate epitopes and hyaluronan in serum from rheumatoid arthritis and osteoarthritis patients. Osteoarthritis Cartilage. 2006;14(3):299-301. [PubMed ID: 16309927]. https://doi.org/10.1016/j.joca.2005.10.005.
-
29.
Shearer MN. Vitamins. In: Lim CK, editor. Hplc of small molecules. Oxford: IRL press; 1986. p. 175-9.
-
30.
Kangelaris KN, Calfee CS, May AK, Zhuo H, Matthay MA, Ware LB. Is there still a role for the lung injury score in the era of the Berlin definition ARDS? Ann Intensive Care. 2014;4(1):4. [PubMed ID: 24533450]. https://doi.org/10.1186/2110-5820-4-4.
-
31.
Mahmood NA, Chaudry FA, Azam H, Ali MI, Khan MA. Frequency of hypoxic events in patients on a mechanical ventilator. Int J Crit Illn Inj Sci. 2013;3(2):124-9. [PubMed ID: 23961457]. https://doi.org/10.4103/2229-5151.114272.
-
32.
Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818-24. [PubMed ID: 7509706]. https://doi.org/10.1164/ajrccm.149.3.7509706.
-
33.
Moison RM, de Beaufort AJ, Haasnoot AA, Dubbelman TM, van Zoeren-Grobben D, Berger HM. Uric acid and ascorbic acid redox ratios in plasma and tracheal aspirate of preterm babies with acute and chronic lung disease. Free Radic Biol Med. 1997;23(2):226-34. [PubMed ID: 9199884]. https://doi.org/10.1016/S0891-5849(97)00033-6.
-
34.
Harwood DT, Darlow BA, Cheah FC, McNeill N, Graham P, Winterbourn CC. Biomarkers of neutrophil-mediated glutathione and protein oxidation in tracheal aspirates from preterm infants: association with bacterial infection. Pediatr Res. 2011;69(1):28-33. [PubMed ID: 20924318]. https://doi.org/10.1203/PDR.0b013e3181ff2378.
-
35.
Boda D, Nemeth I, Pinter S. Surface tension, glutathione content and redox ratio of the tracheal aspirate fluid of premature infants with IRDS. Biol Neonate. 1998;74(4):281-8. [PubMed ID: 9701650]. https://doi.org/10.1159/000014035.
-
36.
Duflo F, Debon R, Goudable J, Chassard D, Allaouchiche B. Alveolar and serum oxidative stress in ventilator-associated pneumonia. Br J Anaesth. 2002;89(2):231-6. [PubMed ID: 12378658]. https://doi.org/10.1093/bja/aef169.
-
37.
Presti D, Scott JE. Hyaluronan-mediated protective effect against cell damage caused by enzymatically produced hydroxyl (OH.) radicals is dependent on hyaluronan molecular mass. Cell Biochem Funct. 1994;12(4):281-8. [PubMed ID: 7834818]. https://doi.org/10.1002/cbf.290120409.
-
38.
Kolleck I, Sinha P, Rustow B. Vitamin E as an antioxidant of the lung: mechanisms of vitamin E delivery to alveolar type II cells. Am J Respir Crit Care Med. 2002;166(12 Pt 2):S62-6. [PubMed ID: 12471091]. https://doi.org/10.1164/rccm.2206019.