Abstract
Background:
Tuberculosis (TB) is a main health problem worldwide. Despite the high incidence of TB in adolescents, studies mainly focus on the risk factors of TB in adults.Objectives:
The current study aimed at comparing the demographic, clinical, and microbiological characteristics of extrapulmonary TB (EPTB) and pulmonary TB (PTB) in adolescents.Methods:
The current retrospective study compared 30 EPTB and 113 PTB cases, aged 10 to 18 years, admitted to Masih Daneshvari Medical center, Tehran, Iran, from March 2006 to March 2011.Results:
The mean age of the patients with PTB and EPTB were 15.4 ± 2.3 and 16.1 ± 1.7 years, respectively. Sixteen (53%) and 74 (65.5%) of the patients with EPTB and PTB, respectively, were female. Multivariate logistic regression analysis showed that contact with adult patients with TB (odds ratios (OR): 0.07; 95% confidence intervals (CI): 0.009 - 0.58) and smear positivity (OR: 0.062; 95% CI: 0.005 - 0.80) were associated with PTB, while having a fever (OR: 21.49; 95% CI: 1.35 - 339.96) was associated with EPTB.Conclusions:
The current study findings in adolescent patients confirmed the quiet onset of EPTB with a lower rate of bacteriologic diagnosis and source detection rate. New strategies are required to improve the early diagnosis and prevention of EPTB in adolescents.Keywords
1. Background
Tuberculosis (TB) is a major health problem worldwide. In 2014, about 9.6 million people were identified with TB of whom 1 million were children (1). The incidence of TB in Iranian population was estimated 21 cases per 100,000 in 2012 (2). The peak of TB incidence in adolescents showed an increase in a recent study in South Africa (3). Adolescents are a recommended target group for new TB vaccines as part of the strategy to control TB (4). Recently, higher risk for TB infection was identified in adolescents, compared with children (5-7). In the literature, few studies focused on TB in adolescents (8). The clinical features of TB are different; although other organs can be affected, lung is the most complicated organ (9). Differences in the risk factors of extrapulmonary TB (EPTB) and pulmonary TB (PTB) are associated with gender, age, underlying diseases, and geographical region. Recently, the proportion of EPTB increased, compared to PTB. For example, EPTB cases increased from 16% to 21%, while PTB cases decreased from 84% to 79% from 1993 to 2013 in the US (10). Additionally, the EPTB rate increased from 16.4% in 2002 to 22.4% in 2011 in Europe (11). Moreover, few studies compared the characteristics of EPTB and PTB (9, 12, 13).
2. Objectives
The current study aimed at comparing the demographic, clinical, and microbiological characteristics of EPTB and PTB in adolescents.
3. Methods
The current retrospective study reviewed 143 adolescents, aged 10 to 18 years, including 113 patients with PTB and 30 with EPTB admitted to the national research institute of tuberculosis and lung disease (NRITLD) in Masih Daneshvari Medical center, Tehran, Iran, from March 2006 to March 2011 with a confirmed diagnosis of TB and extracted the required information. TB was defined as the presence of chronic symptoms (fever, chronic cough, weight-loss, and/or failure to thrive) in relationship with the radiological manifestations (hilar lymphadenopathy and/or consolidation), or histological appearance of biopsy material representing TB-affected tissue (caseous necrosis or granulomatous tubercles), or isolation of Mycobacterium tuberculosis from the sputum, gastric lavage, body secretions, or surgical specimens. The following data were analyzed in the current study: demographic data including age, gender, and nationality, presented symptoms, duration of symptoms, history of TB, bacteriological results, tuberculin skin test (TST), treatment, and drug susceptibility results. For the analysis, patients were divided into 2 groups of EPTB and PTB. EPTB was defined as TB in sites other than the lungs and PTB included patients who only had TB in their lungs. Similar to the previous studies, the patients with coexistence of PTB and EPTB were included in the EPTB group (10, 14).
SPSS version 21 was used for data analysis. The Chi-square or the Fisher exact test was used to evaluate the level of significance. A P value < 0.05 was considered significant.
To recognize the risk factors of EPTB and PTB, all variables related to a level of significance < 0.20 in the univariate analyses were included in a logistic regression model for multivariate analysis (inter methods by likelihood ratio). Odds ratios (OR), 95% confidence intervals (95% CI), and P values were calculated for the possible risk factors.
4. Results
4.1. Demographic Data
Among the patients with EPTB, 16 (53%) were female and 14 (47%) male, and in the patients with PTB, 74 (65.5%) were female and 39 (34.5%) male. The difference between the groups was not statistically significant (P = 0.221) (Table 1).
| Variable | Patients with PTB (N = 113) | Patients with EPTB (N = 30) | P Value |
|---|---|---|---|
| Age, y, mean ± SD | 15.45 ± 2.3 | 16.1 ± 1.7 | 0.158 |
| Age group, y | 0.044 | ||
| 10 - 14 | 36 (32) | 4 (13) | |
| 15 - 18 | 77 (68) | 26 (87) | |
| Gender | 0.221 | ||
| Female | 74 (65.5) | 16 (53) | |
| Male | 39 (34.5) | 14 (47) | |
| Nationality | 0.86 | ||
| Iranian | 62 (55) | 17 (57) | |
| Afghan | 51 (45) | 13 (43) | |
| History of TB | 0.774 | ||
| Yes | 16 (14) | 4 (13.3) | |
| No | 76 (67) | 22 (73.4) | |
| Unknown | 21 (19) | 4 (13.3) | |
| Symptomatic | 0.584 | ||
| Yes | 108 (95.5) | 30 (100) | |
| No | 5 (5) | 0 |
The mean age of the patients with PTB and EPTB were 15.4 ± 2.3 and 16.1 ± 1.7 years, respectively; however, the difference was not statistically significant (P = 0.158).
4.2. Clinical Data
The distribution of symptoms in patients with EPTB and PTB are described in Table 2. The rate of cough was significantly higher in the PTB group (89%), compared with the EXPTB group (73%) (P = 0.024) (Table 2).
Comparison of the Symptoms Between the Adolescents with Pulmonary TB and the Ones with Extrapulmonary TBa
| Variable | Patients with PTB, (N = 113) | Patients with EPTB, (N = 30) | P Value |
|---|---|---|---|
| Cough | 0.024 | ||
| Yes | 101 (89) | 22 (73) | |
| No | 12 (11) | 8 (27) | |
| Fever | 0.054 | ||
| Yes | 78 (69) | 26 (87) | |
| No | 35 (31) | 4 (13) | |
| Loss of appetite | 0.183 | ||
| Yes | 53 (47) | 10 (33) | |
| No | 60 (53) | 20 (67) | |
| Night sweat | 0.489 | ||
| Yes | 57 (50) | 13 (43) | |
| No | 56 (50) | 17 (57) | |
| Weight-loss | 0.755 | ||
| Yes | 75 (66) | 19 (63) | |
| No | 38 (34) | 11 (37) | |
| Fatigue | 0.135 | ||
| Yes | 13 (11.5) | 7 (23) | |
| No | 100 (88.5) | 23 (77) | |
| Chest pain | 0.721 | ||
| Yes | 30 (26.5) | 7 (23) | |
| No | 83 (73.5) | 23 (77) | |
| Dyspnea | 0.424 | ||
| Yes | 40 (35) | 13 (43) | |
| No | 73 (65) | 17 (57) | |
| Hemoptysis | 0.077 | ||
| Yes | 20 (18) | 1 (3) | |
| No | 93 (82) | 29 (97) | |
| Lymphadenopathy | 0.606 | ||
| Yes | 4 (3.5) | 2 (6.7) | |
| No | 109 (96.5) | 28 (93.3) |
The mean intervals between onset of symptoms and diagnosis in adolescents with EPTB and PTB were 3.3 ± 3.4 and 3.2 ± 3.1 months, respectively (P = 0.827). History of pervious TB was recognized in 16 (14%) patients with PTB and 4 (13.3%) patients with EPTB (P = 0.774).
The source of infection was determined in 59 (52%) of the patients with PTB as parents (n = 25), grandparents (n = 8), siblings (n = 8), secondary relatives and neighbors (n = 13), and multiple contact (n = 5). In 9 (30%) patients of the EPTB group, the source of infection was determined as parents (n = 1), grandparents (n = 5), siblings (n = 2), and secondary relatives and neighbors (n = 1). The source detection rate in the PTB group (52%) was significantly higher than that of the EPTB group (30%) (P = 0.039).
There was no significant difference between PTB and EXPTB groups by TST ≥ 10 mm (P = 0.3).
Of the 56 patients tested for HIV; all were HIV negative except 2 patients with PTB (3.5%).
4.3. Microbiological Data
The comparison of positive smear, culture, and polymerase chain reaction (PCR) results between the 2 groups are presented in Table 3.
Histopathology was available in 21 patients. In 5 (24%) patients with PTB and 16 (76%) patients with EPTB, granulomatous inflammation was detected. In addition, caseous necrosis was identified in 4 (80%) patients with PTB and 11 (69%) patients with EPTB.
4.4. Sites of Extrapulmonary
The distribution of EPTB sites based on the age is described in Figure 1.
Age Distribution for Sites of Extrapulmonary Tuberculosis
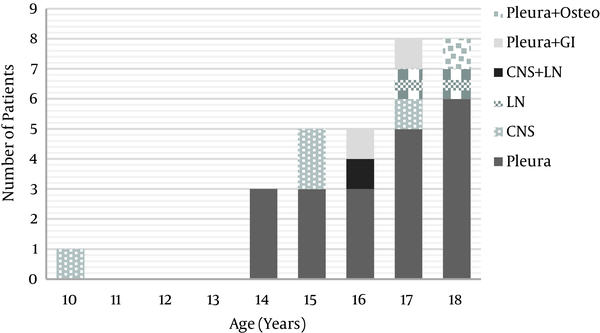
Among the patients with EPTB, 6 out of 22 (27%) with pleura TB and 2 out of 5 (40%) with central nervous system (CNS) TB had concomitant PTB. The other patients had isolated EPTB (Figure 1).
4.5. Multivariate Comparison of the Patients with EPTB and PTB
A multivariate logistic regression analysis model showed that contact with an adult patients with TB (OR 0.07, 95% CI 0.009 - 0.58, P = 0.014) and smear positivity (OR: 0.062, 95% CI: 0.005 - 0.80, P = 0.033) were more likely to be associated with PTB, whereas fever (OR: 21.49, 95% CI: 1.35 - 339.96, P = 0.029) was associated with EPTB (Table 4). The estimated OR of fever was 21.49. However, collinearity was observed between fever and cough variables (P < 0.001) that means this estimate is biased. The study also tested the multivariate logistic regression with only one of these variables and found considerable different OR in both models that approved collinearity. Almost all patients with hemoptysis were diagnosed as PTB (Table 2); therefore, hemoptysis variable could not be included into multivariate analysis due to high standard errors.
| Variable | Patients with PTB (N = 113), | Patients with EPTB (N = 30) | P Value |
|---|---|---|---|
| Contact source | 0.039 | ||
| Known | 59 (52) | 9 (30) | |
| Unknown | 54 (48) | 21 (70) | |
| TST, mm | 0.243 | ||
| < 10 | 23 (20) | 6 (20) | |
| ≥ 10 | 28 (25) | 4 (13) | |
| Unknown | 62 (55) | 20 (67) | |
| Smear | < 0.001 | ||
| Positive | 88 (78) | 8 (27) | |
| Negative | 25 (22) | 21 (70) | |
| Unknown | 0 | 1 (3) | |
| Culture | 0.02 | ||
| Positive | 50 (44) | 5 (17) | |
| Negative | 48 (43) | 20 (66) | |
| Unknown | 15 (13) | 5 (17) | |
| PCR | 0.077 | ||
| Positive | 45 (40) | 7 (23) | |
| Negative | 24 (21) | 12 (40) | |
| Unknown | 44 (39) | 11 (37) |
5. Discussion
In the current study, the characteristics of adolescent patients with EPTB and PTB were assessed. The majority of patients with EPTB were 15 to 18 years old .The detection rate of the source and the rate of smear positivity in the PTB group were significantly higher than those of the EPTB group.
Age was the main risk factor to develop TB disease (15).
In adolescents, the immune system is less responsive against Mycobacterium tuberculosis (16). Recent studies from India (17) and Canada (5) showed a higher rate of EPTB in older adolescents, compared with children. In current study, it was also noted that the majority of patients with EPTB were 15 to 18 years old, even though the age was not significant in the multivariate analysis.
The delay in the diagnosis of EPTB is related to the absence of the classic symptoms and signs of fever, cough, night sweats, loss of appetite, and weight-loss in such patients (13-15). It was found that the number of symptom-free patients was not significantly different between the EPTB and PTB groups. It was reflected by the duration of symptoms in patients with EPTB as well as patients with PTB in the current study. In the current study, approximately two thirds of the patients in the EPTB group had EPTB alone. However, it did not cause lack of symptoms and a delayed diagnosis in such patients.
Fever was associated with EPTB in the current study; a result that was in contrast to those of other studies that found fever was more frequent in patients with PTB (16, 17). This difference may be due to the fact that most of the patients with EPTB in the current study had pleural TB that can commonly present with fever (18), or this could be attributed to the collinearity between fever and cough variables.
It was found that the smear positivity rate was significantly higher in patients with PTB versus EPTB, which was in line with the results of other studies in the literature (20-22). The implementation of rapid diagnosis mycobacterial tests such as the Xpert Mycobacterium tuberculosis/ resistance to rifampin (MTB/RIF) assay would further improve the diagnosis of EPTB (23, 24). Extrapulmonary TB does not receive specific attention in the health control strategy probably due to the lower transmission rate. However, EPTB is associated with high morbidity rate; therefore, early detection reduces the complications of the disease (11).
In the current study, the source detection rate was significantly associated with PTB, which was consistent with those of the studies in children (18) and adults (13) reporting that contact with a TB case was a risk factor for pulmonary TB disease. A study in Rome, Italy, found that children with extrapulmonary TB and a negative history of contact were older than the children with PTB (19).
HIV infection is identified as the main risk factor for EPTB disease (12, 25). Recent studies reported that HIV infection was associated with severe forms of EPTB disease (26) and higher death rates in patients with EPTB (12). Unfortunately, HIV testing was not performed in all patients. However, the relationship between HIV infection and EPTB was not significant in the current study. It is important to screen patients with TB for HIV infection as recommended by the centers for disease control and prevention (CDC) (27).
In conclusion, the current study findings in adolescent patients confirmed the quiet onset of EPTB with a lower rate of bacteriological diagnosis and source detection rate. Early diagnosis of EPTB is critical for treatment to decline morbidity and mortality in such patients. Further larger studies are recommended to determine the relationship between the risk factors such as socioeconomic and cigarette smoking and tuberculosis in Iran, similar to the study conducted by Stevens H. et al., in adolescents (28). TB control programs and new strategies to improve the diagnosis of EPTB in adolescents should be implemented.
Multivariate Logistic Regression Model for Comparing EPTB with PTB
| Variables | P Value | OR | 95% CI |
|---|---|---|---|
| Age group, y | 0.118 | ||
| 15 - 18 | 3.8 | 0.70 - 21.18 | |
| 10 - 14 | 1 | Referent | |
| Contact source | 0.014 | ||
| Known | 0.07 | 0.009 - 0.58 | |
| Unknown | 1 | Referent | |
| Cough | 0.067 | ||
| Yes | 0.103 | 0.009 - 1.17 | |
| No | 1 | Referent | |
| Fever | 0.029 | ||
| Yes | 21.49 | 1.35 - 339.96 | |
| No | 1 | Referent | |
| Fatigue | 0.26 | ||
| Yes | 0.27 | 0.02 - 2.63 | |
| No | 1 | Referent | |
| Loss of appetite | 0.37 | ||
| Yes | 0.48 | 0.09 - 2.39 | |
| No | 1 | Referent | |
| Smear | 0.033 | ||
| Yes | 0.062 | 0.005 - 0.80 | |
| No | 1 | Referent | |
| Culture | 0.44 | ||
| Yes | 2.93 | 0.16 - 53.19 | |
| No | 1 | Referent | |
| PCR | 0.09 | ||
| Yes | 0.10 | 0.007 - 1.48 | |
| No | 1 | Referent |
References
-
1.
World Health Organization. Global tuberculosis report 2015. [cited 27 May]. Available from: http://www.who.int/tb/publications/global_report/gtbr2015_.pdf.
-
2.
World Health Organization (WHO). Global Health Observatory Data Repository 2013. [cited 29 Jun]. Available from: http://apps.who.int/gho/data?theme=main.
-
3.
Wood R, Lawn SD, Caldwell J, Kaplan R, Middelkoop K, Bekker LG. Burden of new and recurrent tuberculosis in a major South African city stratified by age and HIV-status. PLoS One. 2011;6(10). e25098. [PubMed ID: 22016763]. https://doi.org/10.1371/journal.pone.0025098.
-
4.
Brennan MJ, Thole J. Tuberculosis vaccines: a strategic blueprint for the next decade. Tuberculosis (Edinb). 2012;92 Suppl 1:S6-13. [PubMed ID: 22441160]. https://doi.org/10.1016/S1472-9792(12)70005-7.
-
5.
Phongsamart W, Kitai I, Gardam M, Wang J, Khan K. A population-based study of tuberculosis in children and adolescents in Ontario. Pediatr Infect Dis J. 2009;28(5):416-9. [PubMed ID: 19352212]. https://doi.org/10.1097/INF.0b013e3181920d4d.
-
6.
Nejat S, Buxbaum C, Eriksson M, Pergert M, Bennet R. Pediatric tuberculosis in Stockholm: a mirror to the world. Pediatr Infect Dis J. 2012;31(3):224-7. [PubMed ID: 22094631]. https://doi.org/10.1097/INF.0b013e31823d923c.
-
7.
Winston CA, Menzies HJ. Pediatric and adolescent tuberculosis in the United States, 2008-2010. Pediatrics. 2012;130(6):e1425-32. [PubMed ID: 23184110]. https://doi.org/10.1542/peds.2012-1057.
-
8.
Didilescu C, Ibraim E, Tigau M. [The epidemiological profile and current evolutionary trends in tuberculosis in adolescents (15-19 years old) in the capital]. Pneumoftiziologia. 1997;46(3):193-9. [PubMed ID: 9654957].
-
9.
Lin JN, Lai CH, Chen YH, Lee SS, Tsai SS, Huang CK, et al. Risk factors for extra-pulmonary tuberculosis compared to pulmonary tuberculosis. Int J Tuberc Lung Dis. 2009;13(5):620-5. [PubMed ID: 19383196].
-
10.
2013. Atlanta, GA: U.S: Department of Health and Human Services, CDC; Reported Tuberculosis in the United States. Available from: www.cdc.gov/nchstp/tb/.
-
11.
Sandgren A, Hollo V, van der Werf MJ. Extrapulmonary tuberculosis in the European Union and European Economic Area, 2002 to 2011. Euro Surveill. 2013;18(12). [PubMed ID: 23557943].
-
12.
Antony SJ, Harrell V, Christie JD, Adams HG, Rumley RL. Clinical differences between pulmonary and extrapulmonary tuberculosis: a 5-year retrospective study. J Natl Med Assoc. 1995;87(3):187-92. [PubMed ID: 7731067].
-
13.
Sreeramareddy CT, Panduru KV, Verma SC, Joshi HS, Bates MN. Comparison of pulmonary and extrapulmonary tuberculosis in Nepal- a hospital-based retrospective study. BMC Infect Dis. 2008;8:8. [PubMed ID: 18218115]. https://doi.org/10.1186/1471-2334-8-8.
-
14.
Yang Z, Kong Y, Wilson F, Foxman B, Fowler AH, Marrs CF, et al. Identification of risk factors for extrapulmonary tuberculosis. Clin Infect Dis. 2004;38(2):199-205. [PubMed ID: 14699451]. https://doi.org/10.1086/380644.
-
15.
Robert E, Black LB. Committee on Gulf War and Health: Infectious Diseases,. Sivitz - 2006 -Medical.
-
16.
Pickering LK, Baker CJ, Kimberlin DW, Long SS. American Academy of Pediatrics.Tuberculosis. In:. Red Book: 2009 Report of the Committee on Infectious Diseases. 28th ed. Elk Grove Village, IL: American Academy of Pediatrics; 2009. p. 680-701.
-
17.
Poroor J, George D. Tuberculosis in adolescents and children: Data on clinical presentation and treatment outcomes for a period of 4 years, from a tertiary care hospital in South India. J Evid Based Med Healthc. 2015;2(18):2752-7.
-
18.
Devrim I, Akturk H, Bayram N, Apa H, Tulumoglu S, Devrim F, et al. Differences between pediatric extra-pulmonary and pulmonary tuberculosis: a warning sign for the future. Mediterr J Hematol Infect Dis. 2014;6(1). e2014058. [PubMed ID: 25237471]. https://doi.org/10.4084/MJHID.2014.058.
-
19.
Scott LE, Beylis N, Nicol M, Nkuna G, Molapo S, Berrie L, et al. Diagnostic accuracy of Xpert MTB/RIF for extrapulmonary tuberculosis specimens: establishing a laboratory testing algorithm for South Africa. J Clin Microbiol. 2014;52(6):1818-23. [PubMed ID: 24622091]. https://doi.org/10.1128/JCM.03553-13.
-
20.
Buonsenso D, Lancella L, Delogu G, Krzysztofiak A, Testa A, Ranno O, et al. A twenty-year retrospective study of pediatric tuberculosis in two tertiary hospitals in Rome. Pediatr Infect Dis J. 2012;31(10):1022-6. [PubMed ID: 22668805]. https://doi.org/10.1097/INF.0b013e3182615270.
-
21.
Öztop A, Ünsal İ, Günay T, Oğuz VA, Cakmak R. An analysis of adult patients with pulmonary and extrapulmonary tuberculosis. Turkish J Med Sci. 2009;39(5):725-32.
-
22.
Ferjani A, Marzouk M, Kahla IB, Salma WB, Gharbi N, Tarmiz H, et al. Tuberculosis: Bacteriological and epi-demiological aspects in the central region of Tunisia. Int J Microbiol Adv Immunol. 2013;1(2):14-8.
-
23.
Pehme L, Hollo V, Rahu M, Altraja A. Tuberculosis during fundamental societal changes in Estonia with special reference to extrapulmonary manifestations. Chest. 2005;127(4):1289-95. [PubMed ID: 15821207]. https://doi.org/10.1378/chest.127.4.1289.
-
24.
Vadwai V, Boehme C, Nabeta P, Shetty A, Alland D, Rodrigues C. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol. 2011;49(7):2540-5. [PubMed ID: 21593262]. https://doi.org/10.1128/JCM.02319-10.
-
25.
Ade S, Harries AD, Trebucq A, Ade G, Agodokpessi G, Adjonou C, et al. National profile and treatment outcomes of patients with extrapulmonary tuberculosis in Benin. PLoS One. 2014;9(4). e95603. [PubMed ID: 24755603]. https://doi.org/10.1371/journal.pone.0095603.
-
26.
Leeds IL, Magee MJ, Kurbatova EV, del Rio C, Blumberg HM, Leonard MK, et al. Site of extrapulmonary tuberculosis is associated with HIV infection. Clin Infect Dis. 2012;55(1):75-81. [PubMed ID: 22423123]. https://doi.org/10.1093/cid/cis303.
-
27.
Recommendations for Human Immunodeficiency Virus (HIV) Screening in Tuberculosis (TB) Clinics. [cited 21 May]. Available from: http://www.cdc.gov/tb/publications/factsheets/testing/hivscreening.htm.
-
28.
Stevens H, Ximenes RA, Dantas OM, Rodrigues LC. Risk factors for tuberculosis in older children and adolescents: a matched case-control study in Recife, Brazil. Emerg Themes Epidemiol. 2014 Dec 30;11(1):20. [PubMed ID: 25642275]. https://doi.org/10.1186/s12982-014-0020-5.