Abstract
Background:
Escherichia coli is the main causative pathogen in urinary tract infections (UTIs). Antibiotic resistance in this bacterium is an important problem in public health.Objectives:
The aim of this study was to identify the blaTEM, blaSHV, blaOXA, and blaPER genes and AmpC-β-lactamase in clinical isolates of E. coli recovered from patients with UTIs in Kerman, Iran.Methods:
E. coli isolates (N = 105) were analyzed for their antibiotic susceptibility with the disk diffusion method. ESBL and AmpC-producing isolates were detected using phenotypic methods. PCR was used to identify the blaTEM, blaSHV, blaOXA and blaPER genes in ESBL and AmpC-positive isolates.Results:
More than 50% of the isolates were multi-drug resistant. The prevalence of ESBLs, AmpC-β-lactamase, blaTEM and blaOXA in the inpatient isolates was 37.2%, 2%, 37.2% and 5.8%, respectively. Further, the prevalence of ESBLs, blaTEM, blaSHV and blaOXA in the outpatient isolates was 42.5%, 24%, 5.5% and 1.8%, respectively.Conclusions:
The prevalence of ESBL-producing E. coli strains in the community (outpatients) is higher than that in inpatients in Kerman, Iran. An outbreak of ESBL-producing isolates in the community can be a serious problem for public health, as resistance to other classes of antibiotics such as aminoglycoside and fluoroquinolones is often related with ESBL and AmpC production, therefore, detection of ESBL and AmpC-producing isolates in the community and hospitals is very important for the treatment and prevention of such isolates.Keywords
1. Background
β-lactam antibiotics, such as monobactams, cephalosporins and carbapenems, are the important antibiotic for the treatment of gram-negative bacterial infections (1). Extended-spectrum β-lactamase (ESBL) and AmpC-β-lactamase are the main enzymes involved in resistance to β-lactam antibiotics, such as extended-spectrum cephalosporins (1, 2). Furthermore, ESBL and AmpC-β-lactamase-producing isolates usually are multidrug-resistant (MDR) (3-5). Therefore, detection of ESBL and AmpC-positive isolates is very important for proper treatment of bacterial infections (6).
Escherichia coli is a common causative pathogen in urinary tract infections (UTIs) in community and healthcare-associated infections (7, 8). In the last few years, different β-lactamase (bla) genes, such as blaSHV, blaTEM and AmpC-β-lactamase have been identified in E. coli isolates recovered from patients with UTIs (1, 2, 4). As UTIs are one of the most important risk factors for pyelonephritis, cystitis and bacteremia in hospitalized patients (4), it is important to investigate the prevalence of resistance in the causative organism from the viewpoint of treatment and prevention.
2. Objectives
The aim of this study was to identify the ESBL genes blaTEM, blaSHV, blaOXA, and blaPER and AmpC-β-lactamase in clinical isolates of E. coli recovered from patients with UTIs in Kerman, Iran.
3. Methods
3.1. Bacterial Isolates
In a cross-sectional study, we collected 105 non-duplicate isolates of E. coli samples from patients with symptoms of UTI who were admitted to hospitals at Kerman University of Medical Sciences between June 2014 and March 2015. Isolates were identified using standard biochemical tests such as the oxidase test, growth and lactose fermentation in MacConkey and triple sugar iron agar, the MRVP test, and growth in Simmons’ citrate agar (9). All isolates were stored at -80°C in tryptic soy broth containing 40% glycerol (10). All the culture media were purchased from Merck Co., Germany.
3.2. Susceptibility Tests
The antimicrobial susceptibility patterns of the isolates were examined using the disk diffusion method, according to the Clinical & Laboratory Standards Institute (CLSI) guidelines, on Mueller-Hinton agar (MHA) (CONDA, Lab) (11). Susceptibility to the following antibiotics (Mast Diagnostics Ltd., UK) was tested: imipenem (IMI, 10 µg), cefotaxime (CTX, 30 µg), ceftazidime (CAZ, 30 µg), cefepime (CPM, 30 µg), amoxicillin/clavulanic acid (AUG, 20/10 µg), gentamicin (GM, 10 µg), ciprofloxacin (CP, 5 µg), colistin (CO, 10 µg) and trimethoprim/sulfamethoxazole (SXT). E. coli ATCC 25922 was used as the standard control in the susceptibility tests. Isolates that were resistant to at least three classes of antibiotics were considered as MDR isolates according to the recommendations of Magiorakos et al. (12).
3.3. Screening of ESBL-Producing Isolates
ESBL-producing bacteria were detected using the disk synergy test. Briefly, isolates were inoculated on the surface of the MHA plates, with the concentration of the bacterial suspension adjusted to an optical density [OD] of 0.08 - 0.13 at 650 nm, which corresponds to the 0.5 McFarland standard. Then, an AUG disk (20 µg amoxicillin+10 µg clavulanic acid) was placed at the center of the plate, and CAZ (30 µg), CTX (30 µg), cefpodoxime (CPD, 30 µg) and CPM (30 µg) disks were placed 25 - 30 mm apart along an axis passing through the center of the AUG disk. The plates were incubated overnight at 37°C in ambient air. Isolates were considered to be ESBL-positive when a zone of inhibition was observed around each of the disks was expanded to AUG. E. coli ATCC 25922 and Klebsiella pneumoniae ATCC700603 (ESBL-positive isolate) were used as the negative and positive controls, respectively (13, 14).
3.4. Detection of AmpC-Positive Isolates
AmpC-producing isolates were identified using the AmpC disk test (15-17). Briefly, blank paper disks containing 50 × Tris-EDTA (TE) were prepared by applying 20 µL of a 1:1 mixture of saline and 100 × TE to sterile blank paper disks. The surface of an MHA plate was inoculated with a lawn of a cefoxitin (FOX)-susceptible strain (E. coli ATCC 25922) according to the standard disk diffusion method. A FOX (30 µg) (Mast Diagnostics Ltd., UK) disk was placed on the bacterial lawn on the surface of the MHA plate. The paper disks were then rehydrated, and several colonies of each test organism were applied to a disk. The inoculated paper disks were then placed such that they were almost touching the antibiotic disk (FOX) on the MHA plate. The plates were incubated at 37°C in ambient air. Indentation flattening of the zone of inhibition of FOX indicates enzymatic inactivation of the antibiotic and is considered as a positive finding. E. coli ATCC 25922 was used as the negative control in the AmpC disk test method.
3.5. DNA Extraction and Amplification of the bla Genes
Genomic DNA extraction was performed according to the boiling method of Pitout et al. (18). The β-lactamase genes blaTEM, blaSHV, blaPER and blaOXA were detected in the ESBL-positive isolates by PCR using the specific oligonucleotide primers listed in Table 1. PCR amplification was carried out in a FlexCycler PCR thermal cycler (Analytik Jena) with the PCR Master Mix (Ampliqon Inc., Denmark) according to the manufacturer’s guidelines. The PCR reactions were performed in a total volume of 25 µL. The master mix contained 12.5 µL of reaction mixture containing Taq DNA Polymerase Master Mix Red (Ampliqon, Denmark), 0.5 µL of each of the forward and reverse primers at a 10 pmol concentration, 1 µL of the target DNA and 10.5 µL of DNase- and RNase-free distilled water. The PCR conditions were as follows: initial denaturation at 95°C for 5 minutes followed by 30 cycles of denaturation at 95°C for 1 minute, annealing for 1 minute (Table 1), and extension at 72°C for 1 minute. The final extension step was continued for another 5 minutes at 72°C. K. pneumoniae ATCC700603 (positive for blaTEM and blaSHV) and KOAS (positive for blaPER: this strain was provided by Dr. Ali Hashemi, department of microbiology, Shahid Beheshti University of Medical Sciences, Tehran, Iran) were used as positive controls in the PCR experiment (19, 20).
List of Primers for Detection of the bla Gene in This Study
| Target gene | Primer Sequence (5’ - 3’) | Annealing Temperature (°C) | Product Size (bp) | Reference |
|---|---|---|---|---|
| blaSHV | F-TCAGCGAAAAACACCTTG | 56 | 472 | (21) |
| R-TCCCGCAGATAAATCACC | ||||
| blaTEM | F-CTTCCTGTTTTTGCTCAC C | 56 | 636 | (21) |
| R-AGCAATAAACCAGCCAGC | ||||
| blaPER | F-ATGAATGTCATTATAAAAGC | 49 | 925 | (22) |
| R-AATTTGGGCTTAGGGCAGAA | ||||
| blaOXA | F-TCAACTTTCAAGATCGCA | 49 | 610 | (23) |
| R-GTGTGTTTAGAATGGTGA |
3.6. Sequencing of the bla Genes
The sequences of both strands of the amplicons were elucidated at Macrogen (Seoul, South Korea). The sequences were compared with that of the reference genes deposited in GenBank (http://www.ncbi.nih.gov/BLAST), and the full sequence of the bla gene was deposited in GenBank.
3.7. Statistical Analysis
Statistical analysis was performed using the SPSS (v.22.0) statistics software. In this cross-sectional study, SPSS (v.22.0) was used to determine the frequency and spread of antibiotic resistance and resistance-related genes.
4. Results
From the 105 E. coli isolates, 51.5% (n = 54) were recovered from outpatients and 48.5% (n = 51) from inpatients. According to the results of the susceptibility test (Table 2), 50% of the isolates were resistant to at least more than five different classes of antibiotics, including third- and fourth-generation cephalosporins (CAZ, CTX and CPM), amoxicillin-clavulanic acid, aminoglycosides, ciprofloxacin and trimethoprim/sulfamethoxazole. Thus, these isolates were MDR isolates. A total of 42 (40%) isolates were ESBL-positive, only two isolates were AmpC-positive (Figures 1 and 2). The AmpC-producing isolates were ESBL-positive. Among the ESBL and AmpC-producing isolates, 76.1% (n = 32), 14.2% (n = 6) and 2.3% (n = 1) were positive for blaTEM, blaOXA-1 and blaSHV genes, respectively and blaPER gene was not detected in any of the ESBL and AmpC-producing isolates (GenBank accession numbers: blaSHV, KU059762; blaTEM, KU059763; and blaOXA, KU059764). Six isolates were positive for both blaTEM and blaOXA-1 (Number of inpatients = 3, number of outpatients = 3). Table 3 shows the distribution of ESBLs, AmpC, blaTEM, blaOXA-1 and blaSHV in the E. coli isolates from outpatients and inpatients.
Antibacterial Resistance Pattern of E. coli Isolates Recovered from Outpatients and Inpatients with UTIs in Kerman, Iran
| Type of E. coli isolates | Rate of Resistance to Antimicrobial Agents, No. (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUG | CTX | CAZ | CPM | IMP | CO | CIP | NA | SXT | GM | |
| Inpatient isolates (n = 51) | 29 (56.8) | 27 (52.9) | 26 (50.9) | 25 (49) | 4 (7.8) | 4 (7.8) | 38 (74.5) | 39 (76.4) | 38 (74.5) | 11 (21.5) |
| Outpatient isolates (n = 54) | 33 (61.1) | 28 (51.8) | 28 (51.8) | 29 (57.3) | 0 (0) | 0 (0) | 46 (85.1) | 36 (66.6) | 32 (59.2) | 13 (24) |
| Total (n = 105) | 62 (59) | 55 (52.3) | 54 (51.4) | 54 (51.4) | 4 (3.8) | 4 (3.8) | 84 (80) | 75 (71.4) | 70 (66.6) | 24 (22.8) |
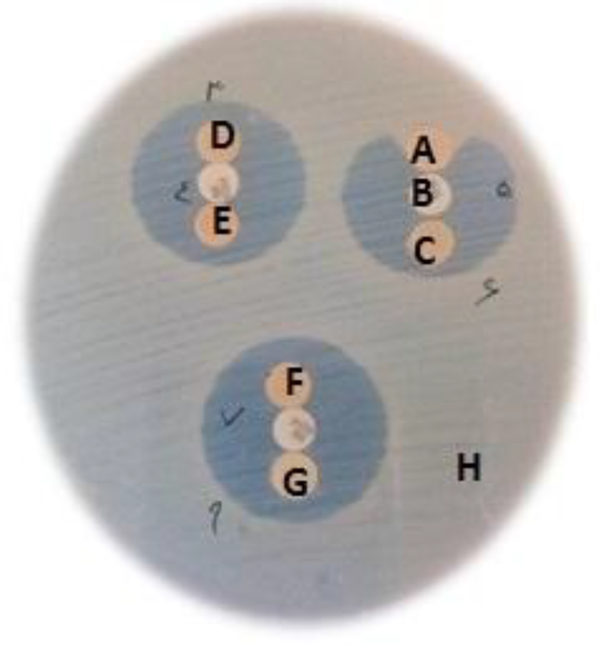
Clinical Isolate Producing ESBLs (Disk Synergy Test)

Results of the AmpC Disk Test
Distribution of ESBLs, AmpC, blaTEM, blaOXA-1 and blaSHV in the isolates from outpatients and inpatientsa
| Patients | Isolates | ESBLs | AmpC | blaTEM* | blaOXA-10* | blaSHV* |
|---|---|---|---|---|---|---|
| Inpatients | 51 (48.5) | 19 (37.2) | 2 (4) | 19 (37.2) | 3 (5.8) | - |
| Outpatients | 54 (51.5) | 23 (42.5) | 0 (0) | 13 (24) | 3 (5.5) | 1 (1.8) |
| Total | 105 (100) | 42 (40) | 2 (1) | 32 (30.4) | 6 (5.7) | 1 (0.9) |
5. Discussion
The findings of previous studies as well our present results indicate that ESBL and AmpC-β-lactamase-producing isolates are usually resistant to others antibiotics such as trimethoprim/sulfamethoxazole and fluoroquinolones (24, 25). The rate of resistance to imipenem was very low; this is similar to the results of others studies conducted in Iran and worldwide. Thus, imipenem is still the most active agent against ESBL and AmpC-β-lactamase-producing E. coli isolates (2, 26-28). However, in the present study, two isolates were found to be resistant to imipenem; thus, resistance to this antibiotic may increase in the future (2, 29). Interestingly, in this study, the diameter of the inhibition zone around the CO disk (colistin) was zero in 4 (7.8%) isolates from inpatients. Usually, colistin not used in the treatment of infections caused by E. coli, therefore outbreaks of infections caused by gram-negative bacilli that are resistant to colistin pose a global threat (30).
ESBL was detected in 23/54 (42.5%) of the isolates recovered from outpatients, and 17/23 (74%) of them harbored the blaTEM and blaSHV genes (Table 3). The prevalence of resistance to five or more antibiotics was higher among the outpatient than the inpatient isolates: more than 70% of the outpatient isolates were MDR. Thus, MDR strains are common in outpatients in Kerman, Iran. Moreover, the prevalence of antibiotic resistance was somewhat higher in the outpatient isolates than in the inpatient isolates. This finding is similar to other studies conducted in Iran. All these studies indicate that in the near future, several antibiotics such as CIP and SXT will be useless in empirical therapy of uncomplicated UTIs in the community (24, 25, 31). The spread of antibiotic resistance is related to healthcare-related behaviors and the environmental policies/laws in place. Inappropriate use of antibiotics, ineffective infection control and hygiene practices are behavioral factors, and the extensive use of antibiotics in agriculture is related to the environmental policies implemented (32). In Iran, it is not clear whether ESBL and AmpC-producing isolates of E. coli are present in the community, but high prevalence of community-acquired ESBL-producing isolates has been reported in other countries in Europe, Asia and USA (24, 25). In our study, we found a high rate of resistance in community uropathogenic E. coli against extended-spectrum cephalosporins (such as CAZ, CTX and CPM) and CIP. Moreover, the prevalence of ESBL-positive isolates in the inpatients was decreased from 63% to 37.25% compared to 2010, but the prevalence was increased in the outpatients (33). Prevalence of ESBL-producing E. coli in other regions of Iran, it is from 15.62% in Mashhad to 89.8% in Tehran (26-28). According to the results of our previous studies, the prevalence of ESBL and AmpC-producing E. coli in hospitalized patients was higher: 63% in inpatients vs. 9.6% in outpatients (26, 27, 29). In this studies, the prevalence of blaTEM was 37.2% and blaSHV was not detected in the inpatient isolates. However, these results are not in agreement with those of Abdi et al., who have reported the prevalence of blaTEM and blaSHV to be 82% and 65%, respectively (34). Moreover, our results are not in agreement with those of Karimi et al., who have reported the prevalence of blaSHV to be 15% in Tehran (35). This difference between different regions of Iran could be related to behavioral factors or environmental policies. To date, AmpC-β-lactamase has been identified in clinical isolates recovered from hospitalized patients (36). In our study, as only two isolates harbored AmpC-β-lactamase, it may imply that AmpC is not prevalent in this particular community in our region. These results indicate that AmpC-β-lactamase-producing community E. coli isolates may not be important for public health, especially in UTI patients in Kerman, Iran. However, the prevalence of AmpC-β-lactamase-producing E. coli in the community in Kerman, Iran, is lower than in other countries such as the Netherlands (23%) (36).
5.1. Conclusion
Most studies in Iran have focused on the outbreak of MDR and ESBL-producing isolates of E. coli and other gram-negative bacilli isolated from hospitalized patients. Our results show a high prevalence of MDR and ESBL-producing isolates of E. coli in hospitalized patients as well as outpatients. The high prevalence of ESBL-producing isolates in the community showed that ESBL-producing E. coli have important implications for public health, especially in relation to UTIs in women. In the future, there may be serious problems in the treatment of infections caused by these bacteria. Since the resistance to other classes of antibiotics, such as aminoglycosides and fluoroquinolones, is often related with ESBL and AmpC production (37, 38) therefore, ESBL-producing isolates must be recognized in community and hospitals for appropriate treatment and prevent the outbreak of ESBL-producing bacteria. It is important to identify infections caused by ESBL-producing bacteria in the community and hospitals for appropriate treatment and prevention outbreak of ESBLs and AmpC producing isolates.
Acknowledgements
References
-
1.
Pfeifer Y, Cullik A, Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int J Med Microbiol. 2010;300(6):371-9. [PubMed ID: 20537585]. https://doi.org/10.1016/j.ijmm.2010.04.005.
-
2.
Mansouri S, Kalantar Neyestanaki D, Shokoohi M, Halimi S, Beigverdi R, Rezagholezadeh F, et al. Characterization of AmpC, CTX-M and MBLs types of beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli producing Extended Spectrum beta-lactamases in Kerman, Iran. Jundishapur J Microbiol. 2014;7(2). eeee8756. [PubMed ID: 25147671]. https://doi.org/10.5812/jjm.8756.
-
3.
Poole K. Pseudomonas aeruginosa: resistance to the max. Front Microbiol. 2011;2:65. [PubMed ID: 21747788]. https://doi.org/10.3389/fmicb.2011.00065.
-
4.
Denton M. Enterobacteriaceae. Int J Antimicrob Agents. 2007;29 Suppl 3:S9-S22. [PubMed ID: 17659212]. https://doi.org/10.1016/S0924-8579(07)72174-X.
-
5.
Falagas ME, Karageorgopoulos DE. Extended-spectrum beta-lactamase-producing organisms. J Hosp Infect. 2009;73(4):345-54. [PubMed ID: 19596491]. https://doi.org/10.1016/j.jhin.2009.02.021.
-
6.
Sundin DR. Hidden Beta-Lactamases in the Enterobacteriaceae – Dropping the Extra Disks for Detection, Part II. Clin Microbiol Newsl. 2009;31(7):47-52. https://doi.org/10.1016/j.clinmicnews.2009.03.001.
-
7.
Soltani J, Poorabbas B, Miri N, Mardaneh J. Health care associated infections, antibiotic resistance and clinical outcome: A surveillance study from Sanandaj, Iran. World J Clin Cases. 2016;4(3):63-70. [PubMed ID: 26989670]. https://doi.org/10.12998/wjcc.v4.i3.63.
-
8.
Navidinia M, Peerayeh SN, Fallah F, Bakhshi B. Phylogenetic groups and pathogenicity island markers in Escherichia coli isolated from children. Jundishapur J Microbiol. 2013;6(10).
-
9.
Aulet de Saab OC, de Castillo MC, de Ruiz Holgado AP, de Nader OM. A comparative study of preservation and storage of Haemophilus influenzae. Mem Inst Oswaldo Cruz. 2001;96(4):583-6. [PubMed ID: 11391434].
-
10.
Martin WJ, Washington II. J. A. Enterobacteriaceace. In: Lennette EH, Balows A, Hausler WJ, Truant JP, editors. Manual of clinical microbiology. 3 ed. Washington. D.C: American Society for Microbiology; 1980. p. 195-219.
-
11.
Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. 34. USA: Clinical and Laboratory Standards Institute; 2014.
-
12.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268-81. [PubMed ID: 21793988]. https://doi.org/10.1111/j.1469-0691.2011.03570.x.
-
13.
Detection of extended-spectrum β-lactamases (ESBLs) in E. coli and Klebsiella species. Available from: http://bsac.org.uk/wp-content/uploads/2012/02/Ecoliklebsiella.pdf.
-
14.
Poorabbas B, Mardaneh J, Rezaei Z, Kalani M, Pouladfar G, Alami MH, et al. Nosocomial Infections: Multicenter surveillance of antimicrobial resistance profile of Staphylococcus aureus and Gram negative rods isolated from blood and other sterile body fluids in Iran. Iran J Microbiol. 2015;7(3):127-35. [PubMed ID: 26668699].
-
15.
Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC beta-lactamases in Enterobacteriaceae lacking chromosomal AmpC beta-lactamases. J Clin Microbiol. 2005;43(7):3110-3. [PubMed ID: 16000421]. https://doi.org/10.1128/JCM.43.7.3110-3113.2005.
-
16.
Mirsalehian A, Kalantar-Neyestanaki D, Nourijelyani K, Asadollahi K, Taherikalani M, Emaneini M, et al. Detection of AmpC-beta-lactamases producing isolates among carbapenem resistant P. aeruginosa isolated from burn patient. Iran J Microbiol. 2014;6(5):306-10. [PubMed ID: 25848519].
-
17.
Doi Y, Paterson DL. Detection of plasmid-mediated class C beta-lactamases. Int J Infect Dis. 2007;11(3):191-7. [PubMed ID: 17339123]. https://doi.org/10.1016/j.ijid.2006.07.008.
-
18.
Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL. Detection of Pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129-35. [PubMed ID: 16000424]. https://doi.org/10.1128/JCM.43.7.3129-3135.2005.
-
19.
Coudron PE. Inhibitor-based methods for detection of plasmid-mediated AmpC beta-lactamases in Klebsiella spp., Escherichia coli, and Proteus mirabilis. J Clin Microbiol. 2005;43(8):4163-7. [PubMed ID: 16081966]. https://doi.org/10.1128/JCM.43.8.4163-4167.2005.
-
20.
Shakibaie MR, Shahcheraghi F, Hashemi A, Adeli NS. Detection of TEM, SHV and PER Type Extended-Spectrum ß-Lactamase Genes among Clinical Strains of Pseudomonas aeruginosa Isolated from Burnt Patients at Shafa-Hospital, Kerman, Iran. Iran J Basic Med Sci. 2008;11(2):104-11.
-
21.
Neyestanaki DK, Mirsalehian A, Rezagholizadeh F, Jabalameli F, Taherikalani M, Emaneini M. Determination of extended spectrum beta-lactamases, metallo-beta-lactamases and AmpC-beta-lactamases among carbapenem resistant Pseudomonas aeruginosa isolated from burn patients. Burns. 2014;40(8):1556-61. [PubMed ID: 24767143]. https://doi.org/10.1016/j.burns.2014.02.010.
-
22.
Weldhagen GF, Poirel L, Nordmann P. Ambler class A extended-spectrum beta-lactamases in Pseudomonas aeruginosa: novel developments and clinical impact. Antimicrob Agents Chemother. 2003;47(8):2385-92. [PubMed ID: 12878494].
-
23.
Colom K, Perez J, Alonso R, Fernandez-Aranguiz A, Larino E, Cisterna R. Simple and reliable multiplex PCR assay for detection of blaTEM, bla(SHV) and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol Lett. 2003;223(2):147-51. [PubMed ID: 12829279].
-
24.
Qi C, Pilla V, Yu JH, Reed K. Changing prevalence of Escherichia coli with CTX-M-type extended-spectrum beta-lactamases in outpatient urinary E. coli between 2003 and 2008. Diagn Microbiol Infect Dis. 2010;67(1):87-91. [PubMed ID: 20227224]. https://doi.org/10.1016/j.diagmicrobio.2009.12.011.
-
25.
Kahlmeter G, Poulsen HO. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: the ECO.SENS study revisited. Int J Antimicrob Agents. 2012;39(1):45-51. [PubMed ID: 22055529]. https://doi.org/10.1016/j.ijantimicag.2011.09.013.
-
26.
Soltan Dallal M, Sabbaghi A, Molla Aghamirzaeie H, Rastegar Lari A, Eshraghian MR, Fallah Mehrabad J, et al. Prevalence of ampc and shv β-lactamases in clinical isolates of escherichia coli from tehran hospitals. Jundishapur J M. 2013;6(2). https://doi.org/10.5812/jjm.5043.
-
27.
Zaniani FR, Meshkat Z, Naderi Nasab M, Khaje-Karamadini M, Ghazvini K, Rezaee A, et al. The Prevalence of TEM and SHV Genes among Extended-Spectrum Beta-Lactamases Producing Escherichia Coli and Klebsiella Pneumoniae. Iran J Basic Med Sci. 2012;15(1):654-60. [PubMed ID: 23493850].
-
28.
Fallah F, Karimi A, Goudarzi M, Shiva F, Navidinia M, Jahromi MH, et al. Determination of integron frequency by a polymerase chain reaction-restriction fragment length polymorphism method in multidrug-resistant Escherichia coli, which causes urinary tract infections. Microb Drug Resist. 2012;18(6):546-9. [PubMed ID: 22816551]. https://doi.org/10.1089/mdr.2012.0073.
-
29.
Kalantar D, Mansouri S. Emergence of multiple β-lactamases produced by Escherichia coli clinical isolates from hospitalized patient in Kerman, Iran. Jundishapur J Microbiol. 2010;3(4):137-45.
-
30.
Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: Mechanisms, frequency and treatment options. Drug Resist Updat. 2010;13(4-5):132-8. [PubMed ID: 20843473]. https://doi.org/10.1016/j.drup.2010.05.002.
-
31.
Navidinia M, Peerayeh SN, Fallah F, Bakhshi B, Sajadinia RS. Phylogenetic grouping and pathotypic comparison of urine and fecal Escherichia coli isolates from children with urinary tract infection. Braz J Microbiol. 2014;45(2):509-14. [PubMed ID: 25242935].
-
32.
Larson E. Community factors in the development of antibiotic resistance. Annu Rev Public Health. 2007;28:435-47. [PubMed ID: 17094768]. https://doi.org/10.1146/annurev.publhealth.28.021406.144020.
-
33.
Mansouri S, Abbasi S. Prevalence of Multiple Drug Resistant Clinical Isolates of Extended-Spectrum Beta-Lactamase Producing Enterobacteriaceae in Southeast Iran. Iran J Med Sci. 2010;35(2):101-8.
-
34.
Abdi S, Ranjbar R, Hakemi VM, Jonaidi N, Baghery BO, Baghery BF. Frequency of bla TEM, bla SHV, bla CTX-M,and qnrAAmong Escherichia coli Isolated From Urinary Tract Infection. Arch Clin Infect Dis. 2014;9(1).
-
35.
Karimi A, Rahbar M, Fallah F, Navidinia M, Malekan MA. Detection of integron elements and gene groups encoding ESBLs and their prevalence in Escherichia coli and Klebsiella isolated from urine samples by PCR method. Afr J Microbiol Res. 2012;6(8). https://doi.org/10.5897/ajmr11.1297.
-
36.
Reuland EA, Halaby T, Hays JP, de Jongh DM, Snetselaar HD, van Keulen M, et al. Plasmid-mediated AmpC: prevalence in community-acquired isolates in Amsterdam, the Netherlands, and risk factors for carriage. PLoS One. 2015;10(1):e0113033. [PubMed ID: 25587716]. https://doi.org/10.1371/journal.pone.0113033.
-
37.
Altoparlak U, Aktas F, Celebi D, Ozkurt Z, Akcay MN. Prevalence of metallo-beta-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii isolated from burn wounds and in vitro activities of antibiotic combinations against these isolates. Burns. 2005;31(6):707-10. [PubMed ID: 16129224]. https://doi.org/10.1016/j.burns.2005.02.017.
-
38.
Kang CI, Wi YM, Lee MY, Ko KS, Chung DR, Peck KR, et al. Epidemiology and risk factors of community onset infections caused by extended-spectrum beta-lactamase-producing Escherichia coli strains. J Clin Microbiol. 2012;50(2):312-7. [PubMed ID: 22162561]. https://doi.org/10.1128/JCM.06002-11.