Abstract
Background:
Langerhans cell histiocytosis (LCH) is a rare histiocytic proliferative disorder of unknown etiology that mainly affects young children. The histological feature is the granuloma-like proliferation of Langerhans-type dendritic cells. The possible role of viruses such as Epstein-Barr virus (EBV, HHV-4), human herpesvirus-6 (HHV-6), herpes simplex virus (HSV) types 1 and 2, and cytomegalovirus (CMV, HHV-5) in the pathogenesis of LCH has been suggested in some studies; however, this still remains under debate.Objectives:
HHV-6 infections are reported to be associated with LCH, but no such reports could be found on Iranian children in the English-language medical literature. This study investigated the presence of HHV-6 in Iranian children with LCH.Methods:
In this retrospective study, we investigated the presence of HHV-6 DNA in 48 patients with LCH, using paraffin-embedded tissue samples, and in 48 controls (age- and tissue-matched) using the nested polymerase chain reaction (nested PCR) method. The patients had been treated at the Department of Pediatric Pathology from 2002 - 2013 and had undergone operations for reasons other than infectious disease. Only the pathology reports were retrospectively reviewed, and the patients were anonymous.Results:
There was no significant difference in the prevalence of HHV-6 detection between the LCH patients and the control subjects. HHV-6 was found by nested PCR in one (2.1%) of the 48 LCH patients and in six (12.5%) of the 48 control cases. P = 0.11 was calculated using Fisher’s exact test (OR: 0.15; 95%CI: 0.02 - 1.29).Conclusions:
Our study is the first to investigate patients with LCH and its possible association with HHV-6 in Iran. Considering the P level of 0.11, which is statistically insignificant, our findings fail to support the hypothesis of a possible role for HHV-6 in the pathogenesis of LCH. These results are in concordance with previous investigations showing negative results.Keywords
Cell Proliferation Dendritic Cells Histiocytosis Langerhans Cell Human Herpesvirus-6 Polymerase Chain Reaction
1. Background
Langerhans cells are a type of non-lymphoid mononuclear cell involved in inflammatory responses, and Langerhans cell histiocytosis (LCH) is their neoplastic proliferation, the clonality of which was first reported by in 1994 (1-3). These immature dendritic cells express lysosomal enzymes, CD1a, cytoplasmic S-100 protein, and langerin (CD207), and contain racket-shaped organelles of Birbeck granules on electron microscopy (4-6).
LCH is an enigmatic histiocytic proliferative disease of unknown etiology; however, a possible etiologic link between viruses or vaccinations and LCH has been proposed, among other environmental agents (7, 8). Epstein-Barr virus (EBV, HHV-4) is known as the etiologic agent of several malignancies, and herpes viruses are reported to cause persistent infections (9, 10). In addition, hemophagocytic syndromes in humans with several inherited immunodeficiencies are proposed to be induced by EBV and cytomegalovirus (CMV, HHV-5) (11-13). Controversial results are reported in the literature regarding the etiologic role of HHV-6 (14-19), but this is yet to be determined. Accordingly, in this study, we investigated the possible association between HHV-6 and LCH in Iranian children.
2. Objectives
Considering the fact that there have been proven cases of cancers with viral etiologies and vaccinations, and the early diagnosis and treatment of viral infections could be of importance in the management of these patients, we investigated the presence of HHV-6 in Iranian children with LCH.
3. Methods
3.1. Patients and Controls
Formalin-fixed, paraffin-embedded (FFPE) tissue samples from 48 patients with a pathologic diagnosis of LCH were extracted from the archive of the pathology department of Mofid children’s hospital in Tehran, Iran (a national referral center) from an 11-year period (2002 - 2013). The diagnosis of LCH was made by a pediatric pathologist, using the histological criteria mentioned in pathology textbooks, i.e. granulomas composed of Langerhans cells with typical grooved nuclei mixed with eosinophils and other inflammatory cells. The diagnoses were confirmed using immunohistochemical techniques for CD1a, S-100 protein, and CD68 when available. After examination of the slides by light microscopy, the tissues with adequate amounts of tumor tissue were used in the study, while the tissues with tumors that were too small were excluded. All patients were Iranian, with an age range of 2 months to 10 years. Forty-eight tissue samples from non-LCH patients who were operated on for reasons other than infectious disease were also selected from the files of the Pathology Department for the same years, as controls (age- and tissue-matched to the LCH cases). These patients’ procedures had been performed for conditions such as hemangioma, cystic hygroma, osteochondroma, dermatitis, emphysema, pilonidal disease, soft tissue cysts, enlarged reactive nodes, and anal fissures. The criterion for inclusion of tissue in the control group was the absence of clinical and microscopic evidence for LCH or any other malignant tumor.
3.2. Paraffin-Embedded Tissue Section Preparation and DNA Extraction
The 5-µm-thick tissue sections were cut from paraffin-embedded blocks on a microtome and placed in sterile screw-cap tubes. It is necessary to completely remove the embedding material before DNA extraction. Xylene and alcohol solutions were used to deparaffinize and rehydrate the tissue sections. The sections were then lysed with a tissue lysis buffer and proteinase K. The samples were subjected to DNA extraction after the tissues were dissolved. The DNA was extracted from lysed-tissue samples according to the manufacturer’s instructions (RTP® DNA/ RNA Virus Mini Kit; Stratec Molecular GmbH, Berlin, Germany). The extracted nucleic acids were stored at -20°C until PCR testing.
3.3. Polymerase Chain Reaction
After quality control of the extracted DNA with the SYBR green real-time PCR (RT-PCR) melting curve for the beta-globin gene (Figure 1), nested PCR was applied to detect the HHV-6 genome in the samples as previously described (20). A 214-bp gene region of the main viral capsid protein was amplified using the nested primer sets (Figure 2). Primers for nested PCR were HHV-1 (outer): 5’-CAATGCTTTTCTAGCCGCCTCTTC-3’ and HHV-2 (outer): 5’- ACATCTATAATTTTAGACGATCCC-3’ and HHV-3 (inner): 5’- TTGTGCGGGTCCGTTCCCATCATA-3 and HHV-4 (inner): 5’-TCGGGATAGAAAAACCTAATCCCT. The limit detection of 50 genome copies of HHV-6 per reaction was determined by the nested PCR assays, using the serial dilutions of AmpliRun® HHV-6 DNA CONTROL (Vircell, S.L., Granada, Spain).
Beta-Globin Gene SYBR Green Real-Time Polymerase Chain Reaction Melting Curve

Electrophoresis of PCR Products of HHV-6 Assay
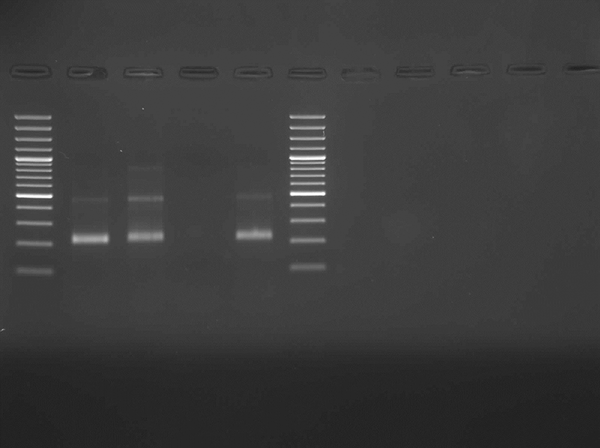
3.4. Statistical and Ethical Considerations
The obtained data were compared using Fisher’s exact test. P < 0.05 was considered to indicate statistical significance.
No ethical issues were involved in this study. Only the pathology reports were retrospectively reviewed, and the patients were anonymous. The articles used as references are valid and the data taken are reported unchanged.
4. Results
Forty-eight patients with a pathologic diagnosis of LCH were included in this study. All patients were Iranian (24 males and 24 females), with an age range of 2 months to 10 years. Age, sex, and biopsy sites for all patients are shown in Table 1. Forty-eight tissue samples from patients with non-LCH diagnoses were also selected as controls (in the same age range and tissue-matched to the LCH cases). The HHV-6 DNA was detected by nested PCR in one of the 48 LCH patients (positive results in 2.1% and negative results in 97.9%). In the control group, we detected HHV-6 in six of the 48 samples (positive results in 12.5% and negative results in 87.5%). This results in OR: 0.15; 95%CI: 0.02 - 1.29, and P = 0.11, which shows no significant difference in HHV-6 prevalence results between the LCH patients and the controls. HHV-6-DNA was found in the lymph node of a two-year-old girl with LCH (Table 1).
Age, Sex, and Biopsy Sites of Patients
| Age | Sex | Biopsy Site | HHV-6 |
|---|---|---|---|
| 2 y | Female | Lymph node | + |
| 2 y | Male | Mediastinal and neck mass | - |
| 10 mo | Male | Skin | - |
| 1 y | Male | Soft tissue, scalp mass | - |
| 2 y | Male | Frontal bone | - |
| 11 mo | Male | Mastoid bone | - |
| 1 y | Female | Skin, chest wall | - |
| 3 y | Male | Iliac bone | - |
| 6 y | Female | Proximal tibia | - |
| 10 y | Male | Skull and soft tissue | - |
| 2 y | Female | Parietal bone and soft tissue | - |
| 2 y | Male | Skin | - |
| 8 y | Male | Soft tissue, scalp | - |
| 2 y | female | Soft tissue, submandibular | - |
| 2 y | Male | Vertebral bone | - |
| 7 y | Male | Clavicle and soft tissue | - |
| 15 mo | Male | Abdominal mass | - |
| 4 y | Female | Base of skull | - |
| 2.5 y | Female | Rt lobe of lung | - |
| 8 y | Female | Skin, abdomen | - |
| 2 mo | Female | Soft tissue | - |
| 2 y | Male | Soft tissue, scalp | - |
| 3 y | Female | Frontal bone and soft tissue | - |
| 3 y | Female | Soft tissue, scalp | - |
| 2 y | Female | Metatarsal bone | - |
| 16 mo | Male | Skin | - |
| 4 mo | Female | Skin | - |
| 1 y | Female | Skin | - |
| 1.5 y | Male | Soft tissue, scalp | - |
| 7 y | Male | Soft tissue, perianal | - |
| 5 y | Female | Skin | - |
| 6 y | Male | Skin | - |
| 1 y | Female | Bone | - |
| 2 y | Female | Soft tissue | - |
| 17 mo | Male | Skin | - |
| 4 y | Female | Lymph node | - |
| 1 y | Female | Skin | - |
| 2 y | Female | Skin | - |
| 22 mo | Male | Bone | - |
| 2 y | Female | Skin | - |
| 1 y | Male | Soft tissue | - |
| 18 mo | Male | Skin | - |
| 2 y | Female | Skin | - |
| 1 y | Male | Skin | - |
| 1 y | Male | Soft tissue | - |
| 2 y | Female | Skin | - |
| 5 y | Male | Skin | - |
| 3 y | Female | Skin | - |
5. Discussion
LCH (histiocytosis X) is an uncommon disease with three overlapping clinical syndromes, including multifocal multisystemic LCH (Letterer-Siwe disease), multifocal unisystemic LCH (Hand-Schüller-Christian disease), and unifocal LCH (solitary eosinophilic granuloma) (2). Hematoxylin-eosin staining of biopsy slides shows granulomas composed of a mixture of Langerhans cells, macrophages, eosinophils, multinucleated giant cells, and lymphocytes (3, 4). The involved organs and the patient’s age determine the prognosis of LCH. Children usually need treatment, whereas most adult patients with lung involvement have an indolent course of regression (5, 6, 21, 22).
Some investigators have tried to identify a link between viruses and LCH. The etiologic role of HHV-6 in LCH is debated, with conflicting results in the literature (14-19). Jeziorski et al. (17) demonstrated no significant association between EBV, CMV, or HHV-6 in the pathogenesis of LCH. McClain et al. (18) also failed to find evidence of genomes for adenovirus, CMV, EBV, herpes simplex virus (HSV), HHV-6, human immunodeficiency virus (HIV), human T-cell leukemia virus types I and II, and parvovirus in 56 cases of LCH, employing in situ hybridization (ISH) and PCR techniques.
In contrast, Leahy et al. (16) detected HHV-6 in 47% of their patients with LCH, using the PCR technique. Glotzbecker et al. (15) used the immunohistochemistry (IHC) method and ISH for detection of HHV-6, and reported a high rate of 71.4% by both methods. However, in another investigation, they reported no significant difference between 13 LCH patients compared to a control group, using qualitative and quantitative real-time PCR (23). Csire and colleagues (14) had an LCH patient with persistently detected HHV-6 through 17 years of follow-up, and suggested that HHV-6 infection may be associated with development or progression of LCH.
In our study, HHV-6-DNA was detected in only one patient (2.1%) out of 48 cases of LCH. The positive result in six (12.5%) of the control samples could be attributed to coincidence. P = 0.11, which shows no significant difference in HHV-6 detection results between the LCH patients and the controls, does not support the hypothesis of a possible role for HHV-6 in the pathogenesis of LCH disease, which is in keeping with some other reports in the literature (17-19).
In previous studies (14-19), authors have declared no limitations, and their methods included serology, IHC, ISH, and PCR. However, the limitations of our study were that IHC and ISH for HHV-6 were not available for use and the patients could not be checked for the evidence of HHV-6 infections serologically.
Our study is of importance because it is the first performed on this subject in Iran. We failed to find any statistically significant differences between patients with LCH and the control group with regard to the presence of HHV-6 DNA. This is in concordance with previous negative results reported in the literature (17-19).
Acknowledgements
References
-
1.
Willman CL, Busque L, Griffith BB, Favara BE, McClain KL, Duncan MH, et al. Langerhans'-cell histiocytosis (histiocytosis X)--a clonal proliferative disease. N Engl J Med. 1994;331(3):154-60. [PubMed ID: 8008029]. https://doi.org/10.1056/NEJM199407213310303.
-
2.
Weiss LM. Histiocytic and dendritic cell proliferations. In: Knowles DM, editor. Neoplastic Hematopathology. 2 ed. Philadelphia: Lippincott Williams & Wilkins; 2001. p. 1815-45.
-
3.
Lichtenstein L. Histiocytosis X; integration of eosinophilic granuloma of bone, Letterer-Siwe disease, and Schuller-Christian disease as related manifestations of a single nosologic entity. AMA Arch Pathol. 1953;56(1):84-102. [PubMed ID: 13057466].
-
4.
The French Langerhans' Cell Histiocytosis Study Group. A multicentre retrospective survey of Langerhans' cell histiocytosis: 348 cases observed between 1983 and 1993. Arch Dis Child. 1996;75(1):17-24. [PubMed ID: 8813865].
-
5.
Ladisch S. Langerhans cell histiocytosis. Curr Opin Hematol. 1998;5(1):54-8. [PubMed ID: 9515204].
-
6.
Laman JD, Leenen PJ, Annels NE, Hogendoorn PC, Egeler RM. Langerhans-cell histiocytosis 'insight into DC biology'. Trends Immunol. 2003;24(4):190-6. [PubMed ID: 12697451].
-
7.
Chen CJ, Ho TY, Lu JJ, Sheu LF, Lee SY, Tien CH, et al. Identical twin brothers concordant for Langerhans' cell histiocytosis and discordant for Epstein-Barr virus-associated haemophagocytic syndrome. Eur J Pediatr. 2004;163(9):536-9. [PubMed ID: 15243808]. https://doi.org/10.1007/s00431-004-1493-y.
-
8.
Sakata N, Toguchi N, Kimura M, Nakayama M, Kawa K, Takemura T. Development of Langerhans cell histiocytosis associated with chronic active Epstein-Barr virus infection. Pediatr Blood Cancer. 2008;50(4):924-7. [PubMed ID: 17474115]. https://doi.org/10.1002/pbc.21249.
-
9.
Purtilo DT. Epstein-Barr-virus-induced oncogenesis in immune-deficient individuals. Lancet. 1980;1(8163):300-3. [PubMed ID: 6101750].
-
10.
Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4(10):757-68. [PubMed ID: 15510157]. https://doi.org/10.1038/nrc1452.
-
11.
Hertel L, Lacaille VG, Strobl H, Mellins ED, Mocarski ES. Susceptibility of immature and mature Langerhans cell-type dendritic cells to infection and immunomodulation by human cytomegalovirus. J Virol. 2003;77(13):7563-74. [PubMed ID: 12805456].
-
12.
Lee AW, Hertel L, Louie RK, Burster T, Lacaille V, Pashine A, et al. Human cytomegalovirus alters localization of MHC class II and dendrite morphology in mature Langerhans cells. J Immunol. 2006;177(6):3960-71. [PubMed ID: 16951359].
-
13.
Senechal B, Boruchov AM, Reagan JL, Hart DN, Young JW. Infection of mature monocyte-derived dendritic cells with human cytomegalovirus inhibits stimulation of T-cell proliferation via the release of soluble CD83. Blood. 2004;103(11):4207-15. [PubMed ID: 14962896]. https://doi.org/10.1182/blood-2003-12-4350.
-
14.
Csire M, Mikala G, Jako J, Masszi T, Janosi J, Dolgos J, et al. Persistent long-term human herpesvirus 6 (HHV-6) infection in a patient with langerhans cell histiocytosis. Pathol Oncol Res. 2007;13(2):157-60. [PubMed ID: 17607379].
-
15.
Glotzbecker MP, Carpentieri DF, Dormans JP. Langerhans cell histiocytosis: a primary viral infection of bone? Human herpes virus 6 latent protein detected in lymphocytes from tissue of children. J Pediatr Orthop. 2004;24(1):123-9. [PubMed ID: 14676546].
-
16.
Leahy MA, Krejci SM, Friednash M, Stockert SS, Wilson H, Huff JC, et al. Human herpesvirus 6 is present in lesions of Langerhans cell histiocytosis. J Invest Dermatol. 1993;101(5):642-5. [PubMed ID: 8228322].
-
17.
Jeziorski E, Senechal B, Molina TJ, Devez F, Leruez-Ville M, Morand P, et al. Herpes-virus infection in patients with Langerhans cell histiocytosis: a case-controlled sero-epidemiological study, and in situ analysis. PLoS One. 2008;3(9):3262. [PubMed ID: 18810271]. https://doi.org/10.1371/journal.pone.0003262.
-
18.
McClain K, Jin H, Gresik V, Favara B. Langerhans cell histiocytosis: lack of a viral etiology. Am J Hematol. 1994;47(1):16-20. [PubMed ID: 8042610].
-
19.
McClain K, Weiss RA. Viruses and Langerhans cell histiocytosis: is there a link? Br J Cancer Suppl. 1994;23:S34-6. [PubMed ID: 8075003].
-
20.
Luiz CR, Machado CM, Canto CL, Christ SC, Pestana JO, Kotton CN, et al. Monitoring for HHV-6 infection after renal transplantation: evaluation of risk factors for sustained viral replication. Transplantation. 2013;95(6):842-6. [PubMed ID: 23354300]. https://doi.org/10.1097/TP.0b013e3182807ab7.
-
21.
Berber I, Erkurt MA, Kuku I, Koroglu M, Kaya E, Unlu S. A Rare Disease in Adult: Langerhans Cell Histiocytosis. World J Oncol. 2013;4(3):165-8. https://doi.org/10.4021/wjon663w.
-
22.
Ninaber M, Dik H, Peters E. Complete pathological resolution of pulmonary Langerhans cell histiocytosis. Respirol Case Rep. 2014;2(2):76-8. [PubMed ID: 25473573]. https://doi.org/10.1002/rcr2.54.
-
23.
Glotzbecker MP, Dormans JP, Pawel BR, Wills BP, Joshi Y, Elkan M, et al. Langerhans cell histiocytosis and human herpes virus 6 (HHV-6), an analysis by real-time polymerase chain reaction. J Orthop Res. 2006;24(3):313-20. [PubMed ID: 16479562]. https://doi.org/10.1002/jor.20039.