Abstract
Background:
Urinary tract infections (UTIs) are common in children. They can lead to hypertension and end stage renal disease (ESRD). Ghrelin is a regulatory hormone that maintains fat tissues and body composition. Ghrelin is mainly produced in the stomach and in smaller amounts in kidneys. It stimulates release of growth hormone (GH), increases food intake, and causes weight gain.Objectives:
The aim of this study was to examine weather urinary Ghrelin concentration is involved in anorexia in patients with UTI and its urinary concentration changes with treatment.Patients and Methods:
This study was performed on 40 hospitalized children with UTI at Mofid children’s hospital during years 2013 to 2014. Ghrelin and Creatinine were measured before and after treatment. statistical analyzes were performed using the SPSS software version 18 by student t test, Wilcoxon test and Spearman coefficient and differences were considered as significant if P < 0.05.Results:
Mean age was 4.5 ± 3.8 years and 34 (85%) were females; 14 children (35%) had proteinuria, nine patients (29%) had Vesicoureteral Reflux (VUR), eight (20%) scare, five (12.5%) hydronephrosis and 33 (82.5%) anorexia. Mean urinary acylated Ghrelin before and after treatment were 138.4 ± 70.9 and 147.2 ± 72.6, respectively (P < 0.001). There was no significant difference between mean urinary Ghrelin before and after treatment in children with UTI with or without anorexia. Acylated Ghrelin had a direct correlation with the incidence of renal scarring (r = 0.37, P = 0.034).Conclusions:
Urinary Ghrelin concentration was lower before treatment of UTI and significantly increased after cessation of inflammation. Further studies are required for more definite results.Keywords
1. Background
Urinary tract infections (UTIs) are among the most common infections and urinary diseases in children. If not diagnosed early and left untreated timely, UTIs can lead to scar formation, hypertension and end stage renal disease (ESRD) (1-7). There have been many efforts to study mediators of inflammation trying to halt deleterious effects of UTI such as scar formation (8-11).
Ghrelin is a regulatory hormone that increases fat tissues and body composition. Ghrelin is a 28 amino acid peptide, which is mainly produced in stomach fundus cells but is also produced in smaller amounts in other organs such as kidneys, lung, adrenals, placenta and brain (12, 13).
Ghrelin stimulates release of Growth Hormone (GH), increases food intake, and causes weight gain, mainly in fat tissue. Ghrelin increases body fat without extra calorie intake. It has been postulated that Ghrelin may make metabolism more efficient (14, 15).
Ghrelin inhibits Leptin receptor, an internal ligand for growth hormone secretion (GHS-R) (16), so it is likely that there is an association between infection and Ghrelin. In the present study, we aimed to compare Ghrelin levels in children with urinary tract infection before and after treatment.
With deterioration of renal function, Ghrelin levels increase in the body. Patients with chronic kidney disease (CKD) experience muscle wasting and high fat tissue (17, 18).
However, despite elevated Ghrelin levels seen in patients with CKD, recent investigations have found that treatment with exogenous Ghrelin or Ghrelin receptor antagonists improve body composition. A study in uremic rats found that treatment with Ghrelin receptor antagonists and exogenous Ghrelin increases lean body mass and growth hormone (GH) levels and decreases inflammation (19).
2. Objectives
In the present study, we aimed to examine weather urinary Ghrelin concentration is involved in anorexia in patients with UTI and weather its urinary concentration changes with treatment; we investigated urine Ghrelin concentration before and after treatment.
3. Patients and Methods
A total of 40 patients admitted with UTI at the pediatric nephrology ward were included in the study. Exclusion criteria were: negative urine culture, no evidence of pyelonephritis on Dimercaptosuccinic acid (DMSA) scan, systemic diseases and chronic kidney disease (CKD).
Before treatment, urine and blood samples for Ghrelin were collected and stored at -20°C. In addition, U/A, U/C, urine Creatinine (Cr), CBC, ESR, CRP, BUN , Cr, BS, TG and cholesterol were measured. On the fifth day of admission, samplings for the above-mentioned tests were repeated. Other investigations were ultrasonography (US), Voiding Cystourethrography (VCUG) and DMSA scan (4, 5). On the last day of study, urine samples were melted and Ghrelin concentration measured with human Acylated Ghrelin Elisa kit, Eurommun company China.
Ethical issues.
The study was approved by the institutional ethics committee of Shahid Beheshti University of Medical Sciences. A written informed consent was obtained from the parents of all the study participants.
3.1. Statistical Analysis
Statistical analysis was performed using paired T test for quantitative data and Wilcoxon test for qualitative data and correlations by spearman coefficient and differences considered significant if P < 0.05.
4. Results
Forty patients including 34 girls (85%) and six boys (15%) were studied. Mean and standard deviation (M ± SD) for age, weight and body mass index (BMI) were 48.3 ± 46.1 (2 - 162 months), 17 ± 10.7 (4.5 - 51 kg) and 15.5 ± 1.9 (12-20), respectively. Pattern of BMI of patients is shown in Figure 1.
Pattern of Body Mass Index of Forty Patients With Urinary Tract Infection Admitted to the Nephrology Ward, Mofid Children Hospital in 2014
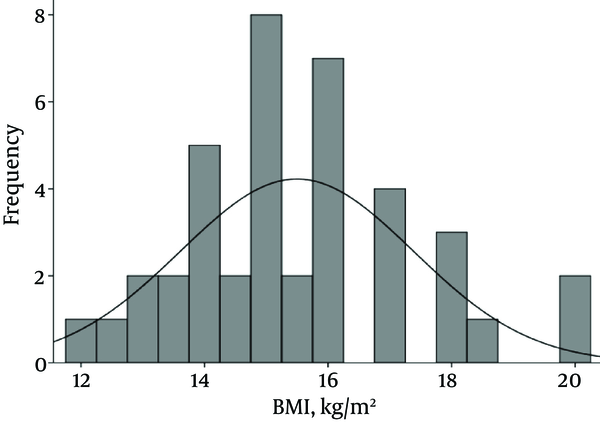
Based on Ultrasonographic (US) studies; hydronephrosis was detected in eight (20.5%) patients. In 29 patients, VCUG was performed, which showed Vesicoureteral Reflux (VUR) in nine cases including four bilateral and five unilateral refluxes. The DMSA scan revealed reduced uptake in 22 patients (66.7%) and scar in nine cases (27.3%).
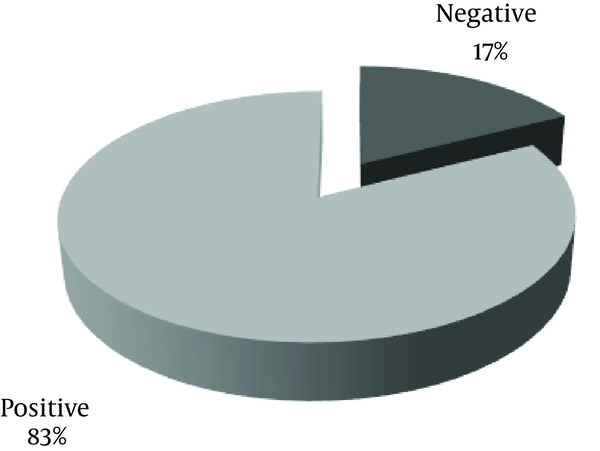
Frequency of anorexia in patients is shown in Figure 2.
Frequency of Anorexia in Forty Patients With Urinary Tract Infection Admitted to the Nephrology Ward, Mofid Children Hospital in 2014
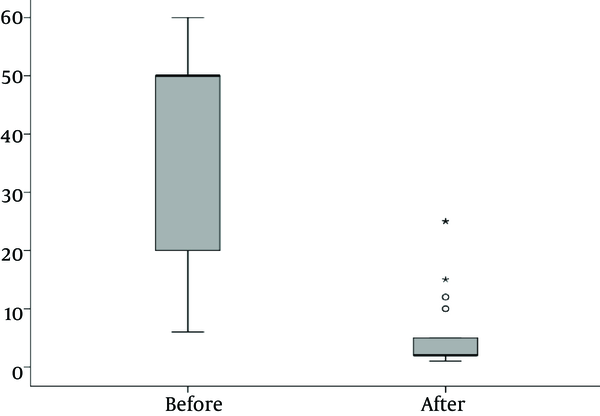
There was a significantly lower WBC and CRP after treatment than before treatment (P < 0.001).
Figure 3 depicts the number of Leukocytes in blood of patients before and after treatment.
Number of Leukocytes in Blood of Forty Patients Before and After Treatment With Urinary Tract Infection Admitted at the Nephrology Ward of Mofid Children Hospital In 2014
Erythrocyte sedimentation rate (ESR) before treatment was 40.3 ± 26.7 (highest: 108) and after treatment 35.2 ± 20.5.
Thus reduction of ESR after treatment was not significant compared to before treatment (P < 0.282).
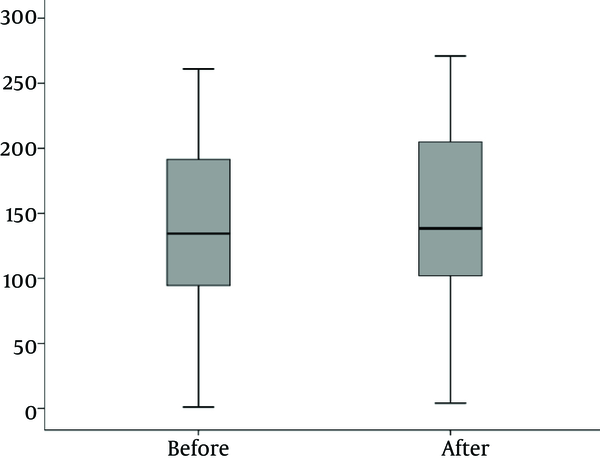
Mean urinary Ghrelin concentration before and after treatment was 138.4 ± 70.9 and147.2 ± 72.6, respectively (P < 0.001).
Mean urinary Ghrelin concentration before and after treatment is shown in Figure 4.
Mean Urinary Ghrelin Concentration Before and After Treatment in 40 Patients Before and After Treatment with UTI Admitted in Nephrology ward, Mofid Children Hospital in 2014
Mean urinary Ghrelin concentration before treatment in patients with anorexia and without anorexia was 135.0 ± 73.6 and 154.6 ± 58.4 respectively (P = 0.51).
Mean urinary Ghrelin concentration after treatment in patients with anorexia and without anorexia was 161.4 ± 59.8 and 144.2 ± 75.5respectively (P = 0.57).
So there was no significant difference in mean urinary Ghrelin concentration before and after treatment in patients with or without anorexia.
5. Discussion
In this study, we investigated urinary Ghrelin before and after treatment of UTI in 40 pediatric patients and its association with renal scar and inflammatory markers.
To the best of our knowledge, this is the first study that investigates urine Ghrelin concentration in pediatric UTIs.
Gunta et al. and Mak et al. studied the pathophysiology of Ghrelin and Leptin in chronic kidney disease and concluded that Ghrelin may have a role in protein energy wasting, cachexia, inflammation and cardiovascular complications in CKD patients (12, 14).
Carrero et al. in their study found that protein-energy wasting modifies the association of Ghrelin with inflammation, Leptin and mortality in hemodialysis patients (13).
In the study of Piatek et al. on urinary tract infection during pregnancy, they found that the level of Leptin, Ghrelin and insulin in maternal and placental blood was lower in pregnant women with UTI than controls (20).
Suzuki et al. in a study entitled, Ghrelin and cachexia in chronic kidney disease, treatment with Ghrelin suggested further investigations (21).
Madison et al. in their study entitled Prostacyclin signaling regulates circulating Ghrelin during acute inflammation suggested that inflammation reduces Ghrelin concentration and supported treatment with Ghrelin in acute and chronic inflammatory diseases (22).
In our study mean urinary Ghrelin concentration after treatment was significantly higher than before treatment. This supports the findings of Carrero et al. (13), Madison et al. (22), Piatek et al. (20) and some other investigators (23-25).
Although in the present study there was no significant difference in mean urinary Ghrelin concentration before and after treatment in patients with or without anorexia, we still think at least it can indirectly have a role through its effects on inflammation.
5.1. Conclusions
In this study we found that urinary Ghrelin concentration is significantly lower before treatment of UTI and significantly increases after cessation of inflammation. Further investigations are needed before suggestion of treatment with Ghrelin in patients with UTI.
5.2. Limitations of the Study
Due to some considerations regarding ethical issues for taking blood from infants and children, we were not able to obtain serum Ghrelin concentrations at the same time as urine tests. Although we had excellent contribution of most of the parents in preparing suitable urine samples, a few cases had limited cooperation.
Acknowledgements
References
-
1.
Elder JS. Kleigman RM, Stanton BF, Geme JWS, Schor NF, Behrman RE, editors. Urinary tract infection and vesicoureteral reflux. 20th ed. Philadelphia: WB Saunders: Nelson textbook of pediatrics; 2016. p. 2556-75.
-
2.
Chevalier RL, Roth JA. Urinary tract disease. Avner ED, Harmon WE, Niaudet P. Pediatric Nephrology. 5th ed. Baltimore, Lippincott Williams & Wilkins. 2004:1049-69.
-
3.
Jantausch B, Kher KK. Kher KK, Schnaper HW, Makker SP, editors. Urinary tract infection. 2nd ed ed. 2007. p. 553-73.
-
4.
Rushton HG, Majd M, Chandra R, Yim D. Evaluation of 99mtechnetium-dimercapto-succinic acid renal scans in experimental acute pyelonephritis in piglets. J Urol. 1988;140(5 Pt 2):1169-74. [PubMed ID: 2846898].
-
5.
Wallin L, Helin I, Bajc M. Follow-up of acute pyelonephritis in children by Tc-99m DMSA scintigraphy: quantitative and qualitative assessment. Clin Nucl Med. 2001;26(5):423-32. [PubMed ID: 11317023].
-
6.
Kumar R, Dayal D, Gupta A, Kumar R, Sodhi K, Bhattacharya A. Acute Focal Bacterial Nephritis Secondary to Vesicoureteric Reflux in a Young Boy. Arch Pediatr Infect Dis. 2015;3(4).
-
7.
Sharifian M, Sharifian S. Diagnostic Challenges in Urinary Tract Infections in Children. Arch Pediatr Infect Dis. 2015;3(2).
-
8.
Cheung WW, Paik KH, Mak RH. Inflammation and cachexia in chronic kidney disease. Pediatric Nephrology. 2010;25(4):711-24. https://doi.org/10.1007/s00467-009-1427-z.
-
9.
Sharifian M, Esmaeli Zand R, Ahmadi M, Ziaee SA, Mohkam M, Reza Dalirani RD, et al. Urinary adrenomedullin level in children with acute pyelonephritis before and after treatment. Iran J Kidney Dis. 2013;7(4):277-81. [PubMed ID: 23880804].
-
10.
Sharifian M, Anvaripour N, Karimi A, Fahimzad A, Mohkam M, Dalirani R, et al. The role of dexamethasone on decreasing urinary cytokines in children with acute pyelonephritis. Pediatric Nephrology. 2008;23(9):1511-6. https://doi.org/10.1007/s00467-008-0864-4.
-
11.
Sharifian M, Karimi A, Mohkam M, Dalirani R, Esfandiar N, Tabatabaei S, et al. Urinary Beta 2-Microglobulin as a Prognostic marker in children with pyelonephritis Running title: Beta 2-Microglobulin a Prognostic marker. Arch Pediatr Infect Dis. 2013;1(1):18-22.
-
12.
Gunta SS, Mak RH. Ghrelin and leptin pathophysiology in chronic kidney disease. Pediatric Nephrology. 2012;28(4):611-6. https://doi.org/10.1007/s00467-012-2380-9.
-
13.
Carrero JJ, Nakashima A, Qureshi AR, Lindholm B, Heimburger O, Barany P, et al. Protein-energy wasting modifies the association of ghrelin with inflammation, leptin, and mortality in hemodialysis patients. Kidney Int. 2011;79(7):749-56. [PubMed ID: 21178976]. https://doi.org/10.1038/ki.2010.487.
-
14.
Mak RH, Cheung W, Purnell J. Ghrelin in chronic kidney disease: too much or too little? Perit Dial Int. 2007;27(1):51-5.
-
15.
Mak RH, Cheung W. Energy homeostasis and cachexia in chronic kidney disease. Pediatr Nephrol. 2006;21(12):1807-14. [PubMed ID: 16897005]. https://doi.org/10.1007/s00467-006-0194-3.
-
16.
Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85(2):495-522. [PubMed ID: 15788704]. https://doi.org/10.1152/physrev.00012.2004.
-
17.
Buscher AK, Buscher R, Hauffa BP, Hoyer PF. Alterations in appetite-regulating hormones influence protein–energy wasting in pediatric patients with chronic kidney disease. Pediatr Nephrol. 2010;25(11):2295-301. https://doi.org/10.1007/s00467-010-1588-9.
-
18.
Arbeiter AK, Buscher R, Petersenn S, Hauffa BP, Mann K, Hoyer PF. Ghrelin and other appetite-regulating hormones in paediatric patients with chronic renal failure during dialysis and following kidney transplantation. Nephrol Dial Transplant. 2008;24(2):643-6. https://doi.org/10.1093/ndt/gfn529.
-
19.
Carrero JJ, Nakashima A, Qureshi AR, Lindholm B, Heimbürger O, Bárány P, et al. Protein-energy wasting modifies the association of ghrelin with inflammation, leptin, and mortality in hemodialysis patients. Kidney Int. 2011;79(7):749-56. https://doi.org/10.1038/ki.2010.487.
-
20.
Piatek J, Gibas-Dorna M, Budzynski W, Krauss H, Marzec E, Olszewski J, et al. Urinary tract infection during pregnancy affects the level of leptin, ghrelin and insulin in maternal and placental blood. Scandinavian journal of clinical and laboratory investigation. 2014;74(2):126-31.
-
21.
Suzuki H, Asakawa A, Amitani H, Nakamura N, Inui A. Ghrelin and cachexia in chronic kidney disease. Pediatric Nephrology. 2012;28(4):521-6. https://doi.org/10.1007/s00467-012-2241-6.
-
22.
Madison LD, Scarlett JM, Levasseur P, Zhu X, Newcomb K, Batra A, et al. Prostacyclin signaling regulates circulating ghrelin during acute inflammation. Journal of Endocrinology. 2008;196(2):263-73. https://doi.org/10.1677/joe-07-0478.
-
23.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656-60. [PubMed ID: 10604470]. https://doi.org/10.1038/45230.
-
24.
Naufel MFS, Bordon M, de Aquino TM, Ribeiro EB, de Abreu Carvalhaes JT. Plasma levels of acylated and total ghrelin in pediatric patients with chronic kidney disease. Pediatric Nephrology. 2010;25(12):2477-82. https://doi.org/10.1007/s00467-010-1628-5.
-
25.
Arvat E, Di Vito L, Broglio F, Papotti M, Muccioli G, Dieguez C, et al. Preliminary evidence that Ghrelin, the natural GH secretagogue (GHS)-receptor ligand, strongly stimulates GH secretion in humans. J Endocrinol Invest. 2000;23(8):493-5. [PubMed ID: 11021763]. https://doi.org/10.1007/BF03343763.
