Abstract
Background:
Between 10 and 20% of children with Kawasaki disease (KD) will not respond to intravenous immunoglobulin (IVIG) treatment, and are prone to coronary abnormalities. A variety of predicting scoring systems, including the Kobayashi system, have been proposed, but have not yet been evaluated using Iranian patients.Objectives:
To evaluate the Kobayashi scoring system with regard to predicting response to IVIG treatment in Iranian children.Patients and Methods:
All patients who received a final diagnosis of KD at Aliasghar children’s hospital between 1982 and 2013, and who met the inclusion criteria, were enrolled in this retrospective cohort study. We excluded patients with missing data, abnormal echocardiographic finding on admission, late admission, atypical or afebrile cases, and those who had received an insufficient amount of IVIG. We compared demographic and echocardiographic data before IVIG, and within 7 days of treatment, as well as C reactive protein (CRP), sodium, aspartate aminotransferase, platelet levels, neutrophil percentage, age of patients, and duration of fever before IVIG administration, in treatment responders and non-responders.Results:
Of the 141 cases, 97 patients met the criteria and were enrolled. Of these, 19 (19.6%) did not respond to IVIG. A total of 61.8% of patients were male, and the mean patient age was 36.9 months (SD = 32.1 months). Echocardiographic evaluation revealed early coronary involvement in 15.3% of patients, and coronary abnormalities were diagnosed in 10% of patients within the first 10 days of presentation and concurrent with their IVIG treatment. A between-groups comparison of quantitative CRP, absolute neutrophil count, and platelet count showed that platelet count alone was significantly higher in nonresponders (P = 0.04). With regard to items of Kobayashi scoring system, data were present for just 41 cases, but a significant difference between the two groups was shown, with the treatment-refractory group having a significantly higher score (P = 0.002). Receiver-operating characteristic curve analysis revealed that the optimum cut-off point for our population would be 2, which makes the sensitivity of the test equal to 75%, with a specificity of 60%.Conclusions:
This preliminary study showed that patients with KD and a high Kobayashi score are at greater risk of being unresponsive to IVIG treatment. Further studies, preferably multicenter evaluations, are required in order to understand the exact application of various scoring systems in the management of people with KD in Iran.Keywords
Kawasaki Disease Immunoglobulins Intravenous Treatment Failure
1. Background
Kawasaki disease (KD) is an acute vasculitis with unknown etiology that occurs in children, and may lead to coronary aneurisms in 25% of untreated cases (1). Resistant cases may need additional doses of intravenous immunoglobulin (IVIG) or corticosteroid treatment, tumor necrosis factor (TNF)-α blockade, cyclosporine A, anti-interleukin (IL)-1, and anti-CD20 therapy (1). As many as 10% - 20% of children with KD have persistent or recurrent fever after IVIG treatment (2), and prediction of nonresponders to this treatment is important, as the nature and pathogenesis of disease is not fully understood, and alternative treatment modalities are limited.
A variety of scoring systems have been proposed for the prediction of IVIG treatment failure (2-4). However, as genetic factors might be important, not only for pathogenesis, but also for treatment response (5), it is necessary to perform further studies in an ethnically different population.
Kobayashi et al. (2006) proposed a risk score in which response to IVIG can be predicted at the onset of disease, according to C reactive protein (CRP), sodium, aspartate aminotransferase (AST), and platelet levels, as well as neutrophil percentage, age of patient, and duration of fever before IVIG administration (3).
2. Objectives
To evaluate the Kobayashi scoring system with regard to predicting response to IVIG treatment in Iranian children. This system was used because it appears to be more practical than other scoring systems, with fewer laboratory findings required for evaluation.
3. Patients and Methods
We enrolled all patients who received a final diagnosis of KD who had been admitted to Aliasghar children’s hospital, a teaching hospital in Tehran, Iran, between 1982 and 2013. During the entire study period, the criteria for a diagnosis of KD was compatible with the diagnostic guidelines for Kawasaki disease (5th revision) (6), that is, fever (a temperature exceeding 38°C for at least 5 days), accompanied by the presence of at least four of the following five findings: bilateral conjunctival infection, changes in the lips and oral cavity, nonpurulent cervical lymphadenopathy, polymorphous exanthema, and changes in the extremities. A retrospective cohort study was designed by reviewing charts, in order to obtain demographic and clinical data relating to the patients at study onset, as well as laboratory findings (CRP, sodium, AST, platelet levels, and neutrophil percentage). IVIG unresponsiveness was defined as persistence of fever for over 48 hours after completion of IVIG infusion (4), and early echocardiographic change was defined as any ectasis or dilation in coronary diameter compared to normal values (internal lumen diameter of ≥ 3 mm in a child < 5 years old or ≥ 4 mm in a child ≥ 5 years old, or if the internal diameter of a segment was at least 1.5 times larger than that of an adjacent segment (3). If a laboratory test was performed at least twice before primary therapy, the highest value was chosen with regard to neutrophil count, AST, and CRP, while the lowest value obtained was chosen for platelet count and sodium. Although Kobayashi et al. proposed a score of ≥ 4 points to designate a high-risk group, we considered scores of ≥ 5 points as high risk for IVIG resistance, as we realized that it may increase specificity (3).
All patients for whom data required for evaluation of risk score was missing, those with abnormal echocardiographic finding on admission or late admission (fever longer than 10 days before admission), atypical patients, and those who had received less than 1 g/kg IVIG as their treatment were excluded.
We classified the remaining patients into two groups: 1) those who clinically responded to IVIG, with cessation of fever and stable normal echocardiographic findings (Group I), and 2) patients who did not respond well to IVIG, showing persistent fever (more than 2 days after IVIG treatment), or relapsing fever, despite being admitted for IVIG treatment in a timely manner, that is, within the first 10 days of illness (Group II). The results of baseline echocardiography (before IVIG treatment) and a second echocardiography (after 7 days of treatment) were collated for both groups, in addition to demographic and laboratory parameters; in cases of multiple laboratory data for a single item, we chose the last to be obtained before IVIG treatment began.
Data were analyzed via statistical package for the social sciences software (version 18), and appropriate statistical tests (the chi square for qualitative parameters and the student’s t-test for quantitative parameters) were used. p values of ≤ 0.05 were considered to be statistically significant.
The research was approved by the ethical committee of Iran University of Medical Sciences; and all aspects of the world medical association declaration of Helsinki were considered.
4. Results
A total of 141 patients had been discharged with a final diagnosis of KD during the study period; after applying exclusion criteria, we enrolled 97 patients, of whom 60 (61.8%) were male and 37 (38.1%) were female, into the study. The mean age of the patients was 36.9 months (SD = 32.1 months). A total of 78 patients (80.4%) responded well to IVIG and constituted group I, while 19 patients (19.6%) did not respond well to this treatment, and were designated as group II. As expected, no significant difference was observed between the two groups with regard to defervescence time after IVIG treatment, being 1.56 days in group I and 3.68 days in group II, with standard deviations of 1.13 and 3.41 days, respectively (P = 0.007). Echocardiographic evaluation revealed early coronary involvement in 15.3% of patients, and coronary abnormalities were diagnosed during the first 10 days of presentation, and concurrent with IVIG treatment, in 10% of patients. All enrolled patients had received two at least 2 g/kg IVIG as their primary treatment, either as a continuous perfusion or in divided doses.
In terms of gender , there was a predominance of males in both groups (49 out of 78 in group I, and 11 out of 19 in group II); however, the observed difference was not significant (P = 0.74). Data for quantitative CRP level, as well as platelet and absolute neutrophil counts on admission, were available for all 97 patients (AST level had been checked in only 41 patients on admission), and the values of these parameters in the two groups are shown in Table 1. Although he differences were not significant (P = 0.21, 0.48, and 0.64, respectively) when they are considered individually, a significantly higher than normal (P = 0.04) platelet count (450000) was observed in group II.
Comparison of Pretreatment C Reactive Protein and Aspartate Aminotransferase Levels, and Platelet and Absolute Neutrophil Counts Between Intravenous Immunoglobulin Responders and Nonresponders in Two Groups of Children with Kawasaki Disease in Aliasghar Children’s Hospital, Tehran, Iran, Between 1982 and 2013a,b
| Group I (n=78) | Group II (n=19) | P Value | |
|---|---|---|---|
| CRP > 10mg/L | 65 (67) | 16 (16.5) | 0.9 |
| CRP < 10 mg/L | 13 (13.4) | 3 (3.1) | |
| Neutrophil count < 1500/mm3 | 2 (2.6) | 0 (0) | 0.48 |
| Neutrophil count > 1500/mm3 | 76 (97.4) | 19 (100) | |
| AST > 48 IU/L c | 9 (22) | 2 (4.9) | 1 |
| AST < 48 IU/L | 24 (58.5) | 6 (14.6) | |
| PLT < 450000/mm3 | 32 (25.8) | 3 (8.61) | 0.04 |
| PLT > 450000/mm3 | 46 (74.2) | 16 (91.4) |
Risk of unresponsiveness to first dose of IVIG, according to the risk score proposed by Kobayashi et al. was then evaluated in the two groups, according to their clinical and echocardiographic findings (Table 2). It was only possible to enroll 41 patients for evaluation, as AST level was required for the scoring system (Table 3). A significant difference between the two groups with regard to scoring ≤ 5 or higher than 5 was observed: the IVIG-refractory group had a significantly higher score (P = 0.002).
The Scoring System Proposed by Kobayashi et al. to Predict Intravenous Immunoglobulin-Refractory Cases of Kawasaki Diseasea
| Risk Score for Prediction of IVIG Resistance | Cut-Off Value | Points Scored |
|---|---|---|
| AST | ≥ 100 IU/L | 2 |
| Sodium | ≤ 133 mmol/L | 2 |
| Duration of illness before initial treatment | ≤ 4 day | 2 |
| Neutrophils 80% | ≥ 80 | 2 |
| CRP | ≥ 10 mg/dL | 1 |
| Age | ≤ 12 mo | 1 |
| Platelet count | ≤ 300000 mm3 | 1 |
There was no significant relationship between the two groups with regard to age (P = 0.78) and gender (P = 69%). The mean score was 1.9 ± 1.9. Considering the cut-point of 5, the test sensitivity and specificity would be 50% and 94%, respectively. The positive predictive value would be 66.6% and the negative predictive value would be 88.5%. The likelihood ratios for a positive test result and a negative test result would be 8.3 and 0.53, respectively.
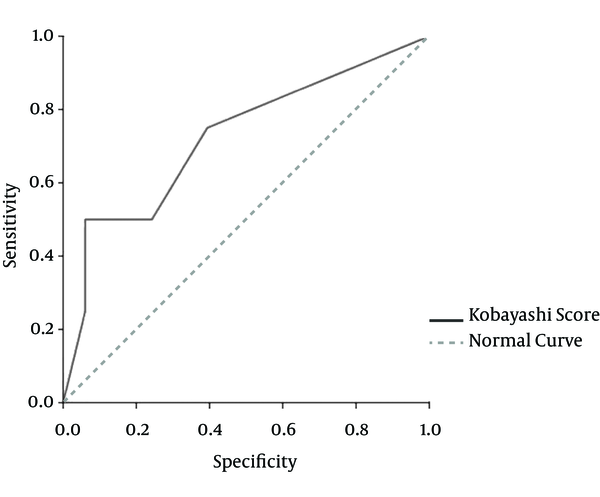
Area under the receiver-operating characteristic (ROC) curve for the Kobayashi score was 0.72 and the best cut-off point for our population would be 2, which makes the sensitivity of the test equal to 75% and the specificity 60%. Considering this cut-off point, the positive and negative predictive value would be 33% and 90%, respectively, with a positive likelihood ratio of 1.8 and a negative likelihood ratio of 0.41 (Figure 1). It can be concluded that if a typical case of KD has none of the risk indexes in this scoring system (i.e. a total risk index of ≤ 1), the probability of unresponsiveness to first IVIG treatment is less than 10%. This could be considered as one of the most important findings since clinical evaluation of KD cases initially began.
Receiver-Operating Characteristic Curve for the Kobayashi Scoring System, Compared with the Patients’ Clinical Outcome
5. Discussion
As KD can present with a varying clinical picture, diagnosis might be difficult and made late, so prediction of unresponsiveness to conventional therapy in patients with this disease is important, not only for the physicians who are caring for the patients, but also for parents who are concerned with regard to cardiac involvement. In addition, when the cost of such a therapy is an issue, as in developing countries, it is crucial to be prepared for further hospitalization and more expensive therapies (2). Sittiwangkul et al. (2006) showed that resistance to IVIG is associated with a higher probability of coronary involvement in the acute phase of disease, and even as long as 2 months after treatment, so it has a poor prognosis with regard to cardiac complications (7). However, the prediction of resistance is likely dependent on a variety of factors, including the race and genetic background of patients. For example, Sleeper et al. found that risk-scoring systems from Japan showed low sensitivity for predicting IVIG resistance in a North American cohort, although they proved to be specific (5). Another US study showed that IVIG resistance appears to be more common in African-Americans (8). Notwithstanding ethnicity, numerous studies have been conducted to predict coronary abnormalities in people with KD, with non-uniform results (2, 3, 5, 7-9).
The Kobayashi et al. study was very large, and evaluated 756 children with KD from 13 hospitals across Japan for primary IVIG resistance. It was found that patients with a high score (≥ 4) were at high risk of IVIG resistance, with sensitivity and specificity being 86% and 67%, respectively. The mean age of the patients was lower than in our study (29 months), but again, approximately 60% of the patients were male. The finding that around 19% of patients were IVIG-unresponsive was similar to the results of our study (2).
Although the mean age of the two groups in our study was not significantly different, some previous studies have considered an age of less than 6 months (10) or 12 months (2, 3, 11) as a determinant for prediction of IVIG unresponsiveness.
Other studies have also included sedimentation rate (7, 12), creatine kinase, creatine kinase MB , N-terminal pro-brain natriuretic peptide (12), and biliribin (13) in resistance prediction. A comparison of various scoring systems shows that the most sensitive (86%) is the Kobayashi system, while the most specific (86%) is the Sano system, in which total bilirubin higher than 0.9 mg/dL, AST level above 200 U/L, and CRP level above 7 mg/dL each get 1 point, but ≥ 2 points has a sensitivity of just 77% (14). Therefore, even with a negative Kobayashi score, IVIG resistance cannot be ruled out (14). Attempts to develop a more sensitive and specific score for patients outside of Japan have thus far been unsuccessful (15).
The mean platelet level in our series of 97 patients was significantly higher in the nonresponsive group, and this level may show not only the magnitude of inflammation, but also the possibility of thrombosis; however, the lowest platelets level has been considered as a predicting factor of nonresponse in most scoring systems (2, 10).
Little is known with regard to the underlying pathogenesis of unresponsiveness, but one study showed that, despite a decrease in IL-6, IL-10 and interferon γ levels following IVIG treatment, the level of TNF-α may increase slightly in patients with coronary abnormalities and IVIG nonresponders. In comparison with responders, the level of aforementioned interleukins was also higher after IVIG treatments in nonresponsive patients, so some cut-off values for serum Th1/Th2 cytokine profile were suggested as predicting the possibility of coronary abnormalities (16).
The selection of a high score for our patients (≥ 5) showed good specificity, but the sensitivity decreased compared with the score proposed by Kobayashi et al (2). The ROC shows that if this score is to be used for excluding the possibility of IVIG unresponsiveness, then those KD patients with a score of < 2 have a very high negative predictive value with regard to Iranian children.
It is important to conduct these studies for prediction of readiness for further therapeutic alternatives. These alternatives include further doses of immunoglubolin, and then methylprednisolone, followed by attempting infliximab, abciximab, cyclosporin A, methotrexate, and cyclophosphamide, in combination with steroids (17), choices that might not be available, especially in remote areas of the country.
Our study had some limitations. First, we did not examine late echocardiographic changes following discharge, as the study was retrospective and cases had been followed up in outpatients in various centers, meaning that late involvement of coronary vessels could not be evaluated. Second, we were unable to include a substantial number of patients, because in these cases, the AST level had not been checked. Third, as different products could be potentially used during the period of study and their effectiveness could potentially be different, we could not have access to identify probably various IVIG products used during the study period.
However, despite these limitations, this preliminary study showed that a Kobayashi score of ≥ 5 can predict IVIG nonresponse in Iranian patients, with 50% sensitivity and 94% specificity. Further studies, preferably prospective multicenter evaluations, are required to understand the exact application of various scoring systems in the management of people with KD in Iran.
References
-
1.
Tacke CE, Burgner D, Kuipers IM, Kuijpers TW. Management of acute and refractory Kawasaki disease. Expert Rev Anti Infect Ther. 2012;10(10):1203-15. [PubMed ID: 23199405]. https://doi.org/10.1586/eri.12.101.
-
2.
Kobayashi T, Inoue Y, Takeuchi K, Okada Y, Tamura K, Tomomasa T, et al. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation. 2006;113(22):2606-12. [PubMed ID: 16735679]. https://doi.org/10.1161/circulationaha.105.592865.
-
3.
Seki M, Kobayashi T, Kobayashi T, Morikawa A, Otani T, Takeuchi K, et al. External validation of a risk score to predict intravenous immunoglobulin resistance in patients with kawasaki disease. Pediatr Infect Dis J. 2011;30(2):145-7. [PubMed ID: 20802375]. https://doi.org/10.1097/INF.0b013e3181f386db.
-
4.
Cha S, Yoon M, Ahn Y, Han M, Yoon KL. Risk factors for failure of initial intravenous immunoglobulin treatment in Kawasaki disease. J Korean Med Sci. 2008;23(4):718-22. [PubMed ID: 18756064]. https://doi.org/10.3346/jkms.2008.23.4.718.
-
5.
Sleeper LA, Minich LL, McCrindle BM, Li JS, Mason W, Colan SD, et al. Evaluation of Kawasaki disease risk-scoring systems for intravenous immunoglobulin resistance. J Pediatr. 2011;158(5):831-835000. [PubMed ID: 21168857]. https://doi.org/10.1016/j.jpeds.2010.10.031.
-
6.
Ayusawa M, Sonobe T, Uemura S, Ogawa S, Nakamura Y, Kiyosawa N, et al. Revision of diagnostic guidelines for Kawasaki disease (the 5th revised edition). Pediatrics International. 2005;47(2):232-4. https://doi.org/10.1111/j.1442-200x.2005.02033.x.
-
7.
Sittiwangkul R, Pongprot Y, Silvilairat S, Phornphutkul C. Management and outcome of intravenous gammaglobulin-resistant Kawasaki disease. Singapore Med J. 2006;47(9):780-4. [PubMed ID: 16924360].
-
8.
Moffett BS, Syblik D, Denfield S, Altman C, Tejtel-Sexson K. Epidemiology of immunoglobulin resistant Kawasaki disease: results from a large, national database. Pediatr Cardiol. 2015;36(2):374-8. [PubMed ID: 25179461]. https://doi.org/10.1007/s00246-014-1016-1.
-
9.
Karimi A, Nateghian AR, KARIMI M, Shirvani B. Association Of Cardiovascular Complications With Acute Phase Response In Kawasaki Patients. Sci J Hamedan Uni Med Sci. 2002;9(3):17-21.
-
10.
Egami K, Muta H, Ishii M, Suda K, Sugahara Y, Iemura M, et al. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J Pediatr. 2006;149(2):237-40. [PubMed ID: 16887442]. https://doi.org/10.1016/j.jpeds.2006.03.050.
-
11.
Rigante D, Valentini P, Rizzo D, Leo A, De Rosa G, Onesimo R, et al. Responsiveness to intravenous immunoglobulins and occurrence of coronary artery abnormalities in a single-center cohort of Italian patients with Kawasaki syndrome. Rheumatol Int. 2010;30(6):841-6. [PubMed ID: 20049445]. https://doi.org/10.1007/s00296-009-1337-1.
-
12.
Lee SM, Lee JB, Go YB, Song HY, Lee BJ, Kwak JH. Prediction of Resistance to Standard Intravenous Immunoglobulin Therapy in Kawasaki Disease. Korean Circ J. 2014;44(6):415-22.
-
13.
Sano T, Kurotobi S, Matsuzaki K, Yamamoto T, Maki I, Miki K, et al. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur J Pediatr. 2007;166(2):131-7. [PubMed ID: 16896641]. https://doi.org/10.1007/s00431-006-0223-z.
-
14.
Eleftheriou D, Levin M, Shingadia D, Tulloh R, Klein NJ, Brogan PA. Management of Kawasaki disease. Arch Dis Child. 2014;99(1):74-83. [PubMed ID: 24162006]. https://doi.org/10.1136/archdischild-2012-302841.
-
15.
Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153(1):117-21. [PubMed ID: 18571548]. https://doi.org/10.1016/j.jpeds.2007.12.021.
-
16.
Wang Y, Wang W, Gong F, Fu S, Zhang Q, Hu J, et al. Evaluation of intravenous immunoglobulin resistance and coronary artery lesions in relation to Th1/Th2 cytokine profiles in patients with Kawasaki disease. Arthritis Rheum. 2013;65(3):805-14. [PubMed ID: 23440694]. https://doi.org/10.1002/art.37815.
-
17.
Shiari R. Kawasaki Disease; A Review Article. Archives of Pediatric Infectious Diseases. 2013;2(1):154-9.