Abstract
Background:
Nosocomial Infection (NI) is one of the leading causes of short- and long-term morbidity and mortality among neonates, especially in Neonatal Intensive Care Units (NICUs).Objectives:
We aimed to evaluate the epidemiology of NIs and associated factors.Methods:
From March 2017 to September 2018, all the neonates who were admitted to the NICUs of Bahrami Children’s Hospital were enrolled. Nosocomial infections were identified based on the definition of CDC-NNIS. Demographic, clinical, and laboratory data of the patients were extracted from the medical records.Results:
A total of 979 neonates were admitted to the NICU, of whom 60 were diagnosed with NI. The incidence of NI was 6.1 per 100 hospitalized patients. The most prevalent NI was bloodstream infection (30%), followed by pneumonia (21.7%). The most frequent presentations were respiratory distress (31.7%) and poor feeding (26.7%). Major pathogens were Gram-positive bacteria such as Staphylococcus aureus (25.7%) and coagulase-negative staphylococci (25.7%). The mean hospital stay was 25.2 ± 20.89 days. The mortality rate of patients with NI was 16.7%. The factors associated with an increased risk of mortality among patients with NI were a lack of ventilation support, low birth weight, and WBCs with an abnormal range.Conclusions:
The results of the present study showed that the incidence of NI was high, and the cultures collected from body fluids had a particular role in the diagnosis and treatment of NI. Standard infection control practices should be applied to reduce the incidence of NI and subsequent morbidity and mortality.Keywords
Neonatal Intensive Care Unit Nosocomial Infection Neonates Infection
1. Background
Nosocomial Infection (NI) is an important cause of mortality among neonates administrated to Neonatal Intensive Care Units (NICUs) (1). Hospitalized neonates, due to their immaturity and weak immune system are at a greater risk for infections (2). It is also found that survivors are vulnerable to long-term neurodevelopmental morbidity (1). Recent progress in taking care of newborns utilizing intensive interventions has led to notable improvements in their survival (3). Nevertheless, prolonged hospitalization and the use of invasive procedures may dispose them to NIs. This clinical condition is usually considered as the infection acquired after 48 hours of hospitalization in patients admitted for reasons apart from that infection (4, 5).
Nosocomial infections are estimated as the cause of 4–56% of all infants’ mortality (6). They also have a great impact on the health-care system by increasing the length of hospital stay and adding to medical expenses, which are very important issues, especially in developing countries. Prematurity, low birth weight, use of invasive devices, and delayed initiation of enteral nutrition are some of the identified risk factors associated with NI (7). Generally, bloodstream infections, mostly caused by central catheterization, and ventilator-related pneumonia are considered the most frequent type of NIs (8-10). Over the last decade, Gram-positive organisms have been reported as the main causes of NIs that can be resistant to multiple drugs (11, 12).
Due to changes in the patterns of antibiotic use, organisms responsible for NI differ among regions and over time. Thus, it is important to monitor the epidemiology of NIs.
2. Objectives
Herein, in the current study, we aimed to evaluate the epidemiology of NIs among neonates in the NICUs of Bahrami Children's Hospital. Moreover, the mortality rate and its related factors were sought in these patients.
3. Methods
3.1. Study Design, Setting, and Population
We conducted a historical cohort study at the NICUs of Bahrami Children’s Hospital of Iran, a tertiary center for high-risk newborns with an average of 900 annual admissions. The hospital consisted of two NICUs, number I and number II, with 17 and 7 inpatient beds, respectively. The NICU No. I was staffed with 17 nurses, four pediatric residents, and two neonatologists while in the NICU No. II, there were 10 nurses, two pediatric residents, and one neonatologist. The average ratio of nurses to hospitalized patients at different shifts was 1:3.
From March 2017 to September 2018, all the neonates who attended the NICUs of the hospital were evaluated. The diagnoses of NIs were coded based on the definition of the Centers for Disease Control and Prevention-Nosocomial Infections Surveillance System (CDC-NNIS) (5). Therefore, an infection acquired after 48 hours of hospitalization without any evidence of that infection at the time of admission was considered an episode of NI. The diagnosis of NI was based on the identification of clinical manifestations of infection (e.g., lethargy, poor feeding, fever, and respiratory signs) or positive cultured specimens (blood, cerebrospinal fluid, urine, eye discharge, tracheal secretion, and wound discharge). Patients older than one-month-old or with incomplete medical records were excluded.
Among 979 admitted neonates during the study period, 336 suspected cases were selected by the supervisor of infectious diseases of the hospital. After gathering the data from patients’ medical records and laboratory databases, NI was confirmed in 60 of the neonates.
3.2. Data Collection and Ethical Consideration
The demographic, clinical, and laboratory features of the neonates were obtained from the medical records. The following data were recorded: gender, gestational age, birth weight, type of delivery, the reason for admission, age at admission, accompanying comorbidity, Total Parenteral Nutrition (TPN), use of ventilation support, site of nosocomial infection, length of hospital stay, clinical manifestation, paraclinical findings, and mortality rate. The protocol of the study was reviewed and approved by the Institutional Review Board of Tehran University of Medical Sciences, Tehran, Iran (ethical code: 9411165037).
3.3. Statistical Analysis
Measurements were reported as absolute numbers and percentages for categorical variables and mean ± Standard Deviation (SD) for quantitative variables. For comparison of proportions, Pearson's chi-square tests and Fisher's exact test were used. For the comparison of quantitative variables, a t-test or nonparametric Mann-Whitney U test was used. Multivariate logistic regression analysis was also applied to identify the potential risk factors for mortality. Analyses were conducted by SPSS version 20.0 statistical software, and P values less than 0.05 were considered statistically significant.
4. Results
4.1. Maternal, Characteristics, and Clinical Features of Neonates
From 979 neonates who were admitted to the NICUs of the hospital, 60 patients who fulfilled the criteria of the study were enrolled. The incidence of infection was 6.1 per 100 hospitalized patients. The three most common reasons for admission to the NICU were respiratory disorders (33.3%), seeking surgical procedures (25%), and icterus (23.3%). The mean age of the neonates at the time of admission was 6.65 ± 6.8 days. Of the neonates, 27 (45%) had concomitant comorbidity. The most common comorbidity was congenital anomalies in 12 (44.4%) patients, followed by cardiac, respiratory, and renal-related comorbidities in seven (25.9%) patients, three (11.1%) patients, and one (3.7%) patient, respectively. Maternal, demographic, and clinical information of the neonates is depicted in Table 1.
Patient’s Demographic and Clinical Data Before Developing Nosocomial Infection
| Variable | No. (%) |
|---|---|
| Gestational Age | |
| < 37 weeks | 32 (53.3) |
| ≥ 37 weeks | 28 (46.7) |
| Birth Weight | |
| ≤ 2500 g | 15 (25) |
| > 2500 g | 45 (75) |
| Gender | |
| Male | 35 (58.3) |
| Female | 25 (41.7) |
| Type of Delivery | |
| Vaginal | 18 (30) |
| Cesarean section | 42 (70) |
| Age at Admission | |
| ≤ 7 days | 44 (73.3) |
| > 7 days | 16 (26.7) |
| Presence of Comorbidity | |
| Yes | 27 (45) |
| No | 33 (55) |
| Ventilation Support | |
| Yes | 26 (43.3) |
| No | 34 (56.7) |
| Total Parenteral Nutrition | |
| Yes | 39 (65) |
| No | 21 (35) |
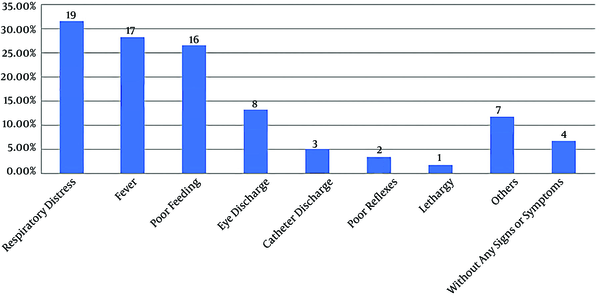
In our study, the mean duration of developing NI was 6.8 ± 5.2 days. The infection was presented in the majority of the neonates as respiratory distress (31.7%; N = 19) and poor feeding (26.7%; N = 16) (Figure 1). The commonest type of infection was bloodstream infection (30%; N = 18), followed by pneumonia (21.7%; N = 13), conjunctivitis (13.3%; N = 8), catheter-related infection (13.3%; N = 8), enterocolitis (10%; N = 6), urinary tract infection (6.6%; N = 4), and meningitis (5%; N = 3).
Distribution of signs and symptoms related to nosocomial infection
4.2. Laboratory and Imaging Findings
Among the NI cases, abnormal WBC was detected in 17 (28.3%) neonates, with 13 (21.6%) having leukocytosis (WBC > 20,000/mm3) and four (6.66%) having leukopenia (WBC < 5,000/mm3). A positive CRP level (> 6 mg/L) was found in 27 (45%) cases. Moreover, in the imaging modalities (chest X-ray, abdominal X-ray, sonography) of 17 neonates, abnormal features were detected.
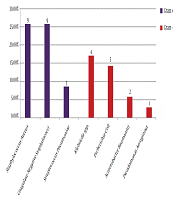
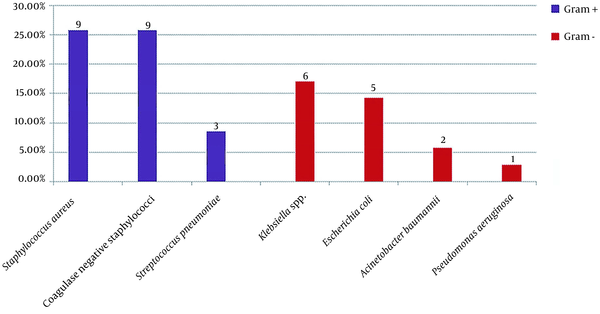
Among 60 cultures collected from body fluids and secretions of the infection site, 35 (58.3%) showed the growth of different microorganisms. The frequencies of the positive cultures obtained from different sites were as follows: blood (21.7%; N = 13), endotracheal tube (11.7%; N = 7), eye discharge (10%; N = 6), urine (6.7%; N = 4), infectious wound discharge (5%; N = 3), and cerebrospinal fluid (3.3%; N = 2). Coagulase-negative staphylococci and Staphylococcus aureus were the most prevalent causes of NI in our study (Figure 2).
Distribution of microorganisms related to nosocomial infection
4.3. Length of Hospital Stay and Mortality Rate
The mean length of hospital stay in the NI cases was 25.2 ± 20.89 days. Furthermore, we assessed the effect of demographic features, clinical data, and laboratory findings on the mean length of hospitalization. The only factors that reached the level of significance were the use of ventilation support and the results of cultures. The mean length of hospital stay was 19.2 ± 9.24 days in neonates with ventilation support and 33.2 ± 28.3 days in neonates with no ventilation support (P value = 0.027). The duration of hospitalization in cultures with negative and positive results was 34.1 ± 27.59 and 18.8 ± 12.23 days, respectively (P value = 0.007).
The overall mortality rate due to NI in our study was 16.7% (N = 10). Multivariable logistic analysis showed that mortality was significantly higher among neonates with LBW (adjusted OR = 6.8, 95% CI: 1.59-29.31), no ventilation support (adjusted OR = 0.05, 95% CI: 0.01-0.49), and WBCs in abnormal range (adjusted OR = 5.31, 95% CI: 1.27-22.25) (Table 2).
Multivariate Analysis of Potential Risk Factors for Mortality in Patients with Nosocomial Infection
| Variables | Mortality, No. (%) | Adjusted OR (95% CI) | P Valuea |
|---|---|---|---|
| Gestational age | 1.38 (0.34 - 5.51) | 0.644 | |
| < 37 weeks | 6 (10) | ||
| ≥ 37 weeks | 4 (6.6) | ||
| Birth weight | 6.83 (1.59 - 29.31) | 0.009 | |
| ≤ 2500 g | 6 (10) | ||
| > 2500 g | 4 (6.6) | ||
| Gender | 1.08 (0.27 - 4.33) | 0.906 | |
| Male | 6 (10) | ||
| Female | 4 (6.6) | ||
| Type of delivery | 1.00 (0.22 - 4.40) | 1.000 | |
| Vaginal | 3 (5) | ||
| Cesarean section | 7 (11.6) | ||
| Age at admission | 1.55 (0.29 - 8.24) | 0.603 | |
| ≤ 7 days | 8 (13.3) | ||
| > 7 days | 2 (3.3) | ||
| Presence of comorbidity | 2.07 (0.51 - 8.26) | 302 | |
| Yes | 6 (10) | ||
| No | 4 (6.6) | ||
| No ventilation support | 17.47 (2.04 - 149.57) | 0.009 | |
| Yes | 1 (1.6) | ||
| No | 9 (15) | ||
| Total parenteral nutrition | 0.16 (0.01 - 1.41) | 0.101 | |
| Yes | 1 (1.6) | ||
| No | 9 (15) | ||
| Fever | 0.58 (0.11 - 3.07) | 0.525 | |
| Yes | 2 (3.3) | ||
| Rectal temperature >38°C | |||
| No | 8 (13.3) | ||
| WBC | 5.31 (1.27 - 22.25) | 0.022 | |
| Abnormal | 6 (10) | ||
| > 20,000/mm3 | |||
| < 5,000/mm3 | |||
| Normal | 4 (6.6) | ||
| CRP | 4.14 (0.75 - 22.58) | 0.103 | |
| Positive (> 6 mg/L) | 6 (10) | ||
| Negative | 4 (6.6) | ||
| Culture | 0.66 (0.17 - 2.60) | 0.559 | |
| Positive | 5 (8.3) | ||
| Negative | 5 (8.3) |
5. Discussion
In recent years, developments in NICUs have increased the survival of neonates with severe pathologic conditions. However, the extensive use of invasive devices may increase the risk of NI. The first step for reducing the NI frequency is to determine the incidence, predisposing factors, and consequences of NI.
In our study, the incidence of NI in NICUs was 6.1 per 100 hospitalized patients. Previous studies in Iran have reported a rate of 5.7% in Hamadan (8), 7.4% in Isfahan (13), and 13.5% in Tehran (14). The reported incidence in some of the developed countries with the advanced health-care system is 11% in the USA (15) and 14% in Canada (16). Contrarily, developing countries have reported higher rates as follows: 8.3% in Turkey (17), 19.2% in Saudi Arabia (18), and 21.4% in Egypt (19). Some reasons can explain this variation in the incidence rate of NIs. The first reason is the different methods for the detection of NI. Although the CDC definition has been mostly used in these studies, some others only have relied on positive cultures. The other reasons may be the differences in underlying conditions, treatment strategy, length of hospital stay, and different methods of study. Thus, making a direct comparison among these studies may be inaccurate. With a brief review, it seems that the prevalence of NIs in the NICUs is lower in Iran than in other parts of the world. However, with the provided data, it is not possible to conclude that Iranian hospitals have a better condition in terms of controlling NIs. Some possible issues, including a lack of a comprehensive information recording system, extensive use of antibiotics, and lack of attention to mild signs and symptoms of infection may contribute to the less diagnosis of NIs.
Bloodstream infections and pneumonia, in sequence, were the most common NIs in our research. Likewise, the most common NIs reported in the relative literature were bloodstream infections with a rate of 26% to 81.7% and pneumonia with a rate of 10.2% to 52% (8, 14, 19, 20). In our study, the majority of the infected patients were on TPN, which is not surprising as it was stated as a risk factor for NI in a recent meta-analysis (7). Thus, to reduce the NI incidence, TPN should be applied only with appropriate indications. Moreover, standard infection control practice should be reconsidered for staff and enteral nutrition should be initiated as soon as possible. We observed that approximately 70% of the neonates with NIs were younger than seven-days-old, which suggests they might be more vulnerable due to the immaturity of their immune systems. Most of the infected neonates were delivered via caesarian sections, which might be related to its preference among Iranian women. Thus, to be considered as a risk factor for NIs, caesarian sections should be evaluated in a controlled study.
In the present study, the majority of the neonates showed clinical signs and symptoms related to infection. Although clinical manifestations were frequently subtle and nonspecific, they can be helpful in the early detection of NIs. Respiratory distress, poor feeding, and fever were the most common presentations of NIs in neonates, which was in line with other studies (21, 22). In our study, 58.3% of the neonates had positive cultures. Moreover, positive CRP levels and abnormalities in WBC were detected in 45% and 28.3% of the neonates, respectively. This shows that cultures are still the best laboratory test for a definite diagnosis of NI.
In the current paper, the most frequently isolated pathogens were Staphylococcus aureus, coagulase-negative staphylococci, and Klebsiella spp. The detection of Staphylococcus spp. as the most common pathogen, could be related to the handling of the neonates by their families and health care providers. Therefore, the adherence of the staff to hand hygiene procedures could interrupt the chain of contamination (23). Moreover, it has been suggested that this microorganism could also have aerosol transmission (24).
The incidence of NI pathogens varies among different regions and over time. In some of the previous studies, Gram-positive cocci were mostly responsible for NIs (8, 12, 13, 20), and in some others, Gram-negative rods were the most frequently detected pathogens (14, 18, 19). Due to antimicrobial resistance increment among Gram-negative rods, their incidence in NICUs is increasing, which has led to several nosocomial outbreaks in NICUs over recent years (25, 26). However, in our study, Gram-positive cocci were still the most prevalent pathogens, as it was responsible for 60% of NI episodes.
The mean duration of hospitalization in our study was 25.2 days. It was revealed that the length of hospitalization was shorter for patients with a positive result for cultures. This suggests that a definite diagnosis would contribute to more efficient treatment strategies and, thus, earlier hospital discharge. The overall mortality rate of neonates due to NI was 16.7% in this study. The reported death rates in several previous studies were as follows: 45% in Greece (10), 40.3% in Saudi Arabia (18), 12.8% in South Korea (20), 13% in Turkey (17), and 29.6% in Iran (14). The result of this study revealed that neonates with low birth weight have a significantly higher risk of mortality, and abnormalities in WBC were associated with a poorer outcome. Although the use of ventilation support is considered a risk factor for NIs, we did not find any association with a poorer prognosis. Due to our observations, the survival of patients with ventilation support was better, and they had a shorter duration of hospitalization, but according to the small sample size and possible effect of confounding factors, it could not be considered a protective factor.
Due to the retrospective design of this study, some patients with NI may not have been registered. Only neonates with a definite diagnosis of NI and those with complete and accurate records were included in the present study. This issue should be considered in interpreting the results, as the reported prevalence might be lower than the actual rate. The selection of the subjects from one center is another limitation of this study, which could restrict the generalization of the findings.
5.1. Conclusion
Nosocomial infection is an important complication of hospitalized neonates with a high incidence at NICUs. The early diagnosis is difficult and both clinical manifestations and laboratory assessments should be considered. The cultures collected from body fluids had a particular role in the diagnosis and treatment of NIs. The distributions of the related pathogens were similar to the reports of previous studies. The mortality rate was relatively high and significantly associated with low birth weight, abnormalities in WBC counts, and lack of ventilation support.
References
-
1.
Fleischmann-Struzek C, Goldfarb DM, Schlattmann P, Schlapbach LJ, Reinhart K, Kissoon N. The global burden of paediatric and neonatal sepsis: a systematic review. Lancet Respir Med. 2018;6(3):223-30. https://doi.org/10.1016/s2213-2600(18)30063-8.
-
2.
Basha S, Surendran N, Pichichero M. Immune responses in neonates. Expert Rev Clin Immunol. 2014;10(9):1171-84. [PubMed ID: 25088080]. [PubMed Central ID: PMC4407563]. https://doi.org/10.1586/1744666X.2014.942288.
-
3.
Shah V, Warre R, Lee SK. Quality improvement initiatives in neonatal intensive care unit networks: achievements and challenges. Acad Pediatr. 2013;13(6 Suppl):S75-83. [PubMed ID: 24268090]. https://doi.org/10.1016/j.acap.2013.04.014.
-
4.
Kouchak F, Askarian M. Nosocomial infections: the definition criteria. Iran J Med Sci. 2012;37(2):72-3. [PubMed ID: 23115435]. [PubMed Central ID: PMC3470069].
-
5.
Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-32. [PubMed ID: 18538699]. https://doi.org/10.1016/j.ajic.2008.03.002.
-
6.
Khan HA, Baig FK, Mehboob R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac J Trop Biomed. 2017;7(5):478-82. https://doi.org/10.1016/j.apjtb.2017.01.019.
-
7.
Wang L, Du KN, Zhao YL, Yu YJ, Sun L, Jiang HB. Risk Factors of Nosocomial Infection for Infants in Neonatal Intensive Care Units: A Systematic Review and Meta-Analysis. Med Sci Monit. 2019;25:8213-20. [PubMed ID: 31675354]. [PubMed Central ID: PMC6849370]. https://doi.org/10.12659/MSM.917185.
-
8.
Basiri B, Sabzehei MK, Shokouhi M, Moradi A. Evaluating the Incidence and Risk Factors of Nosocomial Infection in Neonates Hospitalized in the Neonatal Intensive Care Unit of Fatemieh Hospital in Hamadan, Iran, 2012 - 2013. Arch Pediatr Infect Dis. 2015;3(2). https://doi.org/10.5812/pedinfect.23327.
-
9.
Kilic A, Okulu E, Kocabas BA, Alan S, Cakir U, Yildiz D, et al. Health care-associated infection surveillance: A prospective study of a tertiary neonatal intensive care unit. J Infect Dev Ctries. 2019;13(3):181-7. [PubMed ID: 32040446]. https://doi.org/10.3855/jidc.10688.
-
10.
Christina N, Ioanna P, George L, Konstantinos T, Georgios S. Risk factors for nosocomial infections in neonatal intensive care units (NICU). Health Sci J. 2015;9(2):1.
-
11.
Huynh BT, Padget M, Garin B, Herindrainy P, Kermorvant-Duchemin E, Watier L, et al. Burden of bacterial resistance among neonatal infections in low income countries: how convincing is the epidemiological evidence? BMC Infect Dis. 2015;15:127. [PubMed ID: 25888320]. [PubMed Central ID: PMC4364576]. https://doi.org/10.1186/s12879-015-0843-x.
-
12.
Mariani M, Bandettini R, L. A. Masa D, Minghetti D, Baldelli I, Serveli S, et al. Bacterial invasive infections in a neonatal intensive care unit: a 13 years microbiological report from an Italian tertiary care centre. J Prev Med Hyg. 2020;61(2):E162-6. [PubMed ID: 32803000]. [PubMed Central ID: PMC7419127]. https://doi.org/10.15167/2421-4248/jpmh2020.61.2.1401.
-
13.
Kabiri Samani M, Keivanfar M, Firouzi H, Seyedi SJ, Kianifar H. Bacterial infections and relevant factors in neonates hospitalized at intensive care unit. Iran J Neonatol. 2019;10(3):1-6.
-
14.
Choobdar F, Vahedi Z, Khosravi N, Khalesi N, Javid A, Shojaee S. Nosocomial Infection in an Iranian Neonatal Intensive Care Unit: Hospital Epidemiology and Risk Factors. Arch Pediatr Infect Dis. 2020;8(4). https://doi.org/10.5812/pedinfect.96850.
-
15.
Horbar JD, Edwards EM, Greenberg LT, Morrow KA, Soll RF, Buus-Frank ME, et al. Variation in Performance of Neonatal Intensive Care Units in the United States. JAMA Pediatr. 2017;171(3). e164396. [PubMed ID: 28068438]. https://doi.org/10.1001/jamapediatrics.2016.4396.
-
16.
Lee SK, Aziz K, Singhal N, Cronin CM. The Evidence-based Practice for Improving Quality method has greater impact on improvement of outcomes than dissemination of practice change guidelines and quality improvement training in neonatal intensive care units. Paediatr Child Health. 2015;20(1):e1-9. [PubMed ID: 25722645]. [PubMed Central ID: PMC4333759].
-
17.
Tekin R, Dal T, Pirinccioglu H, Oygucu SE. A 4-year surveillance of device-associated nosocomial infections in a neonatal intensive care unit. Pediatr Neonatol. 2013;54(5):303-8. [PubMed ID: 23643153]. https://doi.org/10.1016/j.pedneo.2013.03.011.
-
18.
Mahfouz AA, Al-Azraqi TA, Abbag FI, Al-Gamal MN, Seef S, Bello CS. Nosocomial infections in a neonatal intensive care unit in south-western Saudi Arabia. East Mediterr Health J. 2010;16(1):40-4. [PubMed ID: 20214156].
-
19.
Abdel-Wahab F, Ghoneim M, Khashaba M, El-Gilany AH, Abdel-Hady D. Nosocomial infection surveillance in an Egyptian neonatal intensive care unit. J Hosp Infect. 2013;83(3):196-9. [PubMed ID: 23374289]. https://doi.org/10.1016/j.jhin.2012.10.017.
-
20.
Jeong IS, Jeong JS, Choi EO. Nosocomial infection in a newborn intensive care unit (NICU), South Korea. BMC Infect Dis. 2006;6:103. [PubMed ID: 16796741]. [PubMed Central ID: PMC1552075]. https://doi.org/10.1186/1471-2334-6-103.
-
21.
Mahallei M, Rezaee MA, Mehramuz B, Beheshtirooy S, Abdinia B. Clinical symptoms, laboratory, and microbial patterns of suspected neonatal sepsis cases in a children's referral hospital in northwestern Iran. Medicine (Baltimore). 2018;97(25). e10630. [PubMed ID: 29923969]. [PubMed Central ID: PMC6024470]. https://doi.org/10.1097/MD.0000000000010630.
-
22.
Hematyar M, Najibpour R, Bayesh S, Hojjat A, Farshad A. Assessing the Role of Clinical Manifestations and Laboratory Findings in Neonatal Sepsis. Arch Pediatr Infect Dis. 2016;5(1). https://doi.org/10.5812/pedinfect.29985.
-
23.
Sypsa V, Psichogiou M, Bouzala GA, Hadjihannas L, Hatzakis A, Daikos GL. Transmission dynamics of carbapenemase-producing Klebsiella pneumoniae and anticipated impact of infection control strategies in a surgical unit. PLoS One. 2012;7(7). e41068. [PubMed ID: 22859965]. [PubMed Central ID: PMC3409200]. https://doi.org/10.1371/journal.pone.0041068.
-
24.
Mirhoseini SH, Didehdar M, Akbari M, Moradzadeh R, Jamshidi R, Torabi S. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia. 2020;36(2):225-32. https://doi.org/10.1007/s10453-020-09624-0.
-
25.
Ferry A, Plaisant F, Ginevra C, Dumont Y, Grando J, Claris O, et al. Enterobacter cloacae colonisation and infection in a neonatal intensive care unit: retrospective investigation of preventive measures implemented after a multiclonal outbreak. BMC Infect Dis. 2020;20(1):682. [PubMed ID: 32942989]. [PubMed Central ID: PMC7500001]. https://doi.org/10.1186/s12879-020-05406-8.
-
26.
Piepenbrock E, Higgins PG, Wille J, Xanthopoulou K, Zweigner J, Jahn P, et al. Klebsiella variicola causing nosocomial transmission among neonates - an emerging pathogen? J Med Microbiol. 2020;69(3):396-401. [PubMed ID: 32125266]. https://doi.org/10.1099/jmm.0.001143.