Abstract
Keywords
COVID-19 Algorithmic Approach Diagnosis Treatment SARS-CoV-2
1. Introduction
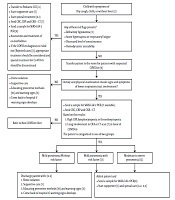
After the outbreak of 2019 novel corona virus infection in China, we faced with the outbreak of disease in other countries such as Iran. Until this moment (March 05, 2020) a total number of confirmed cases more than 3500 with approximately 3.3% deaths have been reported around 31 provinces in Iran. Although there are a few reported cases of corona virus disease 2019 (COVID-19) in children among these cases in Iran and other countries (1-3), we need to have a diagnostic and therapeutic protocol to standardizing prevention and management of COVID-19 in children and differentiation it from other do, which are mimicking it. This algorithm is based on the latest diagnosis and treatment strategies to prevention and treatment of COVID-19 infection (Figure 1) (4, 5).
Algorithmic approach to a child suspected [1] to COVID-19
![Algorithmic approach to a child suspected [1] to COVID-19 Algorithmic approach to a child suspected [1] to COVID-19](https://neoscriber.org/cdn/serve/31514/38784d2b579f7d49f6e051fb03c53cfbfa89d1df/apid-8-2-102400-i001-preview.png)
2. Description of Algorithm
[1] Case Definition
Definite, a child with history, signs and symptoms suggested to COVID-19, together with abnormal chest CT scan and positive PCR test is defined as a definite case of COVID-19.
Suspected, a child with history, signs and symptoms and abnormal chest CT scan (If the other causes for abnormal CT are ruled out) suggested to COVID-19, with negative PCR test is defined as a suspected case of COVID-19.
Rejected, a child with negative PCR test and abnormal chest CT scan due to the other causes except COVID-19 is defined as a rejected case of COVID-19.
[2] Triage
The child suspected of COVID-19 is transferred to a specialized triage room and the child and his parents wear surgical masks. The triage nurse should also have a surgical mask.
[3] Refractory Hypoxemia
There is no clear-cut definition of acute refractory hypoxemia; it generally refers to inadequate arterial oxygenation despite optimal levels of inspired oxygen. O2 saturation < 93% despite O2 therapy or O2 saturation < 90 % at room air is in favor of severe pneumonia (6).
[4] Hospitalization
Patients should be placed in a private room with negative air pressure that has a minimum of 6 to 12 air changes per hour. Doors to the isolation rooms must remain closed, and all individuals who enter must wear a mask with a filtering capacity of 95 percent that allows a tight seal over the nose and mouth, especially during aerosol-generating procedures like endotracheal intubation; therefore, both airborne and contact precautions are warranted (7).
In the absence of a room with negative air pressure, patients are kept in a private isolated room. In the absence of a private room; patients are kept in a cohort at least one meter away.
[5] Supportive Care
Severe cases require appropriate supportive care, including fluid and electrolyte replacement, antipyretics, analgesia, oxygenation and respiratory support (8).
[6] Special Therapy
It should be noted that due to insufficient evidence for COVID-19 treatment in children, recommended treatment protocol is not based on strong clinical evidence, and treatment may be changed if further studies are undertaken.
[6.1] ICU Admitted Patients
Treatment for patients who admitted in intensive care unit is included combined antiviral agents and immunomodulators [oseltamivir + hydroxychloroquine + Kaletra (lopinavir + ritonavir)] ± ribavirin and if necessary antibiotics according to the patient’s situation.
[6.2] Moderate to Severe Pneumonia
Treatment for these patients is included combined antiviral agents and immunomodulators [oseltamivir + hydroxychloroquine + Kaletra (lopinavir + ritonavir)], and if necessary antibiotics according to the patient’s situation.
[6.3] Mild Pneumonia with Risk Factor
Treatment for these patients is included combined antiviral agents and immunomodulators (oseltamivir + hydroxychloroquine), and if necessary antibiotics according to the patient’s situation.
[6.4] Mild Pneumonia Without Risk Factor
These patients should be placed in a watchful waiting program and followed. Treatment in these groups is optional and related to physician decision according to the situation. It may be included (oseltamivir ± hydroxychloroquine), and if necessary antibiotics.
Recommended drug dosages are as follow:
I. Oseltamivir
- Preterm infants consult with a Pediatric Infectious Diseases Specialist.
- Term infants 0 - 12 month, 3 mg/kg/dose, twice daily
- Children ≥ 12 month by body weight
● ≤ 5 kg: 30 mg, twice daily
● > 15 - 23 kg: 45 mg, twice daily
● > 23 - 40 kg: 60 mg, twice daily
● > 40 kg: 75 mg, twice daily
- Adults 75 mg, twice daily
For at least 5 days.
II. Hydroxychloroquine
Infants and children: IV, hydroxychloroquine sulfate: 3 - 5 mg/kg/day (max dose 400 mg), BID For 5 days.
QT interval prolongation, torsades de pointes, and ventricular arrhythmias reported with Chloroquine specially in concurrent use with Kaletra; risk is greater if chloroquine is administered at high doses; use with caution in patients with cardiac disease, a history of ventricular arrhythmias, uncorrected hypokalemia and/or hypomagnesemia, or bradycardia (< 50 bpm), and it can also be used as a single dose in high risk patients.
ECG prior to starting chloroquine and after onset of drug, cardiac monitoring is recommended.
III. Kaletra (Lopinavir + Ritonavir)
● 14 days to 12 months: 16 mg/kg/dose or 300 mg/m2/dose (lopinavir component) orally twice a day
● 12 months to 18 years: Based on BSA: 230 mg/m2/dose (lopinavir component) orally twice a day (maximum dose: lopinavir 400 mg), ritonavir 100 mg/dose, orally twice a day.
Based on weight:
- Less than 15 kg: 12 mg/kg/dose (lopinavir component) orally twice a day
- 15 to 40 kg: 10 mg/kg/dose (lopinavir component) orally twice a day
- Greater than 40 kg/ dose: Lopinavir/ ritonavir 2x200/50 mg tablet, orally twice a day
For 5 - 14 days, depends on physician’s judgment.
Notice: Kaletra should not be administered to neonates before gestational age of 42 weeks and postnatal age of at least 14 days.
IV. Ribavirin (Oral)
For children over 3 years old:
● < 47 kg: 15 mg/kg/day-BID
● 47 - 59: 400 mg-BID
● 60 - 73: 400 mg- in the morning, 600 mg- in the evening
● > 73: 600 mg-BID
For up to 14 days, depends on patient’s response.
[7] Para Clinical Findings
CXR: The sensitivity of chest radiography is low in these patients. In the early stages, the CXR may be normal and does not show ground glass lesions, but in severe cases bilateral multifocal consolidation and even progression to the white lung can be seen (9).
Chest CT Scan: According to recent evidences the CT scan findings of COVID-19 pneumonia manifested as multifocal unilateral or bilateral ground glass opacity (GGO) to mixed GGO and consolidation mostly peripheral located. GGO are usually seen in the early days and progress to the consolidation in the following days. Lymphadenopathy is usually not seen and pleural effusion is rare and mild.
In general, normal chests CT scan maybe helpful in rule out of COVID-19.
Indications of chest CT scan are:
A) Bilateral lung involvement on CXR.
B) ICU admission.
C) In a patient who has not responded to primary treatment and is developing respiratory distress.
D) The CXR is getting worse.
E) Symptomatic patient in contact with definitive patient with COVID-19.
CBC-Diff: Leukopenia or lymphocytopenia less than normal amounts for age (an absolute lymphocyte count < 3000/µLin infants (1 month to 12 months), < 2000/µLin Childs 1 - 5 years of age and < 1100/µLin older than 5 years).
CRP- LDH: Abnormal tests consider based on age and manufacturer’s test kits.
Other Lab Tests: Complementary tests are requested based on the patient’s condition.
[8] Indication of Sampling
In this step, for suspected of COVID-19 case the sample for both COVID-19 and H1N1 should be sent, and in case of access shortage, it should be done at least for the patients admitted in ICU, or with moderate to severe pneumonia, or mild pneumonia with red flag.
How to sampling and send the samples:
● Upper respiratory sampling is done by the nasopharyngeal or oropharyngeal swab. For the upper sampling, contact and droplet precautions, and for the lower sampling, contact and airborne precautions (N95 mask) must be applied.
● All the samples taken from suspected COVID-19 patients must be considered infectious, and all the samplers and sample carriers must follow the standard precautions (10).
● The sampler must use appropriate personal protection equipment (PPE) such as eye protector, medical mask, long sleeves gown, and gloves (7).
● All the persons who play a role in carrying samples must be trained and exercised enough about necessary precautions on transferring samples and emergency cases (like breaking the sample and spreading infection).
● Transferring sample must be done by a three-layer container special for carrying dangerous infectious samples.
● Laboratory must also be noticed about the patients suspected for COVID-19, in order to apply necessary precautions and keep these samples separated from the others.
● Patient’s full name and all needed info must be completed on the form attached to the sample.
Notice: Upper sampling must be done by Dacron or rayon sterile swab (not cotton) and in VTM special perimeter. Samplers should try not to sample from tonsils and uvula. For a severe respiratory patient suspected to coronavirus, disease cannot be rejected only by a negative upper respiratory sample, and upper re-sample or lower respiratory sample is needed.
[9] Precautions in Examination Room
The child suspected to coronavirus shall be transferred to the special examination room at Emergency Department, the patient and accompany shall wear surgical mask. The examiner doctor also must follow the standard and droplet precautions during the examination.
[10] Hygiene Recommendations During Nursing the Patient Suspected to COVID-19 at Home
● To be about personal hygiene including separation of personal hygiene tools like tooth brush and towel, and several times of washing hands.
● To stay at home and to avoid public places.
● Use a separate bedroom where the patient can recover without sharing immediate space with others.
● Rest of the family members must keep at least 1-meter distance with the patient.
● Child nurse must wear appropriately surgical mask on face, and avoid direct contact with respiratory and oral secretions and stool, also keep to wash hands with soap and water, and in case, disposable plastic or latex gloves.
● Toilet, bathroom, table, bed, and all the surfaces which are repeatedly touched must be washed by water, soap and then bleaching liquid 0.5% sodium hypochlorite.
● Patient’s cloths, towels and bed-sheets first must be kept in a nylon carry-bag, and then shall be washed by hot water with temperature between 60°C and 90°C. Wearing gloves for touching unwashed cloths are necessary.
● During surface washing and collecting patient’s cloths, the child nurse must wear protecting apron and disposable gloves. Protecting apron must be washed just like the patient’s cloths (11).
[11] Warning Signs
They include:
● tachypnea (respiratory rate
- > 60 breaths/minute for infants < 2 months of age;
- > 50 breaths/minute for infants 2 - 12 months of age;
- > 40 breaths/minute for Childs 1 - 4 years of age and
- > 30 breaths/minute for Childs older than 5 years of age) (6),
● respiratory distress (chest indrawing, cyanosis, grunting, nasal flaring and tachypnea),
● cyanosis of tongue and lips,
● inability for eating or drinking,
● communication inability during awareness or excessive restlessness,
● dryness of oral mucosa,
● decrease in volume of urine and lack of tear,
● more than 40°C fever or persistent high fever for 3 - 5 days,
● return symptoms after partial recovery.
[12] Severe Pneumonia
- Temperature > 38.5°C,
- moderate to severe respiratory distress (respiratory rate > 70 breaths/minute for infants < 12 months of age and > 50 breaths/minute for older children; suprasternal, intercostal and subcostal retraction; chest indrawing; cyanosis; grunting; nasal flaring; apnea;
- O2 saturation < 93% despite O2 therapy or O2 saturation < 90% at room air;
- lethargy,
- increasing work of breathing, or exhaustion with or without hypercarbia and
- unexplained metabolic acidosis (2, 8).
[13] Risk Factors
History of underlying disease including of any immunodeficiency or immunosuppressive medication; and chronic disease such as diabetes; kidney, heart, respiratory, blood and metabolic disorders (12).
3. Conclusions
Nowadays, the COVID-19 epidemic, especially as a newly emerging disease in East Asia, has caused a great challenge in managing the patients and controlling the disease. This has caused many problems for the health system, as the disease has high prevalence and there are no specific guidelines for diagnostic and therapeutic management, especially in children.
Therefore, at the Research Institute for Children’s Health (RICH), we developed an algorithm for dealing with children suspected of COVID19 and make it available as an easily accessible protocol for all colleagues. It is hoped that with international co-operation, this global dilemma will end with the least burden of disease.
Acknowledgements
References
-
1.
Shen K, Yang Y, Wang T, Zhao D, Jiang Y, Jin R, et al. Diagnosis, treatment, and prevention of 2019 novel coronavirus infection in children: Experts' consensus statement. World J Pediatr. 2020. [PubMed ID: 32034659]. https://doi.org/10.1007/s12519-020-00343-7.
-
2.
Chen ZM, Fu JF, Shu Q, Chen YH, Hua CZ, Li FB, et al. Diagnosis and treatment recommendations for pediatric respiratory infection caused by the 2019 novel coronavirus. World J Pediatr. 2020. [PubMed ID: 32026148]. https://doi.org/10.1007/s12519-020-00345-5.
-
3.
Wei M, Yuan J, Liu Y, Fu T, Yu X, Zhang ZJ. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020. [PubMed ID: 32058570]. [PubMed Central ID: PMC7042807]. https://doi.org/10.1001/jama.2020.2131.
-
4.
NHS England and NHS Improvement. Novel coronavirus (COVID-19) standard operating procedure. Community Pharmacy. Pharmacy Publication; 2020.
-
5.
Interim advice for health professionals: Novel coronavirus (2019-nCoV). 2020, [cited 2020 Feb 3]. Available from: www.health.govt.nz/our-work/diseases-and-conditions/novel-coronavirus-china-2019-ncov.
-
6.
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497-506. [PubMed ID: 31986264]. https://doi.org/10.1016/S0140-6736(20)30183-5.
-
7.
Raimer PL, Han YY, Weber MS, Annich GM, Custer JR. A normal capillary refill time of 2 seconds is associated with superior vena cava oxygen saturations of 70%. J Pediatr. 2011;158(6):968-72. [PubMed ID: 21238980]. https://doi.org/10.1016/j.jpeds.2010.11.062.
-
8.
Davis AL, Carcillo JA, Aneja RK, Deymann AJ, Lin JC, Nguyen TC, et al. American College of Critical Care Medicine Clinical Practice Parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061-93. [PubMed ID: 28509730]. https://doi.org/10.1097/CCM.0000000000002425.
-
9.
Wilder-Smith A, Low JG. Risk of respiratory infections in health care workers: lessons on infection control emerge from the SARS outbreak. Southeast Asian J Trop Med Public Health. 2005;36(2):481-8. [PubMed ID: 15916060].
-
10.
Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: Clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011;53(7):e25-76. [PubMed ID: 21880587]. https://doi.org/10.1093/cid/cir531.
-
11.
Yoon SH, Lee KH, Kim JY, Lee YK, Ko H, Kim KH, et al. Chest radiographic and CT findings of the 2019 novel coronavirus disease (COVID-19): Analysis of nine patients treated in Korea. Korean J Radiol. 2020;21. [PubMed ID: 32100485]. https://doi.org/10.3348/kjr.2020.0132.
-
12.
Jensen PA, Lambert LA, Iademarco MF, Ridzon R. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings, 2005. MMWR Recomm Rep. 2005;54(Rr-17):1-141.