Abstract
Introduction:
Congenital Syphilis (CS) is caused by the vertical transmission of Treponema pallidum during pregnancy. Here, we describe a case of CS, diagnosed solely by serological tests.Case Presentation:
The patient was a newborn with CS, whose mother was infected with Treponema pallidum at the gestational age of 16 - 20 weeks. However, the patient had no signs of early CS, such as low birth weight, nasal congestion, maculopapular rash, or hepatosplenomegaly. The histopathological features in both mother and neonate suggested syphilitic placentitis. Regarding the serological tests, a non-reactive Venereal Disease Research Laboratory (VDRL) test result and a reactive Treponema pallidum haemagglutination (TPHA) test result were reported. After establishing the diagnosis of CS, we initiated treatment with procaine penicillin G (50,000 U/kg body weight), which was injected intramuscularly once a day for 10 days. Six months after discharge, physical examinations showed normal findings and non-reactive results of VDRL and TPHA tests.Conclusions:
It can be concluded that detailed history-taking and serological tests play a vital role in establishing the diagnosis of CS, particularly in patients with asymptomatic congenital syphilis. An early diagnosis of CS can guide clinicians to initiate a standardized treatment and improve patient outcomes.Keywords
1. Introduction
Congenital Syphilis (CS) is a systemic disease caused by maternal infection with Treponema pallidum. Although CS commonly manifests as early congenital syphilis, most cases are asymptomatic, resulting in delayed diagnosis (1). The epidemiological findings show that the incidence of CS has been increasing annually since 2012 in the United States. In 2017, there were 23.3 cases of CS per 100,000 live births in the United States, indicating a 43.8% increase from the previous year or an overall increase of 153.3% relative to 2013 (2).
The common symptoms of CS include maculopapular rash, icterus, enlargement of the liver and spleen, and rhinitis. Nevertheless, more than 50% of infected infants are asymptomatic at birth (3). Accordingly, newborns are subjected to laboratory examinations for CS after a positive serological test during antenatal screening. Clinical guidelines for the prevention of CS suggest that screening should be conducted in the initial prenatal visit (4). Also, women with a high risk of syphilis or those residing in areas with a high prevalence of syphilis must be subject to additional screening tests in the third trimester of pregnancy and at delivery (4). According to protocols established in Indonesia, all pregnant women must be screened for Human Immunodeficiency Virus (HIV) infection, syphilis, and hepatitis at least once during pregnancy.
Generally, the diagnosis and initiation of treatment for CS are based on detailed history-taking, physical examination, previous serological tests, maternal medication use, and serological assays of the newborn and mother at the time of delivery (5). Here, we present the case of an infant with CS, which highlighted the importance of serological tests in establishing the diagnosis of the disease.
2. Case Presentation
The patient was a male infant born to a mother with a reactive syphilis test result. He was born at 38 - 39 weeks of gestation via a cesarean section with an APGAR score of 7 - 9. He cried spontaneously after birth and showed normal muscle tone. His birth weight and height were 2,694 g and 47 cm, respectively. There were no signs of respiratory distress in the post-delivery examination. He was transferred to the neonatology ward after reaching a stable status for further evaluations.
The mother was referred to our clinic from Arjowinangun Primary Health Center (Malang, Indonesia) because of a suspected urinary tract infection. She was subsequently diagnosed with syphilis at the gestational age of 18 - 20 weeks (November 2018) during antenatal screening, based on the reactive Venereal Disease Research Laboratory (VDRL) test (titer of 1:2) and reactive Treponema pallidum haemagglutination (TPHA) test. Antenatal care continued monthly for the mother in the tertiary hospital (Polyclinic of Obstetrics and Gynecology, Saiful Anwar General Hospital, Malang, Indonesia). Also, serological tests, which were conducted at the gestational age of 30 - 32 weeks (February 2019), showed similar results.
The mother received a single dose of intramuscular benzathine penicillin one week before delivery. It should be noted that the delayed treatment of maternal syphilis was due to the mother's delayed antenatal visit. She also routinely consumed multivitamins prescribed by the physician. Detailed history-taking revealed that the maternal history of premarital sex; however, the father's sexual history was unknown. There was no history of vaginal bleeding, vaginal discharge, fever, diabetes mellitus, herb/medicine use, or hypertension. She also had no complaints during pregnancy.
After delivery (April 9, 2019), the newborn received hepatitis B vaccination and vitamin K injection. He showed normal reflexes and behavior and was breastfed exclusively. He was the third child in the family, and both parents were 37-years-old. His father was a meatball seller, and her mother was a housewife. The anthropometric measurements showed the neonate’s good nutritional status, with a bodyweight of 2,694 g, a height of 47 cm, a head circumference of 34 cm, and a chest circumference of 32 cm. His Ballard clinical score was 36, indicating 38 - 39 weeks of gestation. Also, his Lubchenco score was in the 25th to 50th percentile.
At the time of admission to the neonatology ward, the neonate’s vital signs were as follows: body temperature, 36.7˚C; pulse rate, 136 bpm (a steady, regular rhythm); respiratory rate, 42 bpm; and capillary refill time (CRT), < 2 sec. Moreover, the neurological examination revealed negative pathological reflexes (Babinski and Chaddock reflex test). We also conducted laboratory examinations, including complete blood cell count, VDRL test, TPHA test, and long bone X-ray examination for the patient. The initial laboratory examination showed a hemoglobin level of 18.70 g/dL, a leucocyte count of 25.770 cells/μL, a hematocrit level of 51.6%, and a platelet count of 271,000 cells/μL.
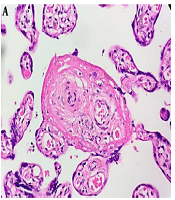
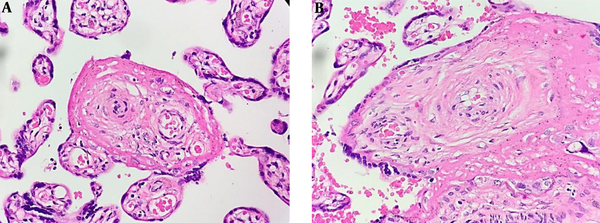
The white blood cell differential test showed eosinophils 1.1%, basophils 0.3%, neutrophils 72.2%, lymphocytes 16.6%, and monocytes 9.8%. Also, the liver function test indicated the normal levels of serum glutamic-oxaloacetic transaminase (SGOT; 27 U/L) and serum glutamic pyruvic transaminase (SGPT; 10 U/L). Regarding the serological tests, the VDRL test result was non-reactive, while the TPHA test result was reactive. The whole-body bone X-ray showed no signs of congenital syphilis. The histopathological analysis of the maternal and fetal sides of the placental tissue suggested syphilitic placentitis represented by hypertrophic vascular and concentric vascular fibrosis (Figure 1).
Histopathological features from the maternal side (A) and fetal side (B) suggesting syphilitic placentitis. (A) chorionic villous coated with floated trophoblastic cells within intervillous spaces and decidual tissues (400X magnification). Hypertrophic vascular and concentric vascular fibrosis with hyalinization is prominently found in both figures.
Based on our history-taking, besides clinical, laboratory, and radiographic examinations, we established the diagnosis of congenital syphilis in a term infant, appropriate for gestational age. Accordingly, the standard management protocol for a term newborn was used, which included ambient oxygen, thermoregulation (warmer temperature, 36.5 - 37.5˚C), hepatitis B immunization, vitamin K injection, and umbilical cord care. Also, the initial nutritional management consisted of breast milk combined with formula milk (almost 20 mL of milk eight times daily). We hospitalized the patient in an isolation room. According to recent clinical guidelines, we treated him with an intramuscular injection of procaine penicillin G 135,000 U (50.000 U/kg body weight) once daily for 10 days.
During 11 days of observation, we consulted an ophthalmologist and an otorhinolaryngologist to screen for any abnormalities caused by syphilis. The screening results showed normal findings in the eye, ear, and nose examinations. During our observations, there were no clinical signs of maculopapular rash, nasal congestion, or other symptoms, suggesting congenital syphilis. However, we found a sign of hyperbilirubinemia on day 4 after admission. The patient looked icteric with a Kramer score of 4. The laboratory examination showed a total bilirubin level of 9.51 mg/dL, a direct bilirubin level of 0.36 mg/dL, and an indirect bilirubin level of 9.15 mg/dL.
We performed double phototherapy with a blanket for 12 hours. Nonetheless, no cerebrospinal fluid (CSF) examination was performed, as the neurological examination showed normal features. Also, head ultrasonography showed normal findings. Six months after discharge (September 26, 2019), physical examinations (including growth and development test) indicated normal findings. Also, both serological tests (VDRL and TPHA) were non-reactive. The patient was scheduled for re-examination by VDRL and TPHA tests at the age of 15 months.
3. Discussion
Here, we presented a case of asymptomatic CS in a newborn, who was diagnosed based on the reactive serological test results and maternal status. An interesting finding of this study was the typical histopathological features of the placenta, indicating syphilitic placentitis; however, this finding is not specific for the diagnosis of CS. Generally, Treponema pallidum can be transmitted vertically into the fetal circulation during pregnancy at 14 weeks of gestation and can increase the inflammatory response in the placental tissue, as shown in the present case (5-8). Clinically, CS has significant effects on various organ systems and may lead to critical conditions and even perinatal mortality (7). In addition to the acute signs of CS, severe in utero or untreated infection may cause blindness, deafness, and various neurological, dental, and orthopedic sequelae (8). However, in approximately 67% of neonates with syphilis, the only clinical sign is the low birth weight (1). In our case, there were no typical signs of CS.
Our patient was born to a mother with reactive VDRL and TPHA test results. Expectedly, he also had a reactive TPHA result, suggesting early CS. Overall, pregnant women with high-risk syphilis during antenatal screening (in the first visit during the first trimester) need to be re-examined at a gestational age of 28 - 32 weeks and after delivery. The two-step process suggested by US Preventive Task Forces includes an initial “nontreponemal” antibody test (VDRL or RPR), followed by a confirmatory "treponemal" antibody detection test (e.g., TPHA) or reverse sequence screening algorithm. The first screening in the first and third trimesters is carried out to prevent the vertical transmission of syphilis, whereas the third-trimester/delivery screening is performed to detect CS and initiate standard treatment (9, 10). In accordance with international standards, a similar clinical guideline is applied in Indonesia. However, in some areas with limited laboratory facilities, the rapid test for syphilis is recommended for being used in pregnant women during antenatal care (11).
The VDRL and Rapid Plasma Reagin (RPR) tests are the most common non-treponemal antibody tests. Other laboratory examinations, such as fluorescent treponemal antibody absorption (FTA-ABS) test, microhemagglutination assay (MHA), and MHA for Treponema pallidum antibodies (MHA-TP) test, are used to confirm the diagnosis of CS after a positive non-treponemal antibody assay (1). However, false-positive treponemal and non-treponemal test results can be found in pregnant women; therefore, confirmatory tests should be performed before establishing the diagnosis of syphilis (11, 12).
Pregnant women with positive serological test results are considered positive for syphilis unless they have an appropriate medication history, and the serological antibody levels are reduced. If adequate treatment is applied, re-treatment is not necessary for patients with serofast antibody titers (11). However, reinfection must be considered in pregnant women with persistently higher antibody titers (e.g., > 1:8 dilutions); therefore, re-treatment may be necessary in such cases (11).
To establish the diagnosis of CS, a detailed history-taking (especially maternal history) and appropriate clinical and laboratory examinations of both mother and infant are essential. All infants born to mothers with reactive syphilis test results must undergo non-treponemal and treponemal tests, together with maternal tests. According to the literature, the cut-off point for neonatal CS is a fourfold change in the non-treponemal titer. However, the absence of a fourfold or greater change in the non-treponemal titer is not an exclusion criterion for positive CS (9). Our newborn showed non-reactive VDRL and reactive TPHA test results. The non-reactive titer of the non-treponemal test (VDRL) in this patient did not exclude congenital infection. The non-reactive result can be attributed to the low titer of the maternal non-treponemal test or maternal infection in late pregnancy. Also, the reactive TPHA test result might be false-positive due to the passive transfer of maternal antibodies.
Recent clinical guidelines suggest the CSF evaluation in neonates with probable CS, since positive CSF results may be necessary to establish the diagnosis of asymptomatic infants. Some CSF examinations include cell count, differential cell count, protein concentration, VDRL test, and FTA-ABS. Nonetheless, the interpretation of CSF tests can be challenging, considering the gestational age. Generally, the signs of Treponema pallidum infection in the central nervous system include pleocytosis (> 18 - 25 leukocytes/µL), reactive VDRL test results, and elevated protein concentration (> 150 mg/dL; 170 mg/dL in preterm infants) (13-16). However, the CSF examination was not carried out for our patients because of financial constraints.
Another essential test for guiding clinicians in the diagnosis of CS is the histopathological examination of the placental tissue. Macroscopically, the placental tissue from a neonate with congenital syphilis is pale, thick, and large. Histopathologically, funisitis with a necrotic sign, villous enlargement, and acute villous inflammation may be found (16). The histopathological examination of the umbilical cord and placenta is essential in all possible cases of syphilis. In our patient, the histopathological features of the placenta, both from the maternal and fetal sides, suggested syphilitic placentitis. The clinical decision to initiate a standardized regimen for syphilis treatment can be made following a comprehensive analysis of clinical manifestations, previous serological tests, and maternal medication use, as well as serological tests and maternal medication use at the time of delivery (5).
According to recent clinical guidelines, syphilis is diagnosed if one of the following criteria is present: (1) Physical examination shows abnormal findings related to CS, (2) The infant’s serum non-treponemal titer is four times the maternal level, and (3) Microscopic analysis of body fluid(s) confirms the diagnosis. These patients must be treated with either intravenous injection of aqueous crystalline penicillin G (50,000 U/kg, twice daily during the first week of life, followed by three doses daily after one week) or intramuscular injection of aqueous procaine penicillin G (50,000 U/kg, one dose daily) for 10 days. Also, infants with probable CS, who have no signs on the physical examination but show incomplete or abnormal laboratory or radiographic results (i.e., CBC, CSF, and radiographic findings of long bones), must be treated with the standardized regimen for CS (4, 16).
In conclusion, detailed history-taking and physical examination are necessary to guide clinicians in the diagnosis of CS. Moreover, in asymptomatic cases, laboratory examinations, particularly serological tests and other assays (e.g., histological examination of the placental tissue), play an essential role in the diagnosis of CS. In this case report, we described the positive findings of maternal and congenital syphilis, based on serological screening tests for syphilis, as well as additional histopathological assays of the placenta. According to the present results, prompt treatment is necessary for both mother and infant. However, this study did not include a long-term follow-up since congenital syphilis can manifest late after the age of two years. Also, we did not report the results of the CSF examination since there were no neurological symptoms in the patient.
References
-
1.
Bembry W, Anderson M, Nelson S. Congenital syphilis: The great pretender strikes back. A case report. Clin Pediatr (Phila). 2018;57(8):992-6. [PubMed ID: 29084433]. https://doi.org/10.1177/0009922817738343.
-
2.
CDC. Sexually transmitted disease surveillance. Centers for disease control and prevention; 2019. Available from: https://www.cdc.gov/nchhstp/newsroom/2019/2018-STD-surveillance-report.html#.
-
3.
Mmeje O, Chow JM, Davidson L, Shieh J, Schapiro JM, Park IU. Discordant syphilis immunoassays in pregnancy: Perinatal outcomes and implications for clinical management. Clin Infect Dis. 2015;61(7):1049-53. [PubMed ID: 26063719]. [PubMed Central ID: PMC4560902]. https://doi.org/10.1093/cid/civ445.
-
4.
Workowski KA, Bolan GA, Centers for Disease C; Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep. 2015;64(RR-03):1-137. [PubMed ID: 26042815]. [PubMed Central ID: PMC5885289].
-
5.
Cooper JM, Sanchez PJ. Congenital syphilis. Semin Perinatol. 2018;42(3):176-84. [PubMed ID: 29627075]. https://doi.org/10.1053/j.semperi.2018.02.005.
-
6.
Fenton KA, Breban R, Vardavas R, Okano JT, Martin T, Aral S, et al. Infectious syphilis in high-income settings in the 21st century. Lancet Infect Dis. 2008;8(4):244-53. [PubMed ID: 18353265]. https://doi.org/10.1016/S1473-3099(08)70065-3.
-
7.
Walker GJ, Walker DG. Congenital syphilis: a continuing but neglected problem. Semin Fetal Neonatal Med. 2007;12(3):198-206. [PubMed ID: 17336171]. https://doi.org/10.1016/j.siny.2007.01.019.
-
8.
Johnson CT, Sheffield JS. Congenital syphilis. Obstetric Imaging: Fetal Diagnosis and Care (Second Edition). 2018. p. 688-6920. https://doi.org/10.1016/b978-0-323-44548-1.00168-6.
-
9.
Singh AE, Levett PN, Fonseca K, Jayaraman GC, Lee BE. Canadian Public Health Laboratory Network laboratory guidelines for congenital syphilis and syphilis screening in pregnant women in Canada. Can J Infect Dis Med Microbiol. 2015;26 Suppl A:23A-8A. [PubMed ID: 25798162]. [PubMed Central ID: PMC4353984]. https://doi.org/10.1155/2015/589085.
-
10.
U. S. Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for syphilis infection in pregnant women: US preventive services task force reaffirmation recommendation statement. JAMA. 2018;320(9):911-7. [PubMed ID: 30193283]. https://doi.org/10.1001/jama.2018.11785.
-
11.
Kementerian Kesehatan Republik Indonesia. Pedoman tata laksana sifilis untuk pengendalian sifilis di layanan kesehatan dasar. Jakarta: Direktorat Jenderal Pengendalian Penyakit dan Penyehatan Lingkungan. 2013.
-
12.
Sena AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51(6):700-8. [PubMed ID: 20687840]. https://doi.org/10.1086/655832.
-
13.
Michelow IC, Wendel GJ, Norgard MV, Zeray F, Leos NK, Alsaadi R, et al. Central nervous system infection in congenital syphilis. N Engl J Med. 2002;346(23):1792-8. [PubMed ID: 12050339]. https://doi.org/10.1056/NEJMoa012684.
-
14.
Shah K, Jagati A, Rathod S, Chaudhary R. Early congenital syphilis: Resurgence of an entity nearing elimination. Indian Journal of Paediatric Dermatology. 2019;20(2):154. https://doi.org/10.4103/ijpd.IJPD_5_18.
-
15.
Gomez GB, Kamb ML, Newman LM, Mark J, Broutet N, Hawkes SJ. Untreated maternal syphilis and adverse outcomes of pregnancy: a systematic review and meta-analysis. Bull World Health Organ. 2013;91(3):217-26. [PubMed ID: 23476094]. [PubMed Central ID: PMC3590617]. https://doi.org/10.2471/BLT.12.107623.
-
16.
CDC. Sexually transmitted diseases treatment guidelines - congenital syphilis. Centers for Disease Control and Prevention; 2015, [cited May 1]. Available from: https://www.cdc.gov/std/tg2015/congenital.htm.