Abstract
Keywords
Anesthesia Bradycardia Controlled Hypotension Dexmedetomidine Rhinoplasty
1. Background
Rhinoplasty, a common cosmetic procedure performed in many countries, in which the application of controlled or permissive hypotension during anesthesia has beneficial effects. By decreasing the blood pressure during operation, blood loss is reduced, and a more visible surgical field is provided. Previously, controlled hypotension was defined as “a reduction in the systolic blood pressure to reach 80-90 mmHg, a reduction in mean arterial pressure (MAP) to reach 50 - 65 mmHg, or a 30% reduction in baseline MAP” (1), but because of reported poor outcomes associated with hypotension and this MAP range during operation, including acute kidney injury (AKI), myocardial injury, and mortality, especially in vulnerable patients, recently higher values are recommended (2-8).
Different types of drugs have been investigated to provide “controlled hypotension” during surgeries, including opioids (9-12), beta-blockers (13, 14), vasodilators (15, 16), and α2-adrenoreceptor agonists (9, 11, 12, 14, 17). Dexmedetomidine, an α2-agonist, has been used as a sedative (18-20), anxiolytic (21), and hypotensive (22, 23) agent for anesthesia. It has also been used as an adjunct analgesic during sedation (24), and general (25), regional (26, 27), and neuraxial anesthesia (28, 29), and also for postoperative pain management (30). The sedative and anxiolytic effects of dexmedetomidine are produced primarily via the stimulation of α2-adrenoceptors in the locus coeruleus of the pons and result in a dose-dependent inhibition of norepinephrine release. The resulting sedation is like stage 2 non-rapid eye movement sleep, with preserved muscle tone and ventilation. The analgesic effect of dexmedetomidine is exerted on the α2-adrenoreceptors mainly in the substantia gelatinosa of the dorsal horn at the spinal cord to reduce the release of nociceptive neurotransmitters (31). The neuroprotective effect of dexmedetomidine following ischemic injury has recently been demonstrated in animal studies (32).
The cardiovascular effects of dexmedetomidine begin with initial hypertension following the administration of a loading dose, due to the activation of α2B receptors located on vascular smooth muscle, with subsequent hypotension and bradycardia due to centrally mediated decrease in sympathetic tone (31, 33). The decrease in cardiac output is due to a negative chronotropic effect of dexmedetomidine, and the systolic and diastolic function is not impaired (34). The hypotensive and heart rate-reducing properties of this agent make it a reasonable choice for procedures requiring “controlled hypotension”, and it has been demonstrated to have favorable effects compared to other drugs in this regard (9, 11, 12, 14, 17). Furthermore, the administration of dexmedetomidine during operation is associated with a decrease in the incidence of postoperative shivering, nausea, vomiting, delirium, and postoperative cognitive dysfunction (35-37).
Apart from all the benefits, dexmedetomidine also has its drawbacks, the most important of which are severe bradycardia and even, in rare cases, cardiac arrest (38-43). Since hemodynamic alterations mostly occur during loading infusion (33), the current study was conducted to determine whether, by decreasing the loading dose of dexmedetomidine, the incidence of bradycardia is reduced while the hypotensive effect is preserved.
2. Objectives
Hence, we compared the effects of three loading doses of dexmedetomidine (1, 0.9, and 0.8 µg/kg) on blood pressure and the incidence and severity of bradycardia during controlled hypotension in rhinoplasty.
3. Methods
3.1. Study Design and Participants
This randomized, prospective, double-blinded, clinical trial was conducted, in accordance with the Declaration of Helsinki, at Firoozgar Hospital (affiliated to the Iran University of Medical Sciences, Tehran, Iran) from April 2020 to February 2021. The study protocol was approved by the Ethics Committee of the university (code: IR. IUMS.FMD.REC 1398.2) and was registered at the Iranian Registry of Clinical Trials (identification code: IRCT20191110045388N1). After obtaining informed consent from the subjects, 81 patients aged 18 to 50 years with the American Society of Anesthesiologists Physical status (ASA-PS) class I and II, scheduled for rhinoplasty, were enrolled. Exclusion criteria were as follows: a history of hypertension, cardiovascular diseases, cerebrovascular disease, or renal dysfunction, morbid obesity, treatment with beta-blockers, scheduled for revision rhinoplasty, allergy to an α2-adrenergic agonist, and mean arterial pressure (MAP) less than 70 mmHg at baseline.
3.2. Randomization and Blinding
All the patients undergoing rhinoplasty in the considered period were assessed for eligibility. Those who were eligible and willing to participate in the study were randomly allocated into three groups by block randomization method, in blocks of six. The random number sequence was obtained via STATA software version 13. Patients were allocated 1:1:1 to receive IV dexmedetomidine with a loading dose of either 1 µg/kg (group 1.0), 0.9 µg/kg (group 0.9), or 0.8 µg/kg (group 0.8) preoperatively. The patient recruitment was continued until the sample size was reached.
An anesthesia resident and an anesthesiologist were involved in performing the study protocol, the first one prepared and set the infusion rate of the loading dose, and the second one, who was blinded to the study group, managed anesthesia, according to the study protocol, and collected the data. To limit inter-observer variability, all the operations and operation field scorings were also performed by only one surgeon who was blinded to the patient’s study group.
3.3. Anesthesia Protocol
Upon the arrival of the patients to the operating room, routine monitoring of electrocardiography, noninvasive blood pressure, peripheral oxygen saturation, temperature, depth of anesthesia (BISPECTRAL VISTA monitoring system; Covedian company, USA), neuromuscular function, and capnography were done. After starting the infusion of 5 mL/kg of a crystalloid solution, a load of dexmedetomidine (Precedex, 200 µg/2 mL, Hospira, USA), diluted in 50 mL of saline 0.9%, with a dose according to the study group, was infused intravenously for 10 min followed by a continuous infusion of 0.3 - 0.7 µg/kg/hour. Fentanyl (2 µg/kg; Caspioan Tamin Pharmaceutical Co. Rasht-Iran) was administered after completion of dexmedetomidine loading infusion, and anesthesia was induced with propofol (1.5 mg/kg; B. Braun Melsungen AG, Germany) and cisatracurium (0.15 mg/kg; Rosamed Co. Iran). Anesthesia was maintained with inhalational sevoflurane (M/s AbbVie S.R.L, Aprilia Campoverde-Italy), adjusted to maintain a bispectral index (BIS) value of 40 - 60. All patients were positioned in 25° reverse Trendelenburg. Five to ten milliliters of a solution containing lidocaine 2% and epinephrine 1:100,000 were locally administered by the surgeon before starting the operation.
The primary goal was to provide a MAP in a range of 60 mmHg - 70 mmHg. If MAP exceeded this limit, provided that the depth of anesthesia was sufficient and dexmedetomidine was infused at the maximum rate, one of the two approaches was applied according to the patient’s HR changes. If the HR was increased simultaneously to more than 20% of the preoperative value, 1 µg/kg of fentanyl was administered, but if MAP increased without significant changes in HR, a bolus of NTG, 50 µg was given.
In case of hypotension, defined as a MAP lower than 60 mmHg, a bolus of 100 mL of crystalloid solution was given, and the infusion of the drug was temporarily discontinued until the return of MAP to the desired value. If the drop in MAP did not improve, a bolus of IV ephedrine 5 mg was administered.
In the case of bradycardia, defined as HR less than 60 beats/min (44) and decreased by more than 20% compared to the baseline value, the infusion of dexmedetomidine was reduced; if the decrease in HR was more than 30%, dexmedetomidine infusion and other anesthetics temporarily discontinued, and in case of persistence of severe bradycardia and/or accompanied by hypotension, a bolus of intravenous atropine 10 µg/kg plus 200 ml of IV crystalloid solution was administered.
3.4. Surgical Field Assessment
The condition of the surgical field was assessed by the surgeon in terms of bleeding and visibility, using a 6-point scale adapted from Fromme et al. (45), named as bleeding score, every 15 minutes during operation: 0 = no bleeding; 1 = minor bleeding, but no aspiration required; 2 = minor bleeding, aspiration required; 3 = minor bleeding, frequent aspiration required; 4 = moderate bleeding, visible only with aspiration; 5 = severe bleeding, continuous aspiration required.
3.5. Data Collection
Hemodynamic variables, including HR, MAP, and BIS were recorded upon arrival of the patient to the operating room 5 and 10 minutes after dexmedetomidine loading dose infusion, before induction of anesthesia, after tracheal intubation, 5, 10, and 15 minutes after intubation, every 15 minutes after tracheal extubation, and 15 minutes afterward. The data were also recorded 2 minutes after local infiltration of epinephrine. The inhaled concentration of sevoflurane was recorded every 15 minutes. The number of patients who received NTG, fentanyl, ephedrine, or atropine, as well as the number of times of the drugs’ administration were recorded for each group. The bleeding score was also recorded every 15 minutes during the operation. The cases of bradycardia were recorded as bradycardia 20% (i.e., a decrease in HR by 20% - 30% of baseline value), bradycardia 30% (i.e., a decrease in HR by more than 30% of baseline value), and bradycardia (i.e., the whole cases of bradycardia). Adverse effects after tracheal extubation and during patients’ stay in the post-anesthesia care unit (PACU) were recorded, as well.
3.6. Study Outcomes
The primary outcome was to provide a MAP of 60- 70 mmHg. The secondary outcomes were bleeding score, the amount of hypotensive (NTG), and extra analgesic agents (fentanyl) required to maintain the blood pressure in the desired range, and the incidence and severity of bradycardia.
3.7. Sample-Size Calculation
Due to the lack of similar articles comparing the effect of different loading doses of dexmedetomidine on blood pressure control during operation, a pilot study including five participants in each group was performed, and the MAP was assessed between the groups. The mean values of MAP ± standard deviations (SDs) in groups 1.0, 0.9, and 0.8 were 65.7 ± 2.5, 67.8 ± 2.6, and 68.5 ± 3, respectively. Considering type I error = 0.05, and statistical power equal to 80%, a sample size of 75 was calculated. Assuming the 8% dropouts, the total sample size was equal to 81 individuals.
3.8. Data Analysis
All statistical analyses were performed using SPSS 25.0. (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, version 25.0. Armonk, NY: IBM Corp.). Mean and standard deviation were used for descriptive and quantitative findings, and frequency and percentage were used for qualitative findings. One-way ANOVA was used to compare the MAPs and HRs between the groups, and one-way ANOVA with repeated measures was used to compare them within the groups. The number of patients who received extra fentanyl or NTG and the times of the drugs’ administration were compared using the Fischers’ exact test. The inhaled concentration of sevoflurane was compared between the groups using one-way ANOVA. The bleeding score was assessed using Fischers’ exact test, and the mean values of the scores were compared between the groups using one-way ANOVA; post hoc binary comparisons were performed based on the LSD test. The Chi-squared test was used for the analysis of bradycardia, and the Fischers’ exact test was used to compare atropine requirements between the groups. P-values of less than 0.05 were considered statistically significant.
4. Results
4.1. Study Population
A total of 85 patients were assessed for eligibility from April 2020 to February 2021. Four patients were unwilling to participate, and eventually, 81 patients were enrolled, and they were divided into three groups of 27 patients (Figure 1). All participants completed the study. The study groups were comparable in terms of age, gender, and ASA-PS (Table 1). The duration of surgery (Table 2) was also similar in all groups.
The study flowchart
| Value | Group 1.0 (N = 27) | Group 0.9 (N = 27) | Group 0.8 (N = 27) | P-Value |
|---|---|---|---|---|
| Duration of surgery, min | 119.00 ± 46.21 | 136.96 ± 42.14 | 130.96 ± 37.42 | 0.285b |
| Sevoflurane, % | 1.59 ± 0.333 | 1.87 ± 0.176 | 1.91 ± 0.181 | < 0.001b |
| Received TNG, n of pts (%) none | 21 (77.8) | 20 (74.1) | 19 (70.4) | 0.980c |
| Once | 2 (7.4) | 3 (11.1) | 4 (14.8) | |
| Twice | 2 (7.4) | 1 (3.7) | 1 (3.7) | |
| 3 times | 2 (7.4) | 3 (11.1) | 3 (11.1) | |
| Received extra fentanyl, n of pts (%) none | 21 (77.8) | 19 (70.4) | 19 (70.4) | 0.964b |
| Once | 4 (14.8) | 5 (18.5) | 5 (18.5) | |
| Twice | 2 (7.4) | 3 (11.1) | 3 (11.1) |
4.2. Primary Outcome
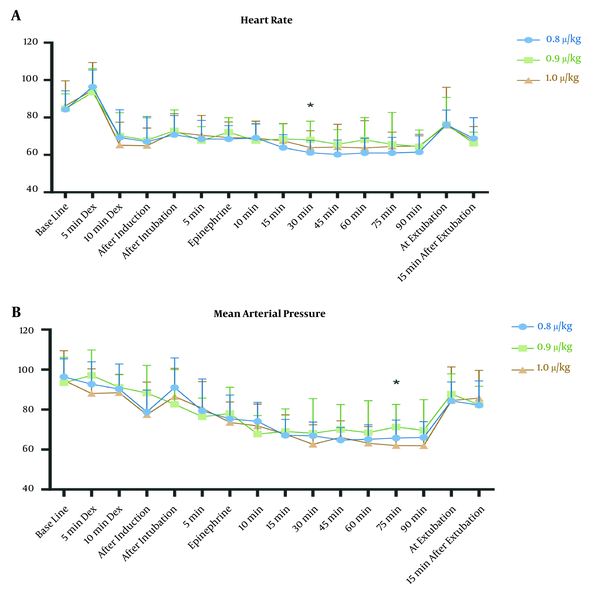
The MAP of 60 mmHg -70 mmHg was successfully achieved in the study groups. The mean values of MAP (Figure 2A) were similar at baseline (P-value = 0.686) and all recorded times (except one) after the intervention. MAP showed a reduction during operation in the studied groups (P < 0.001 for groups 0.8 and 0.9 and P = 0.001 for group 1.0).
The mean arterial pressure (MAP) (A), and heart rate (HR) (B) of participants in group 1.0 (green triangles), group 0.9 (red squares), and group 0.8 (blue circles). 5 min Dex and 10 min Dex: 5 and 10 minutes after starting loading infusion of dexmedetomidine; Epinephrine: after administration of topical epinephrine; and 5, 10, 15, 30, 45, 60, 75, and 90 min are the minutes after intubation. *, P < 0.05.
4.3. Secondary Outcomes
The HRs were also similar in all three groups (Figure 2B), at baseline (P = 0. 784) and post-intervention except 30 after tracheal intubation. The intra-group comparison showed that the HR decreased during the study period (P < 0.001for all three groups).
The number of participants requiring NTG and/or fentanyl and the number of times a bolus of the drugs was administered were similar in all study groups (P = 0.980 and 0.964, respectively). The inhaled concentration of sevoflurane used during anesthesia was different between the study groups (P < 0.001), and it was the lowest in the group 1.0 (Table 2).
The bleeding score (Table 3) was significantly lower in group 1.0 compared to the other two groups (P ≤ 0.001). The post hoc LSD test showed that the mean bleeding score was not significantly different between the groups 1.0 and 0.9 (P = 0.605), and groups 0.9 and 0.8 (P = 0.052), but it was lower in group 1.0 compared to group 0.8 (P = 0.015).
| Score | Group 1.0 | Group 0.9 | Group 0.8 |
|---|---|---|---|
| 0 | 6 (22.6) | 0 (0) | 0 (0) |
| 1 | 6 (22.2) | 13 (48.1) | 7 (25.9) |
| 2 | 10 (37.0) | 7 (25.9) | 12 (44.4) |
| 3 | 2 (7.4) | 5 (18.5) | 1 (3.7) |
| 4 | 2 (7.4) | 2 (7.4) | 0 (0) |
| 5 | 1 (3.7) | 0 (0) | 7 (25.9) |
The overall incidence of bradycardia was the highest in group 1.0 and the lowest in group 0.8 (P = 0.027, Table 4). The incidence of bradycardia with a 20% - 30% decrease in HR was similar in three groups (P = 0.612), whereas the significant difference between the groups was in terms of the incidence of a decrease in HR of more than 30% (P = 0.017); with the highest incidence in group 1.0 and the lowest in group 0.8. All groups were similar in terms of atropine requirement (Table 4). Ephedrine was not required in any of the groups. No significant event occurred after tracheal extubation and in the PACU.
5. Discussion
The current study showed that it is feasible to provide controlled hypotension with the IV administration of dexmedetomidine as an adjunct to other anesthetics. It also showed a lower incidence of bradycardia with lower loading doses. Furthermore, while the hypotensive effect of dexmedetomidine was preserved with all three loading doses, a loading dose of 0.9, but not 0.8 µg/kg, had similar effects of 1.0 µg/kg on the surgical field quality in terms of bleeding and visibility.
Following the recent studies demonstrating the association of MAPs equal to or less than 60 - 65 mmHg with increased risk of postoperative acute kidney injury, cardiac events, and mortality (2-4, 6, 7), the allowable blood pressure decrease during anesthesia has changed over the years. Sun et al. (3), in a study on patients undergoing non-cardiac surgery showed that the AKI that occurred in 6.3% of patients was associated with MAP less than 60 mmHg for 11 to 20 min and MAP less than 55 mmHg for more than 10 min in a graded fashion. Walsh et al. (2) demonstrated that the risk of AKI and myocardial injury was increased at MAPs less than 55 - 60 mmHg. Therefore, generally, a MAP over 60 mmHg -70 mmHg is recommended (5). In most of the studies evaluating the consequences of hypotension, it was not specified whether the episodes of hypotension were induced deliberately or occurred incidentally. Moreover, in some of the studies, above half of the patients had an age of over 60 years, or an ASA-PS equal to three or more, which made them more susceptible to hypotension adverse effects (2-4, 6). Given the eligibility of having a lower age range and limited comorbidities (i.e., ASA-PS limited to class I and II) to participate in our study, we planned to provide a MAP of 60 mmHg - 70 mmHg as a modified definition of controlled hypotension.
Durmus et al. (22) and Ayoglu et al. (23) in different studies have shown that dexmedetomidine reduced bleeding during septoplasty. They administered a loading dose of 1 µg/kg over 10 minutes followed by dexmedetomidine infusion. In their studies, the Map was decreased following the administration of dexmedetomidine and was lower compared to the control group during operation. In the study by Janatmakan et al. (46), with a lower loading dose of dexmedetomidine, 0.5 µg/kg, it has been shown that intraoperative bleeding was less than the control group in spine surgery. The result of our study is consistent with their studies as the MAP reduced after intervention and during operation. The MAPs were similar in our study groups, but the bleeding score was higher in group 0.8 compared to group 1.0.
Bleeding during operation is not necessarily reduced by a decrease in blood pressure alone; however, other contributing factors, such as HR may be involved. Sieśkiewicz et al. (47) demonstrated that to achieve good operative field conditions, in a great proportion of patients, maintaining the HR in a stable low value (i.e., around 60 beats/min) can preclude the need to reduce MAPs to a dangerously low level. In our study, and as shown by Durmus et al. (22) and Ayoglu et al. (23), the administration of a dose of 1 µg/kg followed by the drug’s infusion during surgery resulted in a decrease in blood pressure as well as HR. Other studies showed that dexmedetomidine was as effective as remifentanil infusion in providing controlled hypotension and reduced bleeding (9, 11, 12). Karabayirli et al. (12) demonstrated that the IV dexmedetomidine administration (1 µg/kg) infused over 10 minutes followed by the infusion of 0.7 µg/kg/h has similar effects of a bolus of IV remifentanil (1 µg/kg) followed by 0.25 - 0.5 µg/kg, on the amount of bleeding, surgical field condition, and hemodynamics. In these studies, both HRs and MAPs decreased during operation. Therefore, similar effects of remifentanil and dexmedetomidine might be attributed to their similar effects on reducing MAP and HR. Rokhtabnak et al. (17) reported that compared to magnesium sulfate, dexmedetomidine was more effective in reducing bleeding during rhinoplasty; however, similar MAPs were provided during operation. In their study, the HRs were significantly lower in the dexmedetomidine group. Thus, it seems that the decrease in HR has an impact on the decrease in bleeding during the operation. In the current study, the difference in HR between the groups was not statistically significant, but the incidence of bradycardia was higher in the groups with higher loading doses. The time interval between data recordings might not be short enough to detect more exact values to compare.
The bradycardia associated with IV administration of dexmedetomidine was explained in two phases; the first phase is thought to be vagally mediated reflex bradycardia in response to initial induced hypertension, especially seen in young, healthy patients (48) with high vagal tone (49), and the second phase occurs following the centrally mediated inhibition of sympathetic outflow. (31, 33) There are some reports of cardiac arrests following IV infusion of dexmedetomidine, most of which resolved after a few resuscitative efforts (38-43). However, not all the cases could be attributed to dexmedetomidine alone, as other contributing factors were involved, and most of the reported cases had significant morbidities and/or with some sorts of atrioventricular conduction diseases, though there were reported cases of sinus arrest in young, healthy patients, as well (49).
Adverse events associated with dexmedetomidine occur most frequently during or shortly after a loading infusion (33). Various methods, albeit with controversial results, have been evaluated to moderate hypotension and bradycardia associated with dexmedetomidine administration, either by omitting (50, 51), decreasing (52), or slowing (53) the loading infusion. Ickeringill et al. (50) demonstrated that the undesirable hemodynamic effects of administration of dexmedetomidine infusion were prevented by the elimination of the loading dose, without compromising sedation and analgesia, in patients after major operations. Ibrahim et al. (51) showed that although the infusion of dexmedetomidine without a loading dose decreased the HR and BP, at the recorded time intervals during craniotomy, no significant bradycardia as well as less analgesic and hypnotic requirements, were observed compared to the control group in their study. Sim et al. (52) showed a similar decrease in HR and BP in patients who received either 0.5 or 1 µg/kg dexmedetomidine loading dose during sedation. Kung et al. (53) reported a lower incidence of hypotension and bradycardia with a slower infusion of the loading dose of dexmedetomidine, 20 minutes versus 10 minutes, in elderly patients undergoing spinal anesthesia. The results of our study were consistent with those of Ickeringill et al. (50), Ibrahim et al. (51), and Kung et al. (53), as the decrease in dexmedetomidine loading dose affected the incidence of bradycardia. It is noteworthy that in such studies, the primary outcome was sedation, whereas, in our study, the primary outcome was the provision of controlled hypotension, which results from the cardiovascular effects of dexmedetomidine. Therefore, the elimination of the hypotensive effect of the drug was not our goal as such effects should only be controlled in an acceptable range (i.e., MAP above 60 mmHg); the only concern was to prevent severe bradycardia.
Given that the risk for severe bradycardia, leading to pulseless electrical activity, increases when patients develop a greater than 30% decrease in HR following dexmedetomidine administration (38) we divided the cases of bradycardia into two groups, those with a lesser decrease in HR (i.e., more than 20% and up to 30% decrease in HR), and those with a greater decrease in HR (i.e., more than 30% in HR). In total, 40 patients (49.4% of all) experienced an episode of bradycardia in this study; the incidence was highest in group 1.0 and lowest in group 0.8 (Table 4). However, the incidence of bradycardia cannot be entirely attributed to dexmedetomidine, as many anesthetics, such as propofol and sevoflurane with negative chronotropic effects were administered concomitantly; but as the groups were similar in terms of anesthetic regimen, the difference in the incidence of bradycardia between the groups could be due to the difference in the loading dose of dexmedetomidine. Interestingly, the incidence of bradycardia with a lesser reduction in HR (20% - 30%) was similar between the study groups; the significant difference between the groups was in the incidence of bradycardia with a greater decrease in HR (> 30%), with the higher incidence in the higher loading dose. Therefore, based on the results, reducing the loading dose was associated with a lower incidence of more severe bradycardia.
To our knowledge, no study is available comparing similar loading doses of dexmedetomidine in this regard, which could be a strong point of this study; however, using a larger sample size may lead to more accurate and clear results. We recommend clinical trials with different dexmedetomidine loading doses in different anesthetic settings, and with larger sample sizes. Administering an alternate anesthesia induction agent, not having a considerable effect on HR, may provide more valuable and definite results about the bradycardia effect of dexmedetomidine.
5.1. Conclusions
We conclude that using IV dexmedetomidine as an adjunct to other anesthetics is an acceptable approach to provide controlled hypotension. Administration of a loading dose of 0.9 µg/kg, but not 0.8 µg/kg, compared to 1.0 µg/kg, provides similar surgical field conditions in terms of bleeding and visibility. Furthermore, despite the decrease in heart rate, the hypotensive effect of the drug is preserved.
Acknowledgements
References
-
1.
Degoute CS. Controlled hypotension: a guide to drug choice. Drugs. 2007;67(7):1053-76. [PubMed ID: 17488147]. https://doi.org/10.2165/00003495-200767070-00007.
-
2.
Walsh M, Devereaux PJ, Garg AX, Kurz A, Turan A, Rodseth RN, et al. Relationship between intraoperative mean arterial pressure and clinical outcomes after noncardiac surgery: toward an empirical definition of hypotension. Anesthesiology. 2013;119(3):507-15. [PubMed ID: 23835589]. https://doi.org/10.1097/ALN.0b013e3182a10e26.
-
3.
Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123(3):515-23. [PubMed ID: 26181335]. https://doi.org/10.1097/ALN.0000000000000765.
-
4.
Futier E, Lefrant JY, Guinot PG, Godet T, Lorne E, Cuvillon P, et al. Effect of Individualized vs Standard Blood Pressure Management Strategies on Postoperative Organ Dysfunction Among High-Risk Patients Undergoing Major Surgery: A Randomized Clinical Trial. JAMA. 2017;318(14):1346-57. [PubMed ID: 28973220]. [PubMed Central ID: PMC5710560]. https://doi.org/10.1001/jama.2017.14172.
-
5.
Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth. 2019;122(5):563-74. [PubMed ID: 30916004]. https://doi.org/10.1016/j.bja.2019.01.013.
-
6.
Christensen AL, Jacobs E, Maheshwari K, Xing F, Zhao X, Simon SE, et al. Development and Evaluation of a Risk-Adjusted Measure of Intraoperative Hypotension in Patients Having Nonemergent, Noncardiac Surgery. Anesth Analg. 2021;133(2):445-54. [PubMed ID: 33264120]. [PubMed Central ID: PMC8257473]. https://doi.org/10.1213/ANE.0000000000005287.
-
7.
Wijnberge M, Schenk J, Bulle E, Vlaar AP, Maheshwari K, Hollmann MW, et al. Association of intraoperative hypotension with postoperative morbidity and mortality: systematic review and meta-analysis. BJS Open. 2021;5(1). [PubMed ID: 33609377]. [PubMed Central ID: PMC7893468]. https://doi.org/10.1093/bjsopen/zraa018.
-
8.
Mohammad Shehata I, Elhassan A, Alejandro Munoz D, Okereke B, Cornett EM, Varrassi G, et al. Intraoperative Hypotension Increased Risk in the Oncological Patient. Anesth Pain Med. 2021;11(1). e112830. [PubMed ID: 34221948]. [PubMed Central ID: PMC8241822]. https://doi.org/10.5812/aapm.112830.
-
9.
Lee J, Kim Y, Park C, Jeon Y, Kim D, Joo J, et al. Comparison between dexmedetomidine and remifentanil for controlled hypotension and recovery in endoscopic sinus surgery. Ann Otol Rhinol Laryngol. 2013;122(7):421-6. [PubMed ID: 23951692]. https://doi.org/10.1177/000348941312200702.
-
10.
Kosucu M, Omur S, Besir A, Uraloglu M, Topbas M, Livaoglu M. Effects of perioperative remifentanil with controlled hypotension on intraoperative bleeding and postoperative edema and ecchymosis in open rhinoplasty. J Craniofac Surg. 2014;25(2):471-5. [PubMed ID: 24531244]. https://doi.org/10.1097/SCS.0000000000000603.
-
11.
Javaherforooshzadeh F, Monajemzadeh SA, Soltanzadeh M, Janatmakan F, Salari A, Saeed H. A Comparative Study of the Amount of Bleeding and Hemodynamic Changes between Dexmedetomidine Infusion and Remifentanil Infusion for Controlled Hypotensive Anesthesia in Lumbar Discopathy Surgery: A Double-Blind, Randomized, Clinical Trial. Anesth Pain Med. 2018;8(2). https://doi.org/10.5812/aapm.66959.
-
12.
Karabayirli S, Ugur KS, Demircioglu RI, Muslu B, Usta B, Sert H, et al. Surgical conditions during FESS; comparison of dexmedetomidine and remifentanil. Eur Arch Oto-Rhino-Laryngol. 2017;274:239-45. [PubMed ID: 27470115]. [PubMed Central ID: PMC8257473]. https://doi.org/10.1007/s00405-016-4220-1.
-
13.
Ghodraty M, Khatibi A, Rokhtabnak F, Maleki M, Parsa F. Comparing Labetalol and Nitroglycerine on Inducing Controlled Hypotension and Intraoperative Blood Loss in Rhinoplasty: A Single-Blinded Clinical Trial. Anesth Pain Med. 2017;7(5). e13677. [PubMed ID: 29696111]. [PubMed Central ID: PMC5903219]. https://doi.org/10.5812/aapm.13677.
-
14.
J NS, Kumar S, Vijay T. To Compare the Efficacy of Dexmedetomidine Versus Labetalol in Providing Controlled Hypotension in Functional Endoscopic Sinus Surgery. Anesth Pain Med. 2021;11(1). e108915. [PubMed ID: 34221935]. [PubMed Central ID: PMC8241463]. https://doi.org/10.5812/aapm.108915.
-
15.
Yun SH, Kim JH, Kim HJ. Comparison of the hemodynamic effects of nitroprusside and remifentanil for controlled hypotension during endoscopic sinus surgery. J Anesth. 2015;29(1):35-9. [PubMed ID: 24950745]. https://doi.org/10.1007/s00540-014-1856-0.
-
16.
Cantarella G, La Camera G, Di Marco P, Grasso DC, Lanzafame B. Controlled hypotension during middle ear surgery: hemodynamic effects of remifentanil vs nitroglycerin. Ann Ital Chir. 2018;89:283-6. [PubMed ID: 30588922].
-
17.
Rokhtabnak F, Djalali Motlagh S, Ghodraty M, Pournajafian A, Maleki Delarestaghi M, Tehrani Banihashemi A, et al. Controlled Hypotension During Rhinoplasty: A Comparison of Dexmedetomidine with Magnesium Sulfate. Anesth Pain Med. 2017;7(6). e64032. [PubMed ID: 29696129]. [PubMed Central ID: PMC5903392]. https://doi.org/10.5812/aapm.64032.
-
18.
Barends CR, Absalom A, van Minnen B, Vissink A, Visser A. Dexmedetomidine versus Midazolam in Procedural Sedation. A Systematic Review of Efficacy and Safety. PLoS One. 2017;12(1). e0169525. [PubMed ID: 28107373]. [PubMed Central ID: PMC5249234]. https://doi.org/10.1371/journal.pone.0169525.
-
19.
Kumar CM, Chua AWY, Imani F, Sehat-Kashani S. Practical Considerations for Dexmedetomidine Sedation in Adult Cataract Surgery under Local/Regional Anesthesia: A Narrative Review. Anesth Pain Med. 2021;11(4). e118271. https://doi.org/10.5812/aapm.113750.
-
20.
Akhondzadeh R, Olapour A, Rashidi M, Elyasinia F. Comparison of Sedation with Dexmedetomidine Alfentanil Versus Ketamine-Alfentanil in Patients Undergoing Closed Reduction of Nasal Fractures. Anesth Pain Med. 2020;10(4). e102946. [PubMed ID: 33134144]. [PubMed Central ID: PMC7539046]. https://doi.org/10.5812/aapm.102946.
-
21.
Gu X, Tan X, Chen J, Wang J, Lu Y, Zhang L. The clinical effect of dexmedetomidine combined with parecoxib sodium on sedation, antianxiety and prevention of intubation stress in patients undergoing functional endoscopic sinus surgery: a randomised controlled trial. BMC Anesthesiol. 2020;20(1):166. [PubMed ID: 32631301]. [PubMed Central ID: PMC7336422]. https://doi.org/10.1186/s12871-020-01080-0.
-
22.
Durmus M, But AK, Dogan Z, Yucel A, Miman MC, Ersoy MO. Effect of dexmedetomidine on bleeding during tympanoplasty or septorhinoplasty. Eur J Anaesthesiol. 2007;24(5):447-53. [PubMed ID: 17241505]. https://doi.org/10.1017/S0265021506002122.
-
23.
Ayoglu H, Yapakci O, Ugur MB, Uzun L, Altunkaya H, Ozer Y, et al. Effectiveness of dexmedetomidine in reducing bleeding during septoplasty and tympanoplasty operations. J Clin Anesth. 2008;20(6):437-41. [PubMed ID: 18929284]. https://doi.org/10.1016/j.jclinane.2008.04.008.
-
24.
Amri P, Nahrini S, Hajian-Tilaki K, Hamidian M, Alipour SF, Hamidi SH, et al. Analgesic Effect and Hemodynamic Changes Due to Dexmedetomidine Versus Fentanyl During Elective Colonoscopy: A Double-Blind Randomized Clinical Trial. Anesth Pain Med. 2018;8(6). e81077. [PubMed ID: 30719412]. [PubMed Central ID: PMC6347670]. https://doi.org/10.5812/aapm.81077.
-
25.
Nikoubakht N, Alimian M, Faiz SHR, Derakhshan P, Sadri MS. Effects of ketamine versus dexmedetomidine maintenance infusion in posterior spinal fusion surgery on acute postoperative pain. Surg Neurol Int. 2021;12:192. [PubMed ID: 34084620]. [PubMed Central ID: PMC8168657]. https://doi.org/10.25259/SNI_850_2020.
-
26.
Margulis R, Francis J, Tischenkel B, Bromberg A, Pedulla D, Grtisenko K, et al. Comparison of Dexmedetomidine and Dexamethasone as Adjuvants to Ultra-Sound Guided Interscalene Block in Arthroscopic Shoulder Surgery: A Double-Blinded Randomized Placebo-Controlled Study. Anesth Pain Med. 2021;11(3). https://doi.org/10.5812/aapm.117020.
-
27.
Talebi G, Moayeri H, Rahmani K, Nasseri K. Comparison of Three Different Doses of Dexmedetomidine Added to Bupivacaine in Ultrasound-Guided Transversus Abdominis Plane Block; A Randomized Clinical Trial. Anesth Pain Med. 2021;11(2). e113778. [PubMed ID: 34336630]. [PubMed Central ID: PMC8314081]. https://doi.org/10.5812/aapm.113778.
-
28.
Rahimzadeh P, Faiz SHR, Imani F, Derakhshan P, Amniati S. Comparative addition of dexmedetomidine and fentanyl to intrathecal bupivacaine in orthopedic procedure in lower limbs. BMC Anesthesiol. 2018;18(1):62. [PubMed ID: 29875020]. [PubMed Central ID: PMC5991430]. https://doi.org/10.1186/s12871-018-0531-7.
-
29.
Imani F, Farahmand Rad R, Salehi R, Alimian M, Mirbolook Jalali Z, Mansouri A, et al. Evaluation of Adding Dexmedetomidine to Ropivacaine in Pediatric Caudal Epidural Block: A Randomized, Double-blinded Clinical Trial. Anesth Pain Med. 2021;11(1). e112880. [PubMed ID: 34221950]. [PubMed Central ID: PMC8241816]. https://doi.org/10.5812/aapm.112880.
-
30.
Imani F, Zaman B, De Negri P. Postoperative Pain Management: Role of Dexmedetomidine as an Adjuvant. Anesth Pain Med. 2020;10(6). e112176. [PubMed ID: 34150582]. [PubMed Central ID: PMC8207883]. https://doi.org/10.5812/aapm.112176.
-
31.
Scott-Warren VL, Sebastian J. Dexmedetomidine: its use in intensive care medicine and anaesthesia. BJA Education. 2016;16(7):242-6. https://doi.org/10.1093/bjaed/mkv047.
-
32.
Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front Neurosci. 2020;14:330. [PubMed ID: 32431587]. [PubMed Central ID: PMC7214625]. https://doi.org/10.3389/fnins.2020.00330.
-
33.
Lee S. Dexmedetomidine: present and future directions. Korean J Anesthesiol. 2019;72(4):323-30. [PubMed ID: 31220910]. [PubMed Central ID: PMC6676029]. https://doi.org/10.4097/kja.19259.
-
34.
Lee SH, Choi YS, Hong GR, Oh YJ. Echocardiographic evaluation of the effects of dexmedetomidine on cardiac function during total intravenous anaesthesia. Anaesthesia. 2015;70(9):1052-9. [PubMed ID: 25919658]. https://doi.org/10.1111/anae.13084.
-
35.
Grape S, Kirkham KR, Frauenknecht J, Albrecht E. Intra-operative analgesia with remifentanil vs. dexmedetomidine: a systematic review and meta-analysis with trial sequential analysis. Anaesthesia. 2019;74(6):793-800. [PubMed ID: 30950522]. https://doi.org/10.1111/anae.14657.
-
36.
Hu J, Zhu M, Gao Z, Zhao S, Feng X, Chen J, et al. Dexmedetomidine for prevention of postoperative delirium in older adults undergoing oesophagectomy with total intravenous anaesthesia: A double-blind, randomised clinical trial. Eur J Anaesthesiol. 2021;38(Suppl 1):S9-S17. [PubMed ID: 33122571]. https://doi.org/10.1097/EJA.0000000000001382.
-
37.
Bahr MH, Rashwan DAE, Kasem SA. The Effect of Dexmedetomidine and Esmolol on Early Postoperative Cognitive Dysfunction After Middle Ear Surgery Under Hypotensive Technique: A Comparative, Randomized, Double-blind Study. Anesth Pain Med. 2021;11(1). e107659. [PubMed ID: 34221933]. [PubMed Central ID: PMC8236574]. https://doi.org/10.5812/aapm.107659.
-
38.
Gerlach AT, Murphy CV. Dexmedetomidine-associated bradycardia progressing to pulseless electrical activity: case report and review of the literature. Pharmacotherapy. 2009;29(12):1492. [PubMed ID: 19947809]. https://doi.org/10.1592/phco.29.12.1492.
-
39.
Takata K, Adachi YU, Suzuki K, Obata Y, Sato S, Nishiwaki K. Dexmedetomidine-induced atrioventricular block followed by cardiac arrest during atrial pacing: a case report and review of the literature. J Anesth. 2014;28(1):116-20. [PubMed ID: 23948748]. https://doi.org/10.1007/s00540-013-1676-7.
-
40.
Ohmori T, Shiota N, Haramo A, Masuda T, Maruyama F, Wakabayashi K, et al. Post-operative cardiac arrest induced by co-administration of amiodarone and dexmedetomidine: a case report. J Intensive Care. 2015;3:43. [PubMed ID: 26500779]. [PubMed Central ID: PMC4618359]. https://doi.org/10.1186/s40560-015-0109-0.
-
41.
Kim BJ, Kim BI, Byun SH, Kim E, Sung SY, Jung JY. Cardiac arrest in a patient with anterior fascicular block after administration of dexmedetomidine with spinal anesthesia: A case report. Medicine (Baltimore). 2016;95(43). e5278. [PubMed ID: 27787391]. [PubMed Central ID: PMC5089120]. https://doi.org/10.1097/MD.0000000000005278.
-
42.
Hui C, Cardinale M, Yegneswaran B. Significant Bradycardia in Critically Ill Patients Receiving Dexmedetomidine and Fentanyl. Case Rep Crit Care. 2017;2017:4504207. [PubMed ID: 29038737]. [PubMed Central ID: PMC5606045]. https://doi.org/10.1155/2017/4504207.
-
43.
Aikaterini A, Ioannis D, Dimitrios G, Konstantinos S, Vasilios G, George P. Bradycardia Leading to Asystole Following Dexmedetomidine Infusion during Cataract Surgery: Dexmedetomidine-Induced Asystole for Cataract Surgery. Case Rep Anesthesiol. 2018;2018:2896032. [PubMed ID: 30627445]. [PubMed Central ID: PMC6304573]. https://doi.org/10.1155/2018/2896032.
-
44.
Panchal AR, Bartos JA, Cabanas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2020;142(16_suppl_2):S366-468. [PubMed ID: 33081529]. https://doi.org/10.1161/CIR.0000000000000916.
-
45.
Fromme GA, MacKenzie RA, Gould AJ, Lund BA, Offord KP. Controlled hypotension for orthognathic surgery. Anesth Analg. 1986;65(6):683-6. [PubMed ID: 3706806].
-
46.
Janatmakan F, Nesioonpour S, Javaherforoosh Zadeh F, Teimouri A, Vaziri M. Comparing the Effect of Clonidine and Dexmedetomidine on Intraoperative Bleeding in Spine Surgery. Anesth Pain Med. 2019;9(1). e83967. [PubMed ID: 30881906]. [PubMed Central ID: PMC6408748]. https://doi.org/10.5812/aapm.83967.
-
47.
Sieśkiewicz A, Drozdowski A, Rogowski M. Ocena korelacji średniego ciśnienia tętniczego z krwawieniem śródoperacyjnym przy wolnej czynności serca w trakcie endoskopowej chirurgii zatok przynosowych. Otolaryngologia Polska. 2010;64(4):225-8. https://doi.org/10.1016/s0030-6657(10)70020-2.
-
48.
Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78(5):813-20. [PubMed ID: 8098190]. https://doi.org/10.1097/00000542-199305000-00002.
-
49.
Scheinin H, Aantaa R, Anttila M, Hakola P, Helminen A, Karhuvaara S. Reversal of the sedative and sympatholytic effects of dexmedetomidine with a specific alpha2-adrenoceptor antagonist atipamezole: a pharmacodynamic and kinetic study in healthy volunteers. Anesthesiology. 1998;89(3):574-84. [PubMed ID: 9743392]. https://doi.org/10.1097/00000542-199809000-00005.
-
50.
Ickeringill M, Shehabi Y, Adamson H, Ruettimann U. Dexmedetomidine infusion without loading dose in surgical patients requiring mechanical ventilation: haemodynamic effects and efficacy. Anaesth Intensive Care. 2004;32(6):741-5. [PubMed ID: 15648981]. https://doi.org/10.1177/0310057X0403200602.
-
51.
Ibrahim IM, Hassan R, Mostafa RH, Ibrahim MA. Efficacy of Dexmedetomidine Infusion Without Loading Dose on Hemodynamic Variables and Recovery Time During Craniotomy: A Randomized Double-blinded Controlled Study. Anesth Pain Med. 2021;11(2). e113410. [PubMed ID: 34336625]. [PubMed Central ID: PMC8314083]. https://doi.org/10.5812/aapm.113410.
-
52.
Sim JH, Yu HJ, Kim ST. The effects of different loading doses of dexmedetomidine on sedation. Korean J Anesthesiol. 2014;67(1):8-12. [PubMed ID: 25097732]. [PubMed Central ID: PMC4121500]. https://doi.org/10.4097/kjae.2014.67.1.8.
-
53.
Kung HC, Cheng CC, Kang DH, Jeong HJ, Shin YS, Kim DS, et al. The effects of loading dose administration rate of dexmedetomidine on sedation and dexmedetomidine requirement in elderly patients undergoing spinal anesthesia. Anesthesia and Pain Medicine. 2018;13(3):264-70. https://doi.org/10.17085/apm.2018.13.3.264.