Abstract
Background:
Current evidence on the effect of anesthetic-ECT time interval (AETI) is controversial. This study aimed to investigate the factors affecting the time interval between propofol injection and electro-convulsion induction and the relationship between these factors and the duration of convulsion.Methods:
In this study, 102 patients (616 sessions of ECT) were studied. Demographic and clinical data (age, gender, receiving or not receiving medications that affected the seizure threshold, the total number of ECT sessions, clinical severity of admission scores, clinical diagnosis, propofol dose, seizure duration, and AETI) were collected in special forms and analyzed by appropriate statistical methods.Results:
Sessions with long-term AETI had longer seizure time than sessions with short-term AETI (33.47 ± 8.46 vs. 28.68 ± 9.74, P value < 0.05). The duration of seizures was significantly longer in the group with long AETI in sessions 1, 2, and 4 than in the other group (P value < 0.05). There was a significant relationship between the duration of seizures and propofol dose, AETI, and receiving drugs effective in the seizure threshold (P value < 0.05).Conclusions:
The results showed that increasing AETI and injecting a lower dose of propofol to induce anesthesia would increase the duration of seizures. Also, taking medications that would affect the seizure threshold reduces the duration of seizures.Keywords
1. Background
Electroconvulsive therapy (ECT) is a psychiatric treatment in which seizures are induced electrically in patients to relieve psychiatric disorders (1, 2). It is a simple treatment with limited and transient side effects. It has a rapid therapeutic response and is used in drug-resistant cases and for earlier return to normal life (3). In recent years, ECT has been increasingly used in the treatment of severe and refractory depression, mania, schizophrenia, suicide, and hallucinatory symptoms and has played an important role in the treatment of these disorders (4, 5). In this method, treatment is performed under general anesthesia, in which small electrical currents pass through the brain, deliberately causing short-term seizures. It seems that ECT causes changes in brain chemistry that can immediately reverse the symptoms of mental illness (6). Besides, ECT is usually done two to three times a week. Treatment should continue until the patient receives the maximum appropriate therapeutic response. The duration of the seizure should be at least 25 seconds (4, 7).
A variety of anesthetics are used for electroconvulsive therapy. Each one has its advantages and disadvantages that can create unpleasant experiences for patients (8, 9). The given electrical stimulation in ECT causes seizures under anesthesia. The anesthesia period is short but affects efficacy and seizure threshold. Anesthesia is performed intravenously, which can interfere with the induction of seizures by ECT in addition to exerting anticonvulsant effects. However, these effects depend on several factors such as the drugs used, the concentration of anesthetics in the brain at the time of seizure induction, and their pharmacokinetic dose (7, 10).
The duration of seizures is considered a success factor in seizure therapy. General anesthesia can reduce its effectiveness. Propofol is widely used in most ECT centers (11). The hypnotic effect of propofol begins rapidly after a dose of 2.5 mg/kg and peaks at 90 to 100 seconds. The median effective dose (ED50) of propofol for reducing consciousness is 1 to 1.5 mg/kg after bolus administration. The duration of hypnotics depends on the dose and occurs 5 to 10 minutes after the injection of 2 to 2.5 mg/kg. Age affects the induced dose, the highest of which is under two years of age (2.88 mg/kg). The induced dose decreases with age. Its initial distribution half-life is two to eight minutes (11). This has led propofol to become the main drug used in ECT (12, 13). In the case of propofol, the onset of action and awakening after anesthesia is rapid. However, it may shorten the duration of the seizure (10, 14, 15).
The current evidence on the effect of AETI is controversial (16, 17). Research has shown that the electrical stimulation of the brain causes seizures without effectiveness shortly after the anesthetic is injected when the concentration of the anesthetic in the brain is at its maximum (18). For example, Taylor et al. (17) and Gálvez et al. (19) showed that longer AETI in propofol injection increased the duration and quality of seizures, while Jorgensen et al. presented conflicting results (20). This may be due to long-term ventilation and its effects on end-tidal CO2 (ETCO2). There is evidence suggesting that ventilation may affect the duration and quality of seizures. Research in this field has shown that long-term ventilation is related to the duration of seizures (17, 21). Thus, a full understanding of the factors affecting AETI and the duration of seizures is very important.
2. Objectives
This study aimed to identify the factors affecting the time interval between propofol injection and ECT induction, and the relationship between these factors and the duration of seizures.
3. Methods
This study was performed in the Psychiatric Ward of 22th Bahman Hospital in Qazvin. Demographic and clinical data of patients treated with the standard protocol of anesthesia (propofol and succinylcholine) in ECT sessions were used in the study. These clinical records were reviewed from October 2016 to September 2020. This study was conducted based on the research priorities of Qazvin University of Medical Sciences; it had no ethical issues, and was approved by the Ethics Committee of the University. Inclusion criteria included written consent, age over 18 years, no use of benzodiazepines at least 12 hours before any ECT treatment session, a clinical indication of ECT, bilateral electrode placement, and performing the newly developed ECT technique with the titration of electric current dose. Exclusion criteria included patients treated for outpatient ECT and patients who were not anesthetized with the initial dose of propofol and needed to receive an additional dose to induce anesthesia. In addition, sessions during which patients did not have seizures with their first electrical induction and needed to increase the voltage and re-induce seizures were excluded from the study.
During the study period, due to the use of different methods of anesthesia, it was observed that the time interval between propofol and ECT was different, and this difference would significantly affect the duration of seizures. For example, in the short time between anesthesia and ECT, anesthesiologists and psychiatrists intended to stimulate ECT for a relatively short time. For sessions that required a long interval between anesthesia and ECT, the specialists sought to ensure that ECT stimulation continued for a long time after the anesthetic was administered. This led to the analysis of patients based on AETI. To do this, patients' AETI data were divided into two groups: Short-term (< 2 min) and long-term (≥ 2 min). The short-term group consisted of 43 patients and the long-term group consisted of 59 patients.
Demographic and clinical data (patient code, age, gender, receiving or not receiving medication that would affect the seizure threshold, the total number of ECT sessions, clinical severity scores at admission, clinical diagnosis, propofol dose, seizure duration, and AETI) were collected and registered by special forms. It should be noted that clinical severity scores at admission were calculated by the Clinical Global Impression-Severity (CGI-S) scale. The items of this questionnaire are scored on a seven-point Likert scale. In this test, the doctor needs to assess the severity of the disease at the time of evaluation, using his/her experience. Possible ratings on this scale range from 1 (normal, not sick at all) to 7 (among very severe patients) (22).
Before ECT, all patients underwent pre-anesthesia evaluation and fasted for at least eight hours. They underwent various monitors such as the usual barometer, ECG, and pulse oximetry. After injection of anesthesia, electroshock was performed using a device with bilateral electrodes. The duration of seizures was recorded at each session. These times were recorded by a trained and experienced ECT assessor. In natural ventilation, the anesthesiologist ventilated the patient at approximately 10 breaths per minute. For sessions that required more ventilation, the anesthesiologist ventilated the patient at a rate of 25 breaths per minute. Then, ETCO2 was measured through a nasal catheter as a practical and relatively non-invasive method. The ETCO2 data (in mmHg) of all patients were analyzed. As accepted, ETCO2 is the amount of carbon dioxide released at the end of expiratory respiration. The ETCO2 levels indicate the adequate transport of carbon dioxide from the blood to the lungs and exhalation (23). It should be noted that the ETCO2 levels were measured in all sessions before ECT administration by an anesthesiologist.
After collecting data, the results were analyzed using SPSS software version 22. The Kolmogorov-Smirnov test was used to check the normality of the data. Data were presented using frequency and percentage for stratified variables and mean and standard deviation for continuous variables. The independent t test was used to compare the continuous variables, and the chi-square test was used to compare the stratified variables. The linear regression analysis was used to evaluate the relationship between each of the variables affecting the duration of seizures. The p value < 0.05 was considered as the significance level.
4. Results
In this study, 102 patients (628 sessions of ECT) including 67 men (65.7%) and 35 women (34.3%) were studied. Besides, 43 patients under study had short-term AETI sessions and 59 patients had long-term AETI sessions. Table 1 shows the characteristics of patients based on different clinical and demographic factors. As can be seen, sessions with long AETI were different from sessions with short AETI concerning the seizure time (33.47 ± 8.46 vs. 28.68 ± 9.74, P value < 0.05). No significant relationship was observed in other variables (P value > 0.05, Table 1).
| Variable | AETI, Secs | P Value | |
|---|---|---|---|
| Short Time Interval (n = 43) | Long Time Interval (n = 59) | ||
| Gender | 0.11 | ||
| Male | 32 (74.4) | 35 (59.3) | |
| Female | 11 (25.6) | 24 (40.7) | |
| Age, y | 36.97±6.96 | 35.12±7.55 | 0.21 |
| Duration of seizures, s | 28.68±9.74 | 33.47±8.46 | 0.001 |
| Dosage of succinylcholine, mg/kg | 0.5±.08 | 0.54±.09 | 0.15 |
| End-tidal CO2 mmHg | 28.83±7.64 | 29.67±7.28 | 0.57 |
| Total Sessions, No. | 6.12±0.68 | 6.02±0.64 | 0.44 |
| Propofol, mg/kg | 1.11±0.66 | 1.12±0.62 | 0.95 |
| Diagnosis | 0.28 | ||
| Schizophrenia | 4 (9.3) | 11 (18.6) | |
| Bipolar disorder | 25 (58.1) | 33 (55.9) | |
| Schizoaffective | 1 (2.3) | 1 (1.7) | |
| Major Depression | 12 (27.9) | 9 (15.3) | |
| Other disorders | 1 (2.3) | 5 (8.5) | |
| Using drugs that affected the seizure threshold | 0.68 | ||
| Yes | 26 (60.5) | 38 (64.4) | |
| No | 17 (39.5) | 21 (35.6) | |
| Clinical severity on admission | 5.86 ± 0.70 | 6.08 ± 0.72 | 0.12 |
Table 2 shows data on the duration of seizures in various ECT sessions. There was a statistically significant difference in the duration of seizures between the two groups so that the duration of seizures was significantly longer in the group with long AETI in sessions 1, 2, and 4 than in the other group (P value < 0.05, Table 2).
| Variable | Duration of Seizures | P Value | |||
|---|---|---|---|---|---|
| Number | Short Time Interval | Number | Long Time Interval | ||
| First session | 43 | 24.14 ± 14.86 | 59 | 34.42 ± 12.32 | 0.001 |
| Second session | 41 | 25.95 ± 12.68 | 56 | 30.45 ± 9.15 | 0.04 |
| Third session | 38 | 28.80 ± 13.48 | 53 | 30.23 ± 7.88 | 0.52 |
| Fourth session | 35 | 26.40 ± 7.06 | 49 | 30.65 ± 10.77 | 0.04 |
| Fifth session | 31 | 28.63 ± 13.17 | 44 | 31.77 ± 9.40 | 0.23 |
| Sixth session | 26 | 27.03 ± 8.27 | 37 | 30.46 ± 11.73 | 0.2 |
| Seventh session | 20 | 28.48 ± 10.35 | 29 | 33.07 ± 9.40 | 0.11 |
| Eighth session | 15 | 28.44 ± 10.43 | 20 | 32.85 ± 7.61 | 0.15 |
| Ninth session | 9 | 28.34 ± 12.66 | 10 | 35.80 ± 5.41 | 0.1 |
| 10th session | 4 | 33.00 ± 14.00 | 3 | 30.00 ± 1.00 | 0.73 |
| 11th session | 2 | 34.00 ± 0.00 | 1 | 40.00 ± 0.00 | N |
| 12th session | 2 | 31.00 ± 0.00 | 1 | 42.00 ± 0.00 | N |
The relationship between the duration of seizures and AETI during different electroconvulsive therapy sessions is shown in more detail in Figure 1. As shown in the figure, the increase in seizure duration was greater in the group with long AETI than in the other group (Figure 1).
Relationship between seizure duration and anesthesia-electroconvulsive therapy interval
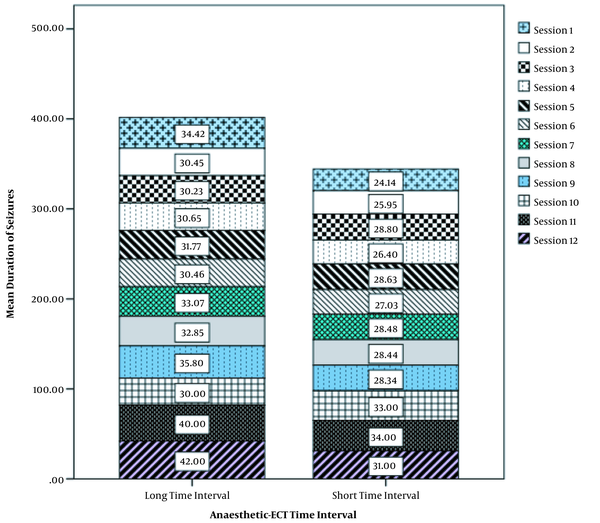
Table 3 shows the relationships between the independent variables and the duration of seizures. The analysis was done both non-adjusted and adjusted for variables of age, sex, and the number of ECT sessions. Among the variables analyzed (anesthesia-ECT interval, receiving drugs affecting seizure threshold, propofol dose, ETCO2, diagnosis, and succinylcholine), only the variables of AETI, receiving drugs affecting seizure threshold, and propofol dose were associated with the duration of seizures (P value < 0.05).
Relationship Between Independent Variables and Duration of Seizures
| Variable | Duration of Seizures | Duration of Seizures a | ||
|---|---|---|---|---|
| Beta | P Value | Beta | P Value | |
| Propofol, mg/kg | -0.203 | 0.041 | -0.204 | 0.047 |
| Receiving medication that affected the seizure threshold | -0.43 | < 0.05 | -0.45 | < 0.05 |
| Anesthesia-ECT interval, s | 0.48 | < 0.05 | 0.51 | < 0.05 |
| Major depression | -0.01 | 0.93 | -0.004 | 0.97 |
| Bipolar disorder | -0.29 | 0.3 | -0.31 | 0.34 |
| Schizophrenia | 0.25 | 0.1 | 0.27 | 0.13 |
| Other disorders | 0.22 | 0.25 | 0.23 | 0.28 |
| Dosage of succinylcholine, mg/kg | -0.007 | 0.94 | -0.007 | 0.95 |
| End-tidal CO2, mmHg | 0.15 | 0.128 | 0.16 | 0.138 |
5. Discussion
Evidence suggests that anesthetics can have a significant effect on seizure duration (24, 25). However, the effect of AETI on the induction of electrical seizures and its relationship with seizure duration and other factors affecting seizure duration (including propofol dose and ETCO2) is indistinct during ECT stimulation and it is in a haze of ambiguity. This study tried to resolve some of the ambiguities by examining the mentioned relationships.
The results showed that increasing AETI and injecting a lower dose of propofol to induce anesthesia would increase the duration of seizures. Perhaps the reason for this is the plasma concentration of the anesthetic (propofol) due to its absorption by peripheral tissues, which, in turn, reduces its amount and causes more propofol to return from the brain to the plasma. Due to the property of propofol in raising the seizure threshold, the electrical induction of the brain causes seizures to be more effective when the concentration of this substance in the brain is low. These results are in line with the studies by Gálvez et al. and contradict the study by Jorgensen et al. (19, 20). Perhaps the reason for this discrepancy is the limited sample size in the study by Jorgensen et al. In a larger sample of patients receiving thiopentone anesthesia, it was also found that an increase in AETI was associated with longer seizure duration and seizure quality (17). The optimal or preferred time interval for AETI probably varies from person to person and is also determined by the anesthetic used and the dose of absolute anesthesia (7). This caused patients to be divided into two groups based on this time interval. Demographic and clinical characteristics of patients were studied in both groups. The results showed that the duration of seizures was longer in people with prolonged AETI. In a more detailed study, the duration of seizures in different ECT sessions was compared, and the results showed that the duration of seizures was significantly longer in the group with long AETI than in the other group in sessions 1, 2, and 4. These results are in line with the study by Aytuluk et al. whose research supported the importance of AETI when used propofol as an anesthetic (26). Besides, alternative agents with different drug profiles may have different results. However, a study by Taylor et al. supports in part the importance of AETI (16).
Many studies assessed the factors that determine the seizure threshold and the change in seizure duration in ECT. It has been shown that changes in seizure threshold or duration of seizures can occur during the ECT period (7, 27). The AETI is very important in determining the duration of seizures; therefore, the monitoring and control of both variables will be important in clinical practice (28, 29). This led to the study of this relationship through regression analysis. The results of this analysis showed a direct relationship between AETI and the duration of seizures and an inverse relationship between the dose of propofol and the duration of seizures. Evidence suggests that propofol can inhibit seizure activity, but it can also have seizure effects. The results of the present study support the first hypothesis and showed that propofol could act as a dose-dependent anticonvulsant (30). A comparative study conducted by Mir et al. in 2017 found that patients receiving propofol had a shorter seizure duration than etomidate and thiopentone receivers (31). In addition, a study by Aytuluk et al. found that ECT with higher doses of propofol was associated with shorter seizure duration than ECT with a lower dose of propofol (26).
In this study, there was no significant difference in ETCO2 between the two groups of long AETI and short AETI. On the other hand, there was no significant relationship between the seizure duration and ETCO2. Thus, seizure duration seemed to be associated with increased AETI and a lower dose of propofol injection. In addition, this study showed that the ventilation method used during AETI may not have a significant effect on the duration of seizures. Taylor et al. achieved similar results and showed that ETCO2 did not affect the quality and duration of seizures (16). Contrary to this study, some studies have shown that excessive ventilation may affect the duration of seizures (32). Perhaps the reason for this discrepancy is that the AETI was not considered in such studies.
In this study, patients taking seizure-boosting drugs such as sodium valproate and carbamazepine had shorter seizure periods; thus, medications received by patients taking ECT should also be considered. Some drugs have positive and negative effects on the seizure threshold. The use of tricyclic, four-ring drugs, monoamine oxidase inhibitors, and antipsychotics does not interfere with ECT, but benzodiazepines and antiepileptic drugs such as sodium valproate and carbamazepine, as well as lidocaine, may elevate the seizure threshold (18).
In this study, unlike previous studies, age had no significant effect on the duration of seizures. Research in this field has shown that age has a significant effect on the duration and quality of seizures, and increasing age was associated with less quality and duration of seizures (19, 33). Concerning the causality of this issue, it should be stated that the mean age of the participants in this study was 35.92 ± 7.32 and ranged from 25 to 49 years, and this difference may be because other age ranges were not considered in this study.
One of the limitations of the present study is that we only examined the effect of these factors on the duration of seizures and neglected to improve patients' cognitive outcomes. This may be a basis for future studies. Another limitation of this study includes its cross-sectional nature. In addition to the limitations mentioned, one of the strengths of the present study is that recognizing the factors affecting the duration of seizures and AETI leads to increasing physicians' knowledge in this field that can be effective in deciding on the optimal dose. In this study, the two groups of AETI studied, despite not being random in terms of demographic and clinical characteristics, were well matched; thus, it was possible to compare the dose and other variables studied in this study. In addition, employing a large number of patients is another strength of this study.
5.1. Conclusions
Based on the results, the longer the time interval between propofol injection and electrical induction in the brain, the longer the induced seizures. It is noteworthy that this study concluded the mentioned result by rejecting the effect of ETCO2 and other factors. In addition, patients treated with seizure-boosting drugs such as sodium valproate and carbamazepine had shorter seizure periods, and it is therefore recommended to omit the overnight dose of anticonvulsants before ECT to induce effective seizures. In addition, patients who required higher doses of propofol to induce anesthesia had shorter seizures. These results indicate that propofol, which is commonly used in ECT, can increase the seizure threshold and thus, exerts anticonvulsant effects, which is contrary to the main purpose of seizures in the process of ECT. By meeting the above-mentioned conditions, especially increasing AETI, this effect can be reduced due to a decrease in the amount of propofol in the brain.
Acknowledgements
References
-
1.
Salik I, Marwaha R. Electroconvulsive therapy. Treasure Island FL: StatPearls Publishing LLC; 2021.
-
2.
Takamiya A, Seki M, Kudo S, Yoshizaki T, Nakahara J, Mimura M, et al. Electroconvulsive therapy for Parkinson's disease: A systematic review and meta-analysis. Mov Disord. 2021;36(1):50-8. [PubMed ID: 33280168]. https://doi.org/10.1002/mds.28335.
-
3.
Biazar G, Khoshrang H, Emir Alavi C, Soleimani R, Atrkarroushan Z, Bayat Z, et al. Electroconvulsive therapy-related anxiety: A survey in an academic hospital in the north of Iran. Anesth Pain Med. 2020;10(1). e99429. [PubMed ID: 32337169]. [PubMed Central ID: PMC7158242]. https://doi.org/10.5812/aapm.99429.
-
4.
Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry Resid J. 2020;16(1):6. https://doi.org/10.1176/appi.ajp-rj.2020.160103.
-
5.
Bowman-Dalley C, Hilliard JG. Perioperative challenges during electro convulsive therapy (ECT). Essentials of Neurosurgical Anesthesia & Critical Care. Springer; 2020. p. 271-7.
-
6.
Hassani V, Amniati S, Kashaninasab F, Niakan M, Moradi Moghadam O, Jafarian AA, et al. Electroconvulsive therapy for a patient with suicide by drinking bleach during treatment of COVID-19: A case report. Anesth Pain Med. 2020;10(6). e107513. https://doi.org/10.5812/aapm.107513.
-
7.
Swift P. The ECT handbook by I. Nicol Ferrier and Jonathan Waite 4th edn.RCPsych/Cambridge University Press. 2019. £45 (pb). 292 pp. ISBN 9781911623168. Br J Psychiatry. 2020;216(3):170. https://doi.org/10.1192/bjp.2020.18.
-
8.
Nazemroaya B, Mousavi SM. Comparison of premedication with low-dose midazolam versus etomidate for reduction of etomidate-induced myoclonus during general anesthesia for electroconvulsive therapy: A randomized clinical trial. Anesth Pain Med. 2019;9(6). e94388. [PubMed ID: 32280614]. [PubMed Central ID: PMC7118685]. https://doi.org/10.5812/aapm.94388.
-
9.
Memarian A, Farhidnia N, Fallahi F. Generalized tonic colonic seizure followed by loss of consciousness early after using low dose of tramadol: A case report. Anesth Pain Med. 2018;8(3). e64707. [PubMed ID: 30214882]. [PubMed Central ID: PMC6119223]. https://doi.org/10.5812/aapm.64707.
-
10.
Uppal V, Dourish J, Macfarlane A. Anaesthesia for electroconvulsive therapy. Continuing Education in Anaesthesia Critical Care & Pain. 2010;10(6):192-6. https://doi.org/10.1093/bjaceaccp/mkq039.
-
11.
Kadiyala PK, Kadiyala LD. Anaesthesia for electroconvulsive therapy: An overview with an update on its role in potentiating electroconvulsive therapy. Indian J Anaesth. 2017;61(5):373-80. [PubMed ID: 28584345]. [PubMed Central ID: PMC5444214]. https://doi.org/10.4103/ija.IJA_132_17.
-
12.
Fernandez-Candil J, Castelltort Masco L, Fabregas Julia N, Urretavizcaya Sarachaga M, Bernardo Arroyo M, Valero Castell R. Anaesthesia in electroconvulsive therapy. Special conditions. Rev Psiquiatr Salud Ment (Engl Ed). 2020;13(1):36-46. [PubMed ID: 30078550]. https://doi.org/10.1016/j.rpsm.2018.05.002.
-
13.
Simpson KH, Halsall PJ, Carr CM, Stewart KG. Propofol reduces seizure duration in patients having anaesthesia for electroconvulsive therapy. Br J Anaesth. 1988;61(3):343-4. [PubMed ID: 3263142]. https://doi.org/10.1093/bja/61.3.343.
-
14.
Kang E, Lee KH, Park JH. Comparison of two methods of anesthesia using patient state index: Propofol versus sevoflurane during interventional neuroradiology procedure. Anesth Pain Med. 2019;9(2). e87518. [PubMed ID: 31341825]. [PubMed Central ID: PMC6614782]. https://doi.org/10.5812/aapm.87518.
-
15.
Saeidi M, Alikhani R, Hormati A, Sabouri SM, Aminnejad R. Propofol-induced masseter muscle spasm in a woman with a major depressive disorder. Anesth Pain Med. 2018;8(3). e78748. [PubMed ID: 30214889]. [PubMed Central ID: PMC6119344]. https://doi.org/10.5812/aapm.78748.
-
16.
Taylor R, Wark H, Leyden J, Simpson B, McGoldrick J, Hadzi-Pavlovic D, et al. Effects of the anaesthetic-ECT time interval and ventilation rate on seizure quality in electroconvulsive therapy: A prospective randomised trial. Brain Stimul. 2020;13(2):450-6. [PubMed ID: 31889671]. https://doi.org/10.1016/j.brs.2019.12.012.
-
17.
Taylor R, Hadzi-Pavlovic D, Nikolin S, Bull M, Wark H, Leyden J, et al. The anaesthetic-ECT time interval with thiopentone-Impact on seizure quality. J Affect Disord. 2019;252:135-40. [PubMed ID: 30981950]. https://doi.org/10.1016/j.jad.2019.04.027.
-
18.
Kaplan BJ. Kaplan and sadock's synopsis of psychiatry. Behavioral sciences/clinical psychiatry. Tijdschr Psychiatr. 2016;58(1):78-9.
-
19.
Gálvez V, Hadzi-Pavlovic D, Wark H, Harper S, Leyden J, Loo CK. The anaesthetic-ect time interval in electroconvulsive therapy practice-is it time to time? Brain stimul. 2016;9(1):72-7. [PubMed ID: 26452698]. https://doi.org/10.1016/j.brs.2015.09.005.
-
20.
Jorgensen A, Christensen SJ, Jensen AEK, Olsen NV, Jorgensen MB. The influence of the anesthesia-to-stimulation time interval on seizure quality parameters in electroconvulsive therapy. J Affect Disord. 2018;231:41-3. [PubMed ID: 29428352]. https://doi.org/10.1016/j.jad.2018.01.022.
-
21.
Gundogdu O, Avci O, Gursoy S, Kaygusuz K, Kol IO. The effects of hyperventilation on seizure length and cerebral oxygenation during electroconvulsive therapy. North Clin Istanb. 2020;7(3):246-54. [PubMed ID: 32478296]. [PubMed Central ID: PMC7251261]. https://doi.org/10.14744/nci.2019.70893.
-
22.
Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37. [PubMed ID: 20526405]. [PubMed Central ID: PMC2880930].
-
23.
Eckstein M, Hatch L, Malleck J, McClung C, Henderson SO. End-tidal CO2 as a predictor of survival in out-of-hospital cardiac arrest. Prehosp Disaster Med. 2011;26(3):148-50. [PubMed ID: 22107764]. https://doi.org/10.1017/S1049023X11006376.
-
24.
Sartorius A, Beuschlein J, Remennik D, Pfeifer AM, Karl S, Bumb JM, et al. Empirical ratio of the combined use of S-ketamine and propofol in electroconvulsive therapy and its impact on seizure quality. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):457-63. [PubMed ID: 32699969]. [PubMed Central ID: PMC7981301]. https://doi.org/10.1007/s00406-020-01170-7.
-
25.
Francis-Taylor R, Ophel G, Martin D, Loo C. The ictal EEG in ECT: A systematic review of the relationships between ictal features, ECT technique, seizure threshold and outcomes. Brain Stimul. 2020;13(6):1644-54. [PubMed ID: 32998055]. https://doi.org/10.1016/j.brs.2020.09.009.
-
26.
Aytuluk HG, Simsek T, Yilmaz M, Turan AZ, Saracoglu KT. Can propofol lead to an increase in seizure threshold over the course of electroconvulsive therapy? Clin Psychopharmacol Neurosci. 2019;17(4):523-30. [PubMed ID: 31671490]. [PubMed Central ID: PMC6852674]. https://doi.org/10.9758/cpn.2019.17.4.523.
-
27.
Rasimas JJ, Stevens SR, Rasmussen KG. Seizure length in electroconvulsive therapy as a function of age, sex, and treatment number. J ECT. 2007;23(1):14-6. [PubMed ID: 17435566]. https://doi.org/10.1097/01.yct.0000263254.21668.f0.
-
28.
Ferrier IN, Waite J. The ECT handbook. Cambridge University Press; 2019.
-
29.
Stripp TK, Jorgensen MB, Olsen NV. Anaesthesia for electroconvulsive therapy - new tricks for old drugs: a systematic review. Acta Neuropsychiatr. 2018;30(2):61-9. [PubMed ID: 28462732]. https://doi.org/10.1017/neu.2017.12.
-
30.
Wang B, Bai Q, Jiao X, Wang E, White PF. Effect of sedative and hypnotic doses of propofol on the EEG activity of patients with or without a history of seizure disorders. J Neurosurg Anesthesiol. 1997;9(4):335-40. [PubMed ID: 9339406]. https://doi.org/10.1097/00008506-199710000-00008.
-
31.
Mir AH, Shah NF, Din MU, Langoo SA, Reshi FA. Effectiveness of sodium thiopentone, propofol, and etomidate as an ideal intravenous anesthetic agent for modified electroconvulsive therapy. Saudi J Anaesth. 2017;11(1):26-31. [PubMed ID: 28217049]. [PubMed Central ID: PMC5292848]. https://doi.org/10.4103/1658-354X.197339.
-
32.
Gomez-Arnau J, de Arriba-Arnau A, Correas-Lauffer J, Urretavizcaya M. Hyperventilation and electroconvulsive therapy: A literature review. Gen Hosp Psychiatry. 2018;50:54-62. [PubMed ID: 29054017]. https://doi.org/10.1016/j.genhosppsych.2017.09.003.
-
33.
Perera TD, Luber B, Nobler MS, Prudic J, Anderson C, Sackeim HA. Seizure expression during electroconvulsive therapy: relationships with clinical outcome and cognitive side effects. Neuropsychopharmacology. 2004;29(4):813-25. [PubMed ID: 14735129]. https://doi.org/10.1038/sj.npp.1300377.