Abstract
Background:
Postoperative pain following laparoscopic cholecystectomy (LC) arises from incision sites and residual intraperitoneal CO2 gas. Opioids as a class of pain-relieving drugs are broadly used to control pain after LC; however, these drugs can cause various side effects.Objectives:
The purpose of this study was to compare the efficacy of intraperitoneal injection of bupivacaine with that of intravenous ketorolac in managing postoperative pain in patients who had undergone LC.Methods:
This randomized, double-blind clinical trial was carried out on patients who had undergone LC. Ninety patients who had undergone elective LC were randomly divided into 3 groups (n = 30 for each group). Group A received 40 mL of 0.25% bupivacaine solution intraperitoneally at the end of the operation; group B received 30 mg of ketorolac intravenously 30 minutes before surgery and every 8 hours after surgery, and patients in group C received normal saline intraperitoneally and intravenous injection. The patients were postoperatively assessed for Visual Analog Scale (VAS) scores, postoperative opioid consumption, shoulder pain, side effects (sedation, nausea, and vomiting), and satisfaction. The data were analyzed using SPSS. P values < 0.05 were considered significant.Results:
The intraperitoneal injection of bupivacaine and intravenous injection of ketorolac were significantly effective in reducing postoperative abdominal pain, shoulder pain, and incidence of nausea and vomiting compared to the placebo group (P < 0.001). Although intraperitoneal bupivacaine and intravenous ketorolac had no significant difference in pain relief compared with each other, patients in both bupivacaine and ketorolac groups were significantly more satisfied with their analgesia compared to the control group (P < 0.001).Conclusions:
Intraperitoneal injection of bupivacaine and intravenous injection of ketorolac both are safe and effective methods to control pain, nausea, and vomiting after LC.Keywords
Ketorolac Bupivacaine Intraperitoneal Injection Postoperative Pain Laparoscopic Cholecystectomy
1. Background
Laparoscopic cholecystectomy (LC), the most common abdominal surgical procedure worldwide, is the current gold standard for the treatment of cholelithiasis (1, 2). Postoperative pain is one of the major reasons for rehospitalization after surgery, as well as the chief complaint of prolonged recovery after cholecystectomy and other abdominal surgeries; in this regard, various methods have been proposed to treat them (3-6).
Opioids are the most powerful analgesic drugs in controlling and treatment of pain after cholecystectomy; however, opioid-related side effects (such as drowsiness, postoperative nausea and vomiting (PONV), constipation, and respiratory depression) lead to poor pain control and decreased patient satisfaction (7-10). To reduce the side effects of administered opioids, other methods of perioperative pain management, such as non-opioids and nerve blocks, have been considered by anesthesiologists (11-17). Intraperitoneal injection is one of the effective methods to control pain after LC (18). A number of studies reported the efficacy of intraperitoneal injection of local anesthetics following LC, while others reported their ineffectiveness (19).
Bupivacaine is the most common local anesthetic that has been used for local anesthesia after LC (20). Bupivacaine inhibits pain transmission by binding to voltage-gated sodium channels and blocking sodium entry into the cell (21). However, the optimum volume or concentration of bupivacaine to reduce postoperative pain is still unclear.
As a first-generation non-steroidal anti-inflammatory drug (NSAID), Ketorolac exerts its actions by blocking the prostaglandin synthesis by inhibiting the cyclooxygenase enzyme (22, 23). The role of NSAIDs in controlling postoperative pain after LC has been studied in several studies.
Many studies have shown that the preventive use of NSAIDs reduces the need for opioids after surgery and delays the first dose of opioids for pain management (12, 24, 25). However, the administration of NSAIDs may be associated with adverse effects such as inhibition of platelet aggregation and a higher risk of intraoperative and postoperative bleeding (26, 27).
2. Objectives
Considering the importance of postoperative pain management after LC, this study was designed to compare the efficacy of intraperitoneal bupivacaine and intravenous ketorolac in managing postoperative pain following LC. Also, we aimed to achieve a more effective and useful dose of intraperitoneal bupivacaine.
3. Methods
3.1. Study Design
This randomized, double-blind clinical trial was conducted at Imam Khomeini Hospital of Ardabil, Iran, from 2015 to 2017. The effects of intraperitoneal bupivacaine and intravenous ketorolac on postoperative pain were assessed after LC. Based on ASA physical status I-II, the inclusion criteria were patients between 20 to 60 years old who had undergone LC. In addition, patients with a history of hospitalization at ≤ 1 month before the study, diabetes mellitus, hypersensitivity or reaction to the drugs, besides heart diseases and dysrhythmias, PONV, gastrointestinal disorder, infectious diseases, chronic respiratory diseases, chronic renal failure, hepatitis, cancer, and long-term use of NSAIDs or opioids, were excluded from the study.
3.2. Randomization and Intervention
In the current study, all patients underwent LC with the general anesthesia method. All patients received the same anesthetic method. First, they received 3 - 5 mL/kg Ringer’s lactate solution. Induction of anesthesia was established by administering midazolam 20 µg/kg, fentanyl 3 µg/kg, and propofol 2 mg/kg. For muscle relaxation and tracheal intubation, atracurium 0.5 mg/kg was given. After 3 minutes, endotracheal intubation was performed with tube sizes 7.5 to 8 mm. Maintenance of anesthesia was done with propofol infusion (75 - 125 µg/kg/min) and O2 (100%).
During surgery, fentanyl (1 µg/kg) and atracurium (0.2 mg/kg) were injected intravenously every half hour. At the end of surgery and spontaneous breathing of patients, atropine 0.02 mg/kg and neostigmine 0.05 mg/kg were injected intravenously to reverse muscle relaxation.
Patients then underwent LC. All the operations were performed by 1 surgeon. At the end of surgery, patients were randomly divided into 3 groups based on a one-to-one allocation (30 patients in each group) using sealed envelopes. Group A received 40 mL of bupivacaine HCL (0.5%; Aspen Co, France) with 0.25% of concentration at the end of the operation. Group B received 30 mg of ketorolac (Caspian Tamin, Rasht, Iran) injected intravenously 30 minutes before surgery and every 8 hours after surgery. Moreover, group C (the placebo group) received 40 mL of normal saline intraperitoneally and 2 mL of normal saline intravenously. In all 3 groups, patients received patient-control analgesia (PCA) pump (Zhejiang Fert, Pouyan Tajhiz Teb of Asia Co Ltd, China). This device had 15 µg/mL bolus dosing, 15-minute lockout time, and none infusion rate with 100 mL reservoir volumes.
The principal investigator, patients, and outcome analyzer were blinded to group status, knowing only randomization group codes.
3.3. Variables of the Study
In the current study, demographic characteristics, including age, sex, body weight, ASA physical status, and duration of surgery, were recorded. In addition, patients’ abdominal and shoulder pain, nausea, vomiting, sedation, satisfaction, and opioid consumption were evaluated.
3.4. Patients Follow-up
Postoperative pain and PONV were recorded 1, 6, 12, and 24 hours after surgery. The postoperative pain and PONV were assessed by Visual Analog Scale (VAS) and PONV scores, respectively.
VAS scores express the severity of pain ranging from 0 to 10 [0, pain free; 1 - 3, mild pain (does not affect sleep); 4 - 6, moderate pain; 7 - 9, severe pain (cannot sleep or wake up because of pain); and 10, sharp pain]. PONV scores evaluate nausea and vomiting ranging from 0 to 4 (if the patient had no nausea or vomiting, a score of 0 was given, and if the patient had severe nausea and vomiting, a score of 4 was given). Moreover, vomiting was defined as forceful expulsion of gastric contents from the mouth or retching. If events of vomiting or retching were separated by more than 1 minute, they were considered as separate episodes. For cases with VAS ≥ 4, patients used fentanyl loading (bolus) doses and, if needed, followed by slowly intravenous 0.5 mg/kg meperidine injection. For cases with nausea and vomiting, 10 mg of metoclopramide was administered slowly and intravenously. Overall patient satisfaction scores (ranging from 1 to 5; 1, very dissatisfied and 5, very satisfied) were recorded 24 hours after surgery. Average opioid consumption was calculated based on the use of fentanyl and meperidine.
3.5. Statistical Analysis
The results are given as mean ± SD or median and 25th - 75th percentiles. Continuous variables were compared using the Student t test. Data were compared between different groups using 1-way analysis of variance (ANOVA) with the Tukey-Kramer post hoc test or by the Kruskal-Wallis test. Analysis of variance was also used for repeated measurements. All data were processed using SPSS version 21 (SPSS Inc, Chicago, Ill, USA). P values of < 0.05 were considered significant.
3.6. Ethical Considerations
The current research was performed with informed consent and ethical approval from the Ethics Committee of Ardabil University of Medical Sciences (code: IR.ARUMS.REC.1394.70). Moreover, this study was registered on the Iranian Registry for Clinical Trials website (code: IRCT201606064131N2).
4. Results
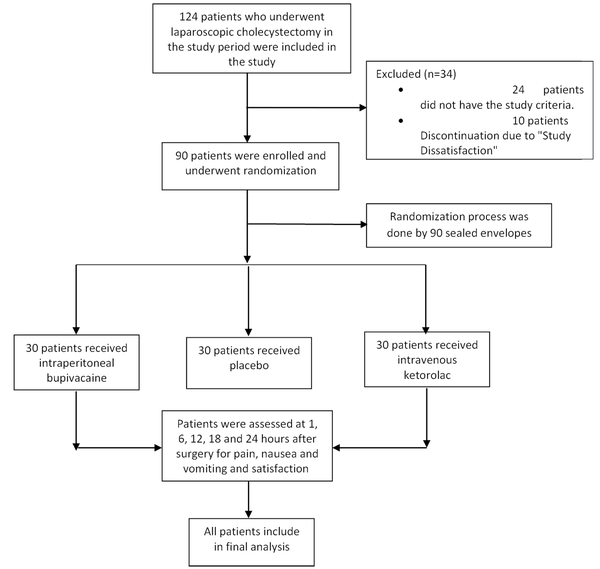
The flowchart of the study is presented in Figure 1. A total of 124 patients who had undergone LC were included in the study; 24 patients did not meet the inclusion criteria and were excluded from the study, and 10 patients discontinued due to “study dissatisfaction.” The study population consisted of 90 patients (30 patients in each group), of whom 64 were female (71.11%), and 26 were male (28.89%). Demographic characteristics of the study population are represented in Table 1. There were no significant differences in age, sex, weight, ASA status (I/II), and operation time among the 3 groups at each time point (P > 0.05; Table 1).
Flowchart of the study
| Variables | Placebo Group | Bupivacaine Group | Ketorolac | P Value |
|---|---|---|---|---|
| Age (y) | 45.1 ± 14.2 | 39.4 ± 15.2 | 42.7 ± 13.9 | 0.32 |
| Sex (male/female) (n) | 4/26 | 6/24 | 3/27 | 0.53 |
| Weight (kg) | 74.5 ± 10.2 | 72.7 ± 11.3 | 76.4 ± 12.1 | 0.44 |
| ASAPS I/II (n) | 27/3 | 29/1 | 29/1 | 0.42 |
| Operation time (min) | 46.7 ± 13.7 | 50.8 ± 15.2 | 48.9 ± 17.5 | 0.59 |
4.1. Postoperative Abdominal Pain
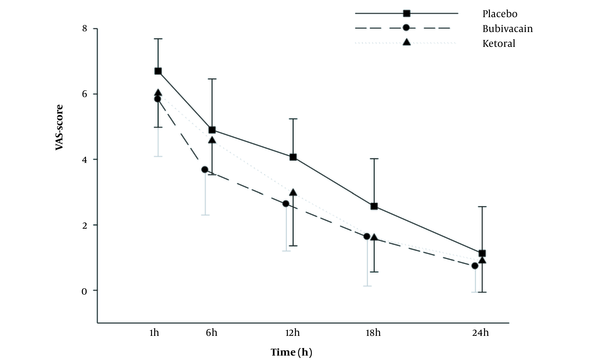
VAS score values are shown in Figure 2 and Table 2. VAS score values in all 3 groups increased 1 hour after surgery. The results showed that VAS score values significantly increased in the placebo group than in the bupivacaine and ketorolac groups from 30 minutes to 24 hours after the operation (P < 0.01 to P < 0.001; Figure 2). In addition, ANOVA with repeated measurements was used to compare the mean pain severity 1, 6, 12, 18, and 24 hours after surgery in all groups. According to the Greenhouse-Geisser test, the mean pain intensity varied significantly at different times (regardless of groups A, B, and C; F 4,38 = 446.23; P < 0.001). To show the effect of time, the comparison of the mean pain intensity between different times showed a significant difference in mean pain intensity between all hours (P < 0.001 for all).
Comparison of the pain scores (Visual Analog Scale) in the experimental groups at postoperative times. Values are represented as mean ± SD. Differences between the results of the placebo group and other groups (*** P < 0.001; n = 30 for each group).
| Variables (h) | Placebo Group | Bupivacaine Group | Ketorolac | P Value (Within Groups) |
|---|---|---|---|---|
| VAS 1 | 6.7 ± 0.9 | 5.8 ± 1.7 | 6.0 ± 1.0 | < 0.001 |
| VAS 6 | 4.9 ± 1.56 | 3.6 ± 1.3 | 4.5 ± 1.0 | |
| VAS 12 | 4.0 ± 1.1 | 2.6 ± 1.4 | 2.9 ± 1.6 | |
| VAS 18 | 2.5 ± 1.4 | 1.6 ± 1.5 | 1.6 ± 1.0 | |
| VAS 24 | 1.1 ± 1.4 | 0.7 ± 0.7 | 0.9 ± 0.9 | |
| P value (between groups) | 0.001 | |||
A paired comparison of mean pain severity between groups A, B, and C showed no significant difference between groups A and B in terms of the mean pain intensity after the operation; however, the mean pain severity was significantly lower in the bupivacaine and ketorolac groups than in the placebo group (P < 0.001 and P < 0.05, respectively).
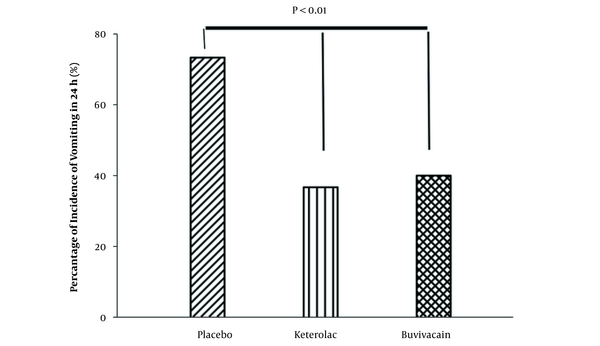
Considering the rate of nausea, the results showed that the rate of nausea was significantly higher in the placebo group than in the bupivacaine and ketorolac groups (P < 0.001). There were no significant differences between bupivacaine and ketorolac groups in terms of nausea 1 - 24 hours after surgery (Figure 3).
Percentage of the nausea incidence in the experimental groups 24 hours after surgery (n = 30 for each group).
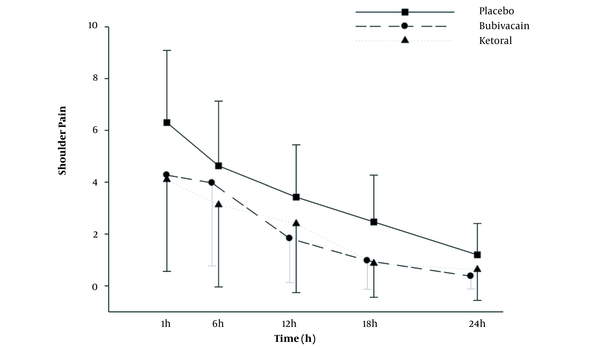
The analysis of shoulder pain showed a significant time effect (P < 0.001) with a moderate effect size of partial η2 = 0.54. The groups had significantly different means (P = 0.012; partial η2 = 0.10; Figure 4).
Comparison of the shoulder pain in the experimental groups at postoperative times. Values are represented as mean ± SD. Differences between the results of the placebo group and other groups (*** P < 0.001; n = 30 for each group).
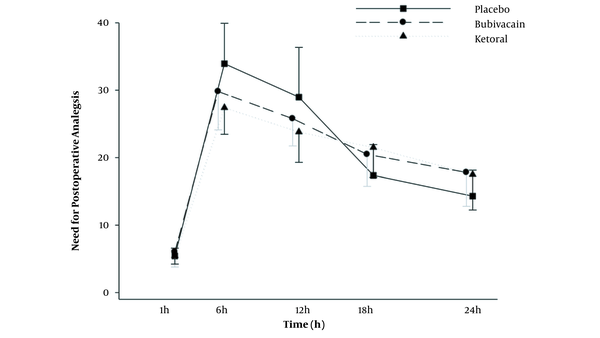
In terms of the need for fentanyl after surgery, the results showed that 6 and 12 hours after surgery, the need for fentanyl analgesia was significantly higher in the placebo group than in the bupivacaine and ketorolac groups (P < 0.001; Table 3). Furthermore, the need for fentanyl was more in the bupivacaine group than in the ketorolac group 6 and 12 hours after surgery, but this difference was not statistically significant (Figure 5). Moreover, according to the Greenhouse-Geisser test, the average consumption of fentanyl was significantly different at various times (regardless of groups A, B, and C). A comparison of the mean fentanyl consumption was made between different times, and there was a significant difference in mean fentanyl consumption between different time points (P < 0.001 for all; Table 3).
The Average Consumption of Fentanyl (µg) in the Ketorolac, Bupivacaine, and Placebo Groups 24 Hours After Surgery a
| Variables (h) | Placebo Group | Bupivacaine Group | Ketorolac | P Value (Within Groups) |
|---|---|---|---|---|
| 1 | 5.4 ± 1.1 | 6.0 ± 2.2 | 5.4 ± 1.2 | < 0.001 |
| 6 | 33.9 ± 5.9 | 29.8 ± 5.6 | 27.4 ± 3.9 | |
| 12 | 28.9 ± 7.3 | 25.7 ± 4.0 | 23.8 ± 4.5 | |
| 18 | 17.3 ± 4.5 | 20.4 ± 4.7 | 21.5 ± 4.4 | |
| 24 | 14.3 ± 3.8 | 17.8 ± 5.0 | 17.5 ± 5.2 | |
| P value (between groups) | < 0.001 | |||
Comparison of the postoperative analgesic consumption in the experimental groups at postoperative times. Values are represented as the number of frequency. Differences between the results of the placebo group and other groups (*** P < 0.001; n = 30 for each group).
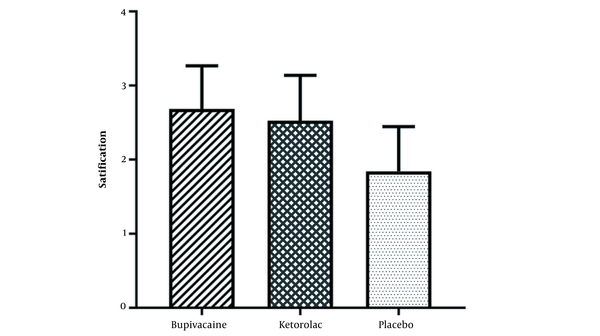
The results of patients’ satisfaction with analgesia in different groups showed that bupivacaine and ketorolac groups were highly satisfied compared with the placebo group (P < 0.001; Figure 6). There was no significant difference between bupivacaine and ketorolac groups in terms of patients’ satisfaction with analgesia (Figure 6).
Patients’ satisfaction with analgesia in the studied groups. Values are represented as mean ± SD (n = 30 for each group).
5. Discussion
Although opioids are frequently used for postoperative pain management, these drugs are associated with significant side effects, which can affect patients’ treatment (12, 28-30). Recently, multiple studies have been designed to produce effective pain relief to eliminate the need for opioids (31-33). The current study was conducted to evaluate the efficacy and complications of intraperitoneal injection of bupivacaine with those of intravenous injection of ketorolac in managing postoperative pain after LC. The results of the current study showed that intraperitoneal bupivacaine and intravenous ketorolac reduced pain in the surgical site and shoulder in the first 24 hours after LC compared with the placebo group; however, bupivacaine and ketorolac had no significant difference in pain relief compared with each other. In addition, according to the nausea and vomiting score, it was shown that the incidence of nausea was significantly higher in the placebo group than in the bupivacaine and ketorolac groups. Furthermore, various methods have been studied to reduce PONV in these patients (34).
Similar to our findings, Khurana et al. reported that intraperitoneal bupivacaine reduced pain in the first 24 hours after surgery compared with the placebo group (35). Sharma et al. also reported that intraperitoneal bupivacaine and ropivacaine resulted in pain relief in the first 24 hours after surgery (18). In addition, Wasim and Shafqatullah and Nupur et al. showed that intraperitoneal bupivacaine in LC significantly reduced pain in the first 24 hours after surgery compared with the control group (36, 37). Overall, the results of the above studies on the effect of bupivacaine on pain relief were consistent with the current study. However, Zmora et al. reported that bupivacaine was not associated with significant pain relief after LC (38). In their study, bupivacaine was injected into the gallbladder bed and right sub-diaphragm, which may cause different results.
Concerning the effects of ketorolac in patients with LC, various studies have indicated that ketorolac can reduce pain, which is consistent with our results (39). Our findings revealed that the rate of nausea and vomiting was lower in the bupivacaine and ketorolac groups than in the placebo group. Sharma et al. reported a 15 - 20% incidence of nausea and vomiting in the intraperitoneal bupivacaine group (18), which was lower than our study results, but Murdoch et al. reported a 59% incidence of nausea in the ketorolac group, which was higher than our study results (39).
The results of our study also showed that fentanyl consumption did not show a significant difference between the studied groups. Unlike our study, some previous studies have reported that bupivacaine and ketorolac can reduce the use of opioids in managing postoperative pain (39, 40). Various factors may be involved in this discrepancy, related to the use of fentanyl after surgery, such as sample size, duration of surgery, type of injection (intravenous with topical), site of injection (sub-diaphragm against sublingual), anesthetic solution, time of injection (before or after surgery), residual carbon dioxide volume, and dosage and type of analgesic medications (19).
The current study also evaluated patients’ satisfaction with analgesia. Patients in both intraperitoneal bupivacaine and intravenous ketorolac groups were significantly more satisfied with their analgesia than the control group. Generally, postoperative pain relief in patients improves respiration, facilitates movements, accelerates the return to normal functioning, and increases patient satisfaction with postoperative analgesia.
The low sample size and duration of follow-up were the limitations of the present study. Accordingly, further studies with larger sample sizes are needed to confirm these results.
5.1. Conclusions
Intraperitoneal bupivacaine and intravenous ketorolac both are safe and effective techniques for controlling pain, reducing nausea and vomiting, and increasing the satisfaction of patients who are candidates for LC.
References
-
1.
Marino MV, Mirabella A, Guarrasi D, Lupo M, Komorowski AL. Robotic-assisted repair of iatrogenic common bile duct injury after laparoscopic cholecystectomy: Surgical technique and outcomes. Int J Med Robot. 2019;15(3). e1992. [PubMed ID: 30773791]. https://doi.org/10.1002/rcs.1992.
-
2.
Ghomeishi A, Mohtadi AR, Behaeen K, Nesioonpour S, Bakhtiari N, Khalvati Fahlyani F. Comparison of the Effect of Propofol and Dexmedetomidine on Hemodynamic Parameters and Stress Response Hormones During Laparoscopic Cholecystectomy Surgery. Anesth Pain Med. 2021;11(5). e119446. https://doi.org/10.5812/aapm.119446.
-
3.
Norozi V, Ghazi A, Amani F, Bakhshpoori P. Effectiveness of Sublingual Buprenorphine and Fentanyl Pump in Controlling Pain After Open Cholecystectomy. Anesth Pain Med. 2021;11(3). e113909. [PubMed ID: 34540635]. [PubMed Central ID: PMC8438705]. https://doi.org/10.5812/aapm.113909.
-
4.
Aditianingsih D, Mochtar CA, Chandra S, Sukmono RB, Soamole IW. Comparison of Three-Quadrant Transversus Abdominis Plane Block and Continuous Epidural Block for Postoperative Analgesia After Transperitoneal Laparoscopic Nephrectomy. Anesth Pain Med. 2018;8(5). e80024. [PubMed ID: 30533391]. [PubMed Central ID: PMC6240789]. https://doi.org/10.5812/aapm.80024.
-
5.
Davari-Tanha F, Samimi S, Khalaj Z, Bastanhagh E. Comparison of Intraperitoneal Normal Saline Infusion with Pulmonary Recruitment Maneuver in Reducing Shoulder and Upper Abdomen Pain Following Gynecologic Laparoscopic Procedures: A Randomized, Controlled, Triple-Blind Trial. Anesth Pain Med. 2019;9(3). e92444. [PubMed ID: 31497525]. [PubMed Central ID: PMC6712360]. https://doi.org/10.5812/aapm.92444.
-
6.
Imani F, Rahimzadeh P, Faiz HR, Abdullahzadeh-Baghaei A. An Evaluation of the Adding Magnesium Sulfate to Ropivacaine on Ultrasound-Guided Transverse Abdominis Plane Block After Abdominal Hysterectomy. Anesth Pain Med. 2018;8(4). e74124. [PubMed ID: 30250819]. [PubMed Central ID: PMC6139531]. https://doi.org/10.5812/aapm.74124.
-
7.
Mottaghi K, Safari F, Sezari P, Salimi A, Nashibi M. Effect of Intrapleural Meperidine on Post-Operative Pain after Open Cholecystectomy. Tanaffos. 2019;18(1):79-83. [PubMed ID: 31423145]. [PubMed Central ID: PMC6690319].
-
8.
Rupniewska-Ladyko A, Malec-Milewska M. A High Dose of Fentanyl May Accelerate the Onset of Acute Postoperative Pain. Anesth Pain Med. 2019;9(5). e94498. [PubMed ID: 31903331]. [PubMed Central ID: PMC6935250]. https://doi.org/10.5812/aapm.94498.
-
9.
Edinoff AN, Kaplan LA, Khan S, Petersen M, Sauce E, Causey CD, et al. Full Opioid Agonists and Tramadol: Pharmacological and Clinical Considerations. Anesth Pain Med. 2021;11(4). e119156. [PubMed ID: 34692448]. [PubMed Central ID: PMC8520671]. https://doi.org/10.5812/aapm.119156.
-
10.
Malik KM, Imani F, Beckerly R, Chovatiya R. Risk of Opioid Use Disorder from Exposure to Opioids in the Perioperative Period: A Systematic Review. Anesth Pain Med. 2020;10(1). e101339. [PubMed ID: 32337175]. [PubMed Central ID: PMC7158240]. https://doi.org/10.5812/aapm.101339.
-
11.
Suksompong S, von Bormann S, von Bormann B. Regional Catheters for Postoperative Pain Control: Review and Observational Data. Anesth Pain Med. 2020;10(1). e99745. [PubMed ID: 32337170]. [PubMed Central ID: PMC7158241]. https://doi.org/10.5812/aapm.99745.
-
12.
Urits I, Jung JW, Amgalan A, Fortier L, Anya A, Wesp B, et al. Utilization of Magnesium for the Treatment of Chronic Pain. Anesth Pain Med. 2021;11(1). e112348. [PubMed ID: 34221945]. [PubMed Central ID: PMC8236839]. https://doi.org/10.5812/aapm.112348.
-
13.
Edinoff AN, Girma B, Trettin KA, Horton CC, Kaye AJ, Cornett EM, et al. Novel Regional Nerve Blocks in Clinical Practice: Evolving Techniques for Pain Management. Anesth Pain Med. 2021;11(4). e118278. [PubMed ID: 34692446]. [PubMed Central ID: PMC8520672]. https://doi.org/10.5812/aapm.118278.
-
14.
Johnson AC, Colakoglu S, Reddy A, Kerwin CM, Flores RA, Iorio ML, et al. Perioperative Blocks for Decreasing Postoperative Narcotics in Breast Reconstruction. Anesth Pain Med. 2020;10(5). e105686. [PubMed ID: 34150564]. [PubMed Central ID: PMC8207839]. https://doi.org/10.5812/aapm.105686.
-
15.
Alimian M, Imani F, Rahimzadeh P, Faiz SHR, Bahari-Sejahrood L, C. Hertling A. Adding Dexmedetomidine to Bupivacaine in Ultrasound-guided Thoracic Paravertebral Block for Pain Management after Upper Abdominal Surgery: A Double-blind Randomized Controlled Trial. Anesth Pain Med. 2021;11(6). e120787. https://doi.org/10.5812/aapm.120787.
-
16.
Mahmoudi K, Rashidi M, Soltani F, Savaie M, Hedayati E, Rashidi P. Comparison of Intercostal Nerve Block with Ropivacaine and Ropivacaine-Dexmedetomidine for Postoperative Pain Control in Patients Undergoing Thoracotomy: A Randomized Clinical Trial. Anesth Pain Med. 2021;11(6). e118667. https://doi.org/10.5812/aapm.118667.
-
17.
Rokhtabnak F, Sayad S, Izadi M, Djalali Motlagh S, Rahimzadeh P. Pain Control After Mastectomy in Transgender Patients: Ultrasound-guided Pectoral Nerve Block II Versus Conventional Intercostal Nerve Block: A Randomized Clinical Trial. Anesth Pain Med. 2021;11(5). e119440. https://doi.org/10.5812/aapm.119440.
-
18.
Sharma CS, Singh M, Rautela RS, Kochhar A, Adlakha N. Comparison of intraperitoneal and periportal bupivacaine and ropivacaine for postoperative pain relief in laparoscopic cholecystectomy: a randomized prospective study. Anaesth Pain Intensive Care. 2019;18(4):350-4.
-
19.
Boddy AP, Mehta S, Rhodes M. The effect of intraperitoneal local anesthesia in laparoscopic cholecystectomy: a systematic review and meta-analysis. Anesth Analg. 2006;103(3):682-8. [PubMed ID: 16931681]. https://doi.org/10.1213/01.ane.0000226268.06279.5a.
-
20.
Suma S, Suresh M, Nikhil M, Sreeramulu PN. Intra-Peritoneal Bupivacaine Instillation for Post-Operative Pain Relief after Laparoscopic Cholecystectomy: A Prospective Study. Int J Contemp Surg. 2019;7(2). https://doi.org/10.5958/2321-1024.2019.00033.3.
-
21.
Paganelli MA, Popescu GK. Actions of bupivacaine, a widely used local anesthetic, on NMDA receptor responses. J Neurosci. 2015;35(2):831-42. [PubMed ID: 25589775]. [PubMed Central ID: PMC4293426]. https://doi.org/10.1523/JNEUROSCI.3578-14.2015.
-
22.
Tuncer F, Knackstedt R, Murthy A, Patel N. Postoperative Ketorolac Administration Is Not Associated with Hemorrhage in Cranial Vault Remodeling for Craniosynostosis. Plast Reconstr Surg Glob Open. 2019;7(8). e2401. [PubMed ID: 31592008]. [PubMed Central ID: PMC6756670]. https://doi.org/10.1097/GOX.0000000000002401.
-
23.
Imani F, Rahimzadeh P, Faiz HR, Nowruzina S, Shakeri A, Ghahremani M. Comparison of the Post-Caesarean Analgesic Effect of Adding Dexmedetomidine to Paracetamol and Ketorolac: A Randomized Clinical Trial. Anesth Pain Med. 2018;8(5). e85311. [PubMed ID: 30538943]. [PubMed Central ID: PMC6252045]. https://doi.org/10.5812/aapm.85311.
-
24.
Tully J, Jung JW, Patel A, Tukan A, Kandula S, Doan A, et al. Utilization of Intravenous Lidocaine Infusion for the Treatment of Refractory Chronic Pain. Anesth Pain Med. 2020;10(6). e112290. [PubMed ID: 34150583]. [PubMed Central ID: PMC8207879]. https://doi.org/10.5812/aapm.112290.
-
25.
De Oliveira GJ, Agarwal D, Benzon HT. Perioperative single dose ketorolac to prevent postoperative pain: a meta-analysis of randomized trials. Anesth Analg. 2012;114(2):424-33. [PubMed ID: 21965355]. https://doi.org/10.1213/ANE.0b013e3182334d68.
-
26.
Niemi TT, Backman JT, Syrjala MT, Viinikka LU, Rosenberg PH. Platelet dysfunction after intravenous ketorolac or propacetamol. Acta Anaesthesiol Scand. 2000;44(1):69-74. [PubMed ID: 10669275]. https://doi.org/10.1034/j.1399-6576.2000.440113.x.
-
27.
Rezabakhsh A, Mahmoodpoor A, Soleimanpour M, Shahsavarinia K, Soleimanpour H. Clinical Applications of Aspirin as a Multi-potent Drug Beyond Cardiovascular Implications: A Proof of Concept for Anesthesiologists- A Narrative Review. Anesth Pain Med. 2021;11(5). e118909. https://doi.org/10.5812/aapm.118909.
-
28.
Trang T, Al-Hasani R, Salvemini D, Salter MW, Gutstein H, Cahill CM. Pain and Poppies: The Good, the Bad, and the Ugly of Opioid Analgesics. J Neurosci. 2015;35(41):13879-88. [PubMed ID: 26468188]. [PubMed Central ID: PMC4604226]. https://doi.org/10.1523/JNEUROSCI.2711-15.2015.
-
29.
Imani F, Zaman B, De Negri P. Postoperative Pain Management: Role of Dexmedetomidine as an Adjuvant. Anesth Pain Med. 2021;10(6). e112176. [PubMed ID: 34150582]. [PubMed Central ID: PMC8207883]. https://doi.org/10.5812/aapm.112176.
-
30.
Ali H, Ismail AA, Wahdan AS. Low-Dose Ketamine Infusion Versus Morphine Infusion During Abdominoplasty to Change the Postoperative Pain Profile. Anesth Pain Med. 2020;10(6). e108469. [PubMed ID: 34150574]. [PubMed Central ID: PMC8207844]. https://doi.org/10.5812/aapm.108469.
-
31.
Imani F, Varrassi G. Ketamine as Adjuvant for Acute Pain Management. Anesth Pain Med. 2019;9(6). e100178. [PubMed ID: 32280623]. [PubMed Central ID: PMC7119219]. https://doi.org/10.5812/aapm.100178.
-
32.
Rahimzadeh P, Faiz SHR, Hoseini M, Mousavie SH, Imani F, Negah AR. Comparison of intraperitoneal bupivacaine, acetazolamide, and placebo on pain relief after laparoscopic cholecystectomy surgery: A clinical trial. Med J Islam Repub Iran. 2018;32:112. [PubMed ID: 30815407]. [PubMed Central ID: PMC6387819]. https://doi.org/10.14196/mjiri.32.112.
-
33.
Imani F, Faiz HR, Sedaghat M, Hajiashrafi M. Effects of adding ketamine to fentanyl plus acetaminophen on postoperative pain by patient controlled analgesia in abdominal surgery. Anesth Pain Med. 2014;4(1). e12162. [PubMed ID: 24660145]. [PubMed Central ID: PMC3961015]. https://doi.org/10.5812/aapm.12162.
-
34.
Albooghobeish M, Ghomeishi A, Adarvishi S, Neisi A, Mahmoodi K, Asadi M, et al. The Effect of Preoperative Zintoma Capsule on Postoperative Nausea and Vomiting After Laparoscopic Cholecystectomy. Anesth Pain Med. 2018;8(5). e67132. [PubMed ID: 30533389]. [PubMed Central ID: PMC6241159]. https://doi.org/10.5812/aapm.67132.
-
35.
Khurana S, Garg K, Grewal A, Kaul TK, Bose A. A comparative study on postoperative pain relief in laparoscopic cholecystectomy: Intraperitoneal bupivacaine versus combination of bupivacaine and buprenorphine. Anesth Essays Res. 2016;10(1):23-8. [PubMed ID: 26957685]. [PubMed Central ID: PMC4767078]. https://doi.org/10.4103/0259-1162.164731.
-
36.
Wasim M, Shafqatullah S. Role of intraperitoneal bupivacaine in laparoscopic cholecystectomy. HBD. 2015;17:32-3.
-
37.
Nupur C, Shubha S, Vinayak SR. Evaluation of intraperitoneal bupivacaine for postoperative analgesia in patients undergoing laparoscopic cholecystectomy: a prospective randomized trial. Anaesth Pain Intensive Care. 2019:361-6.
-
38.
Zmora O, Stolik-Dollberg O, Bar-Zakai B, Rosin D, Kuriansky J, Shabtai M, et al. Intraperitoneal bupivacaine does not attenuate pain following laparoscopic cholecystectomy. JSLS. 2000;4(4):301-4. [PubMed ID: 11051189]. [PubMed Central ID: PMC3113191].
-
39.
Murdoch J, Ramsey G, Day AG, McMullen M, Orr E, Phelan R, et al. Intraperitoneal ketorolac for post-cholecystectomy pain: a double-blind randomized-controlled trial. Can J Anaesth. 2016;63(6):701-8. [PubMed ID: 26864193]. https://doi.org/10.1007/s12630-016-0611-4.
-
40.
Melidi E, Papadima A, Pandazi A, Zografos G. Efficacy of Repeated Intraperitoneal Administration of Levobupivacaine in Pain and Opioid Consumption After Elective Laparoscopic Cholecystectomy: A Prospective Randomized Placebo-controlled Trial. Surg Laparosc Endosc Percutan Tech. 2016;26(4):295-300. [PubMed ID: 27380615]. https://doi.org/10.1097/SLE.0000000000000297.