Abstract
Background:
Maintaining hemodynamic stability during intracranial surgery is one of the most important tasks. There is no general agreement regarding which anesthetics are optimal for craniotomy. Propofol and short-acting opioids are usually used, but their use is not without side effects. Recently, dexmedetomidine has been considered a safe alternative in different surgeries.Objectives:
We aimed to assess the efficacy of 0.5 µg/kg/h dexmedetomidine infusion without loading dose as an adjunct to general anesthesia for craniotomy.Methods:
A prospective, randomized, double-blinded, parallel-group, placebo-controlled trial was conducted. Setting: Single university teaching hospital’s operating rooms and postoperative intensive care unit. Patients: A total of 50 patients scheduled for elective supratentorial craniotomy participated in this study. Interventions: Patients were randomly divided into either control group (group C) and Dexmedetomidine group (group D). Main outcome measure: Intraoperative hemodynamics measurements at specific timings.Results:
We found that dexmedetomidine had significantly maintained mean arterial blood pressure and heart rate (P-value < 0.001); with lower intraoperative fentanyl and propofol consumption in group D (132 ± 35 µg and 14 ± 30 mg, respectively) when compared to group C (260 ± 38 µg and 534 ± 66 mg, respectively). Finally, a lesser sedation level was noticed in the dexmedetomidine group, together with a significantly lesser recovery time of 10.3 ± 4 min.Conclusions:
Dexmedetomidine infusion without loading dose could be an efficacious and safe agent in achieving hemodynamic stability with intraoperative opioid-sparing effect and lesser recovery time.Keywords
1. Background
Maintaining hemodynamic stability during intracranial surgery is one of the most important tasks because good anesthesia increases the success rate of surgery together with improving postoperative prognosis. As known to all, intraoperative hypertension may lead to hemorrhage, and vasogenic edema and low blood pressure may result in cerebral ischemia in areas of impaired autoregulation (1). Dexmedetomidine, a potent α2-adrenoreceptor agonist, has been observed to be able to provide good perioperative hemodynamic stability when given as adjunct general anesthesia (GA) (2). Its usual loading dose is 1 µg/kg, while its maintenance infusion has the range of 0.2 to 0.7 µg/kg/hour until 20 - 30 minutes till the end of surgery. The activation of different subtypes of α2-adrenergic receptors will determine its various effects observed during infusion (3).
Opioids provide effective analgesia and prevent hemodynamic responses to surgical stimulation. But higher doses may be associated with delayed recovery, respiratory depression, increased intracranial tension, and postoperative nausea and vomiting (PONV). Dexmedetomidine, as a promising alternative, effectively reduces opioid requirements and potentiate analgesia. Also, its sympatholytic and antinociceptive properties are advantageous for neurosurgical patients, especially at critical moments. The commonest adverse event associated with Dexmedetomidine infusion is bradycardia, especially if patients receive its loading dose rapidly or given in high maintenance dose (4).
2. Objectives
We designed this randomized, double-blinded study to evaluate the effectiveness of Dexmedetomidine infusion at a dose of 0.5 µg/kg/h without loading dose during supratentorial craniotomies under GA. We compared intraoperative hemodynamics, perioperative analgesic consumption, intraoperative propofol consumption, postoperative sedation scores in both groups. We hypothesize that dexmedetomidine would have a favorable effect on hemodynamic with negligible side effects.
3. Methods
3.1. Ethics
This was a prospective, randomized, parallel-group, placebo-controlled, single-center trial. The work was approved by the Ethics committee of University hospital (FMASU R 115/ 2020) on 18/11/2020 (Ain Shams University Hospital). It was also registered at Clinical Trial Registry ClinicalTrials.gov Identifier: NCT04607525. Written informed consent was obtained from all patients before the study. This trial followed the CONSORT statement.
3.2. Study Population
Fifty American Society of Anesthesiologists-Physical Status (ASA-PS) I and II patients, aged 18 to 65 years, 70 - 80 kg, both sexes, undergoing elective supratentorial craniotomy for tumor resection were included in the study. The study exclusion criteria were emergency surgery, patients with a Glasgow Coma Score (GCS) < 15. Patients on antihypertensive medication (α-methyldopa, clonidine, or other α2-adrenergic agonists) and patients with preoperative heart rate (HR) less than 45 beats per minute or any degree of heart block were also excluded from the study.
3.3. Study Groups
The patients were randomized into two groups: Dexmedetomidine group (group D): Patients received 0.5 µg/kg/h of dexmedetomidine and control group (group C): Patients received equal volume and rate of normal saline 0.9%. Infusion of blinded solutions was started after intubation. Dexmedetomidine dosing regimen was in accordance with existing guidelines (1, 5-7).
3.4. Patients’ Recruitment and Randomization
Randomization was performed using a computer‐generated random number table in opaque sealed envelopes with 1:1 allocation ratio by an anesthesiologist not directly involved in the trial. An anesthesia technician prepared the study drug syringes according to the sequence number and assigned patients to the trial groups.
3.5. Anesthesia
Patients received midazolam as a preanesthetic medication, and 1 mg granisetron together with 8 mg dexamethasone as PONV prophylaxis after application of routine standard monitoring. A standard anesthetic technique was followed after adequate preoxygenation with: propofol 2-3 mg/kg and fentanyl 1 µg/kg. Atracurium besylate 0.5 mg/kg was used to provide muscle relaxation. After intubation and securing the endotracheal tube, we started the blinded study solution. We also started a variable-rate propofol infusion up to a maximum limit of 0.15 mg/kg/min. Propofol infusion dosage was in accordance with previous existing guidelines (8). We then placed other invasive monitoring for rigorous patient follow-up. A maximum of 1.2% (vaporizer setting) of isoflurane mixed with oxygen (50%) and air (50%) was used for the maintenance of anesthesia. Notably, low-dose isoflurane may negatively affect the diseased cortical tissue (9). Additionally, prolonged high-dose of most volatile anesthetic agents may lead to delayed recovery. Accordingly, an anesthetic regimen with a 1.2% (vaporizer setting) of isoflurane was our maximum limit. Following both of skull pinning and skin incision, a bolus of 50 μg fentanyl was given intravenously to attenuate the expected hemodynamic response. Additionally, 0.5 µg/kg intravenous (IV) fentanyl was titrated intraoperatively at the discretion of the attending anesthesiologists till the start of the closure of dura. No other intraoperative adjunct analgesics were given. In the present study, we used fentanyl because it has little effect on cerebral blood flow (CBF) regulation (10).
Mean arterial blood pressure (MAP) and heart rate (HR) were measured at specific time points. Any increase or decrease in HR or blood pressure was managed as required after exclusion of a surgical cause. For example, MAP or HR rise of > 20% above baseline was treated by administering a 0.5 μg/kg intravenous bolus of fentanyl and increasing propofol infusion rate. At the end of the procedure, the neuromuscular blockade was reversed. Patients were extubated and then transferred to the intensive care unit (ICU). Postoperatively, 5 mg intravenous Nalbuphine was prescribed for all patients of both groups when a patient’s pain visual analogue scale score was > 4. Also, a fixed dose of 1 g intravenous acetaminophen was given /8h IV.
3.6. Outcome Measurements
The primary outcome was intraoperative hemodynamic changes in response to dexmedetomidine infusion, and the secondary outcomes included the total need for rescue agents (fentanyl and propofol).
Summary of outcome measurements:
1) Demographic data and patients’ characteristics.
2) The duration of the surgery.
3) Recovery time in minutes (Time interval between stopping of isoflurane and extubation).
4) Intraoperative hemodynamics were recorded at the following timings:
T0: Preoperatively as baseline
T1: After intubation
T2: During pinning
T3: At skin incision
H1: After 1 hour from surgical incision
H2: After 2 hours
H3: After 3 hours
H4: After 4 hours
T-end: At skin closure
ICU H1: after 1 hour from ICU admission
ICU H2: after 2 hours from ICU admission
5) Total intraoperative fentanyl consumption in microgram. (Induction dose and predetermined bolus doses given at skin incision and pinning were not included).
6) Total intraoperative propofol consumption in mg.
7) Total postoperative Nalbuphine consumption in mg during 1st postoperative day.
8) Assessment of postoperative sedation level using University of Michigan Sedation Scale (UMSS) (11) (Table 1).
| Score | Patient’s State |
|---|---|
| 0 | Awake and alert |
| 1 | Minimally sedated |
| 2 | Moderately sedated |
| 3 | Deeply sedated |
| 4 | Unarousable |
It was assessed every hour for 2 hours, starting from extubation.
3.7. Sample Size Calculation
The sample size was calculated using STATA program, setting the type-1 error (α) at 0.05 and the power (1- β) at 0.8. The results of the previous study (12) showed that the mean MAP in the Dexmedetomidine group (60 minutes after pinning) was 99.3 ± 6.2 compared to the control group that was 107.2 ± 2.46. Accordingly, a sample size of 25 cases in the Dexmedetomidine group and 25 cases in the control group would achieve 100% power to detect the observed difference.
3.8. Data Management and Analysis
The collected data were revised, coded, tabulated, and introduced to a PC using Statistical package for social science (SPSS 20). Data were presented, and suitable analysis was done according to the type of data obtained for each parameter. Descriptive statistics: mean, standard deviation (± SD) for numerical data, frequency, and percentage of non-numerical data. Analytical statistics: Student t-test was used to assess the statistical significance of the difference between two study group means and, chi-square test was used to examine the relationship between two qualitative variables.
4. Results
All of the 50 patients successfully completed the study (Figure 1). Demographic data and duration of surgery were statistically comparable in both groups (Tables 2 and 3).
Flow diagram of the patients
Demographic Data
| Control, No. (%) | Dexmedetomidine, No. (%) | Chi-Square Test | |||
|---|---|---|---|---|---|
| χ2 | P-Value | Sig. | |||
| Gender | 0.085 | 0.771 | NS | ||
| Male | 16 (64.0) | 15 (60.0) | |||
| Female | 9 (36.0) | 10 (40.0) | |||
| ASA-PS | 0 | 1 | NS | ||
| I | 13 (52.0) | 13 (52.0) | |||
| II | 12 (48.0) | 12 (48.0) | |||
Patients’ Characteristics and Perioperative Data
| Control, Mean (SD) | Dexmedetomidine, Mean (SD) | t-Test | |||
|---|---|---|---|---|---|
| T | P-Value | Sig. | |||
| Age (y) | 49.9 (13.9) | 49.8 (13.0) | 0.021 | 0.983 | NS |
| Body mass index (kg/m2) | 26.8 (2.2) | 26.6 (2.4) | 0.245 | 0.808 | NS |
| Surgery duration (min) | 393.20 (54.37) | 365.60 (57.01) | 1.752 | 0.086 | NS |
| Recovery time (min) | 31.4 (5.1) | 10.3 (4.1) | 16.140 | < 0.001 | S |
| Total intraoperative fentanyl (µg) | 260.0 (38.2) | 132.0 (35.0) | 12.355 | < 0.001 | S |
| Total intraoperative propofol (mg) | 534.4 (66.3) | 14.0 (30.7) | 35.599 | < 0.001 | S |
| Postoperative nalbuphine (mg) | 7.4 (2.9) | 7.4 (2.9) | 0 | 1 | NS |
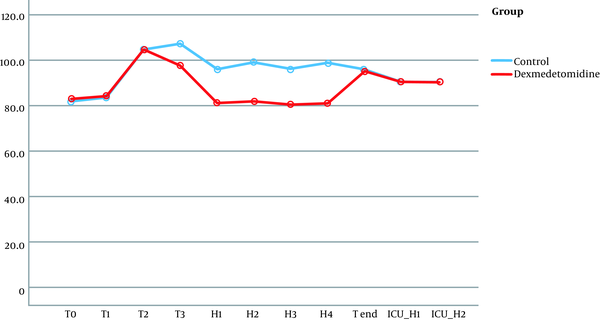
No significant differences were observed in terms of hemodynamic parameters at the following timings: baseline readings, after intubation, and during pinning. Although patients in the dexmedetomidine group did not have a significant rise in MAP together with lower HR during surgery (at H1, H2, H3, H4), the patients of the control group had a significant rise in MAP and HR (Figures 2 and 3).
Mean arterial blood pressure follow-up in both groups.
Heart rates follow-up in both groups
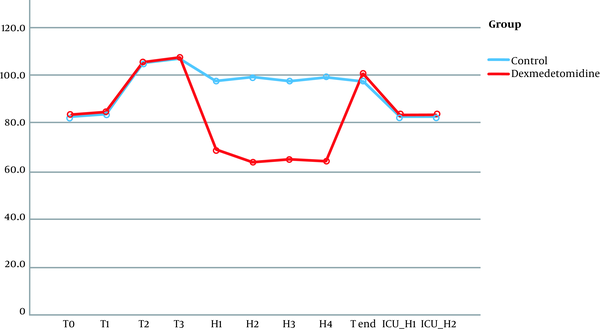
Table 3 also shows higher mean intraoperative additional fentanyl requirements and mean intraoperative propofol doses in the control group. Additionally, recovery time was found to be significantly shorter in the dexmedetomidine group (10.3 ± 4 min) when compared to the control group (31.4 ± 5 min) (P < 0.001). Finally, a higher sedation level was observed in the control group rather than the dexmedetomidine group at the time of extubation and at 60 min after extubation, and this difference was statistically significant (Table 4).
University of Michigan Sedation Scale
5. Discussion
Craniotomy surgeries are usually characterized by brief periods of intense stimulation that are interposed with long periods of little stimulation, which makes them a challenging type of surgery for anesthesiologists. Intraoperative hypertensive episodes consequent to noxious stimuli may be complicated postoperatively by intracranial hemorrhage and cerebral edema. It is known that a change of more than 20 - 25% in MAP may have deleterious effects (13).
The current study demonstrated that perioperative use of dexmedetomidine without a loading dose for supratentorial craniotomy operations provided stable intraoperative hemodynamics at various time intervals. An intravenous bolus of dexmedetomidine leads to a biphasic blood pressure response (3). Dexmedetomidine infusion induces an initial transient increase in MAP (due to activation of postsynaptic α2B receptors), followed by a decrease in MAP and HR (by the activation of α2A receptors in the central nervous system). The omission of the dexmedetomidine loading bolus can prevent initial hypertension (3)
Regarding dexmedetomidine intraoperative hemodynamic stability, our results are supported by the findings of many studies (1, 2, 10, 14-20) that established dexmedetomidine, given by infusion, attenuated intraoperative hemodynamic stress response whether given as 0.4 µg/kg/h without loading dose (1) or in a ranging dose of 0.4 to 0.6 µg/kg/h after loading dose of 1 µg/kg/h (2, 12, 14-18). The same conclusion was made even when it was given in a target-controlled infusion manner (10) or in a high maintenance dose (19). Additionally, previous studies (15-17) suggested that the use of dexmedetomidine improved the hemodynamic stability in patients with bispectral index (BIS)-guided anesthesia.
Dexmedetomidine side effects are usually in the form of hemodynamic alterations. In our study, we did not observe significant bradycardia requiring intervention, nor hypotension due to the low dose of dexmedetomidine infusion used.
Dexmedetomidine is known to have analgesic potential (21). Consistent with this study, we observed significantly lower intraoperative requirements of analgesics. Our study also confirmed the findings of previous studies (2, 5, 10, 14-16, 18, 19, 22)regarding anesthetic and analgesic sparing effects of dexmedetomidine during surgeries. For example, Chakrabati et al. (5) noticed a significant reduction of intraoperative -BIS guided- fentanyl and propofol utilization in patients undergoing cerebellopontine angle surgeries. Also, the same finding is consistent with studies done on other types of surgeries (23, 24).
In dis-concordance to our results, Sriganesh and their colleagues (6, 7) demonstrated that a dexmedetomidine infusion of 0.5 µg/kg/h without loading dose was not superior to fentanyl. This could be attributed to the bilateral scalp block group that was given in their patients, which may have influenced intraoperative surgical stress response independent of study drugs given, and so abolishing hemodynamic differences between both groups. Also, Rajan et al. (13) observed the same findings that dexmedetomidine infusion of 0.5 - 1 µg/kg/h after a loading dose was not superior to remifentanil infusion during brain tumor surgery. Their results could be attributed to the high potency of remifentanil’s analgesic properties.
One of the main anesthesia goals after craniotomy is rapid awakening from anesthesia to allow early neurosurgical assessment and subsequent early detection of cerebral complications. We reported that the mean time to extubation was less in group D compared to group C. The low requirement of intraoperative narcotics and propofol due to dexmedetomidine usage may have fastened recovery from anesthesia as observed in previous studies (1, 6). Additionally, many studies (10, 14, 18, 19) also observed that the dexmedetomidine infusion resulted in faster recovery after general anesthesia without causing any respiratory depression. On the contrary to our findings, Chakrabati et al. (5) and Mathew et l. (17) noticed prolonged recovery in the dexmedetomidine group; however, it was statistically insignificant when compared to the control group. One could attribute these findings to a global decrease in inhalational and narcotic consumption due to their strict titration, guided by BIS monitor. It is known that hemodynamic variability, when used as an anesthetic titration guide, usually leads to overdosing of used anesthetics (5). Also, Rajan et al. (13), Turgut et al. (25), and Javaherforooshzadeh et al. (26) observed longer extubation time in the dexmedetomidine group when compared to the remifentanil group. This could be attributed to remifentanil’s rapid onset of action and ultra-short duration. Finally, we observed a lesser sedation score, adding to more benefits of dexmedetomidine usage in supratentorial craniotomies, which is supported by previous results (1, 15, 18).
5.1. Limitations
There were some limitations in the present study. First, we could use devices for monitoring the depth of anesthesia, but there was a lack of reports regarding its effectiveness in neurosurgical patients; as the intracranial air may interfere with BIS monitor and result in poor signal transfer (27). Additionally, bispectral index monitoring was not available in our hospital. Second, the effect of dexmedetomidine on cerebral perfusion and intracranial pressure was not studied. Neuroprotective effect of dexmedetomidine is controversial (28, 29). So further studies need to be done to observe if any neuroprotection is provided by dexmedetomidine. Finally, it is plausible to use targeted plasma concentrations. This will permit attending anesthesiologist to titrate the dexmedetomidine dose leading to improvement in hemodynamic stability and even shorter awakening times.
5.2. Conclusions
Dexmedetomidine at a dose of 0.5 μg/kg/h infusion without loading dose provided stable intraoperative hemodynamics in patients undergoing supratentorial craniotomy. Furthermore, it was accompanied by its reduced intraoperative requirement and rapid recovery.
Acknowledgements
References
-
1.
Batra A, Verma R, Bhatia VK, Chandra G, Bhushan S. Dexmedetomidine as an anesthetic adjuvant in intracranial surgery. Anesth Essays Res. 2017;11(2):309-13. [PubMed ID: 28663612]. [PubMed Central ID: PMC5490096]. https://doi.org/10.4103/0259-1162.194555.
-
2.
Kaushal RP, Gupta D, Kaushal B, Mathur A. Study to assess the role of dexmedetomidine in patients with intracranial tumors undergoing craniotomy under general anesthesia. J Evol Med Dent Sci. 2013;2(43):8305-14.
-
3.
Castillo RL, Ibacache M, Cortinez I, Carrasco-Pozo C, Farias JG, Carrasco RA, et al. Dexmedetomidine improves cardiovascular and ventilatory outcomes in critically ill patients: Basic and clinical approaches. Front Pharmacol. 2019;10:1641. [PubMed ID: 32184718]. [PubMed Central ID: PMC7058802]. https://doi.org/10.3389/fphar.2019.01641.
-
4.
Peng K, Wu S, Liu H, Ji F. Dexmedetomidine as an anesthetic adjuvant for intracranial procedures: meta-analysis of randomized controlled trials. J Clin Neurosci. 2014;21(11):1951-8. [PubMed ID: 24974190]. https://doi.org/10.1016/j.jocn.2014.02.023.
-
5.
Chakrabarti D, Kamath S, Madhusudan Reddy KR, Srinivas DB, Manohar N, Masapu D. Effect of adjunctive dexmedetomidine on anesthesia and analgesia requirement and recovery characteristics during Bispectral Index-guided anesthesia for cerebello-pontine angle surgeries: A randomized clinical trial. J Anaesthesiol Clin Pharmacol. 2018;34(4):496-502. [PubMed ID: 30774230]. [PubMed Central ID: PMC6360882]. https://doi.org/10.4103/joacp.JOACP_55_18.
-
6.
Sriganesh K, Syeda S, Shanthanna H, Venkataramaiah S, Palaniswamy SR. Effect of opioid versus non-opioid analgesia on surgical pleth index and biomarkers of surgical stress during neurosurgery for brain tumors: Preliminary findings. Neurol India. 2020;68(5):1101-5. [PubMed ID: 33109859]. https://doi.org/10.4103/0028-3886.294559.
-
7.
Sriganesh K, Syeda S, Shanthanna H, Venkataramaiah S, Palaniswamy SR. Comparison of intraoperative fentanyl with dexmedetomidine for perioperative analgesia and opioid consumption during craniotomies: A randomised controlled pilot study with non-inferiority design. Int J Clin Pract. 2019;73(6). e13338. [PubMed ID: 30829429]. https://doi.org/10.1111/ijcp.13338.
-
8.
Chan VW, Chung FF. Propofol infusion for induction and maintenance of anesthesia in elderly patients: recovery and hemodynamic profiles. J Clin Anesth. 1996;8(4):317-23. [PubMed ID: 8695136]. https://doi.org/10.1016/0952-8180(96)00041-4.
-
9.
Lee EJ, Lee MY, Shyr MH, Cheng JT, Toung TJ, Mirski MA, et al. Adjuvant bupivacaine scalp block facilitates stabilization of hemodynamics in patients undergoing craniotomy with general anesthesia: a preliminary report. J Clin Anesth. 2006;18(7):490-4. [PubMed ID: 17126775]. https://doi.org/10.1016/j.jclinane.2006.02.014.
-
10.
Tanskanen PE, Kytta JV, Randell TT, Aantaa RE. Dexmedetomidine as an anaesthetic adjuvant in patients undergoing intracranial tumour surgery: a double-blind, randomized and placebo-controlled study. Br J Anaesth. 2006;97(5):658-65. [PubMed ID: 16914460]. https://doi.org/10.1093/bja/ael220.
-
11.
Malviya S, Voepel-Lewis T, Tait AR, Merkel S, Tremper K, Naughton N. Depth of sedation in children undergoing computed tomography: validity and reliability of the University of Michigan Sedation Scale (UMSS). Br J Anaesth. 2002;88(2):241-5. [PubMed ID: 11878656]. https://doi.org/10.1093/bja/88.2.241.
-
12.
Jadhav N, Wasekar N, Wagaskar V, Kondwilkar B, Patil R. Use of dexmedetomidine in patients undergoing craniotomies. J Clin Diagn Res. 2017;11(1):UC01-8. [PubMed ID: 28274022]. [PubMed Central ID: PMC5324467]. https://doi.org/10.7860/JCDR/2017/24002.9235.
-
13.
Rajan S, Hutcherson MT, Sessler DI, Kurz A, Yang D, Ghobrial M, et al. The effects of dexmedetomidine and remifentanil on hemodynamic stability and analgesic requirement after craniotomy: A randomized controlled trial. J Neurosurg Anesthesiol. 2016;28(4):282-90. [PubMed ID: 26325514]. https://doi.org/10.1097/ANA.0000000000000221.
-
14.
Ilhan O, Koruk S, Serin G, Erkutlu I, Oner U. Dexmedetomidine in the supratentorial craniotomy. Eurasian J Med. 2010;42(2):61-5. [PubMed ID: 25610125]. [PubMed Central ID: PMC4261342]. https://doi.org/10.5152/eajm.2010.19.
-
15.
Tripathi M, Kumar V, Malviya D, Singh M, Kumar MTyagi A. Hemodynamic and recovery profile with dexmedetomidine and fentanyl in intracranial supratentorial surgeries: a comparative study. IOSR J Pharm. 2015;5(11):43-8.
-
16.
Bekker A, Sturaitis M, Bloom M, Moric M, Golfinos J, Parker E, et al. The effect of dexmedetomidine on perioperative hemodynamics in patients undergoing craniotomy. Anesth Analg. 2008;107(4):1340-7. [PubMed ID: 18806050]. https://doi.org/10.1213/ane.0b013e3181804298.
-
17.
Mathew PJ, Jain A, Wig J, Mukherjee KK, Gupta A. Randomized controlled trial of intravenous dexmedetomidine for control of hemodynamics, surgical bleeding, and recovery profile during transsphenoidal pituitary surgery. JNACC. 2019;7(2):91-5. https://doi.org/10.1055/s-0039-1697558.
-
18.
Soliman RN, Hassan AR, Rashwan AM, Omar AM. Prospective, randomized study to assess the role of dexmedetomidine in patients with supratentorial tumors undergoing craniotomy under general anaesthesia. Middle East J Anaesthesiol. 2011;21(3):325-34. [PubMed ID: 22428485].
-
19.
Gupta A, Dwivedi Y, Saxena S, Srivastava U, Mangla S, Mishra S. A randomized control study of dexmedetomidine versus fentanyl as an anesthetic adjuvant in supratentorial craniotomies. Anaesth Pain Intensive Care. 2019:307-11.
-
20.
Janatmakan F, Nesioonpour S, Javaherforoosh Zadeh F, Teimouri A, Vaziri M. Comparing the effect of clonidine and dexmedetomidine on intraoperative bleeding in spine surgery. Anesth Pain Med. 2019;9(1). e83967. [PubMed ID: 30881906]. [PubMed Central ID: PMC6408748]. https://doi.org/10.5812/aapm.83967.
-
21.
Imani F, Zaman B, De Negri P. Postoperative pain management: Role of dexmedetomidine as an adjuvant. Anesth Pain Med. 2021;10(6). https://doi.org/10.5812/aapm.112176.
-
22.
Asri S, Hosseinzadeh H, Eydi M, Marahem M, Dehghani A, Soleimanpour H. Effect of dexmedetomidine combined with inhalation of isoflurane on oxygenation following one-lung ventilation in thoracic surgery. Anesth Pain Med. 2020;10(1). e95287. [PubMed ID: 32309196]. [PubMed Central ID: PMC7145426]. https://doi.org/10.5812/aapm.95287.
-
23.
J N S, Kumar S, Vijay T. To compare the efficacy of dexmedetomidine versus labetalol in providing controlled hypotension in functional endoscopic sinus surgery. Anesth Pain Med. 2021;11(1). https://doi.org/10.5812/aapm.108915.
-
24.
Amri P, Nahrini S, Hajian-Tilaki K, Hamidian M, Alipour SF, Hamidi SH, et al. Analgesic effect and hemodynamic changes due to dexmedetomidine versus fentanyl during elective colonoscopy: A double-blind randomized clinical trial. Anesth Pain Med. 2018;8(6). e81077. [PubMed ID: 30719412]. [PubMed Central ID: PMC6347670]. https://doi.org/10.5812/aapm.81077.
-
25.
Turgut N, Turkmen A, Ali A, Altan A. Remifentanil-propofol vs dexmedetomidine-propofol--anesthesia for supratentorial craniotomy. Middle East J Anaesthesiol. 2009;20(1):63-70. [PubMed ID: 19266828].
-
26.
Javaherforooshzadeh F, Monajemzadeh SA, Soltanzadeh M, Janatmakan F, Salari A, Saeed H. A comparative study of the amount of bleeding and hemodynamic changes between dexmedetomidine infusion and remifentanil infusion for controlled hypotensive anesthesia in lumbar discopathy surgery: A double-blind, randomized, clinical trial. Anesth Pain Med. 2018;8(2). e66959. [PubMed ID: 30009153]. [PubMed Central ID: PMC6035495]. https://doi.org/10.5812/aapm.66959.
-
27.
Akavipat P, Hungsawanich N, Jansin R. Alternative placement of bispectral index electrode for monitoring depth of anesthesia during neurosurgery. Acta Med Okayama. 2014;68(3):151-5. [PubMed ID: 24942793]. https://doi.org/10.18926/AMO/52655.
-
28.
Engelhard K, Werner C, Kaspar S, Mollenberg O, Blobner M, Bachl M, et al. Effect of the alpha2-agonist dexmedetomidine on cerebral neurotransmitter concentrations during cerebral ischemia in rats. Anesthesiology. 2002;96(2):450-7. [PubMed ID: 11818781]. https://doi.org/10.1097/00000542-200202000-00034.
-
29.
Arulvelan A, Manikandan S, Easwer HV, Krishnakumar K. Cerebral vascular effects of loading dose of dexmedetomidine: A Transcranial Color Doppler study. Indian J Crit Care Med. 2016;20(1):9-13. [PubMed ID: 26955211]. [PubMed Central ID: PMC4760000]. https://doi.org/10.4103/0972-5229.173680.